Abstract
Innate regulation through Toll-like receptor (TLR) signaling has been shown to be important for promoting T cell subset development and function. However, limited information is known about whether differential TLR signaling can selectively inhibit Th17 and/or Th1 cells, important for controlling excessive inflammation and autoimmune responses. Here we demonstrate that activation of TLR7 signaling in T cells can inhibit both Th17 cell differentiation from naïve T cells and IL-17 production in established Th17 cells. We further report that down-regulation of STAT3 signaling is responsible for TLR7-mediated inhibition of Th17 cells due to induction of SOCS3 and SOCS5. TLR7-mediated suppression of Th17 cells does not require DC involvement. In addition, we show that TLR7 signaling can suppress Th1 cell development and function due to a mechanism different from Th17 cell suppression. Importantly, our complementary in vivo studies demonstrate that treatment with the TLR7 ligand imiquimod can inhibit both Th1 and Th17 cells, resulting in prevention of and immunotherapeutic reduction in experimental autoimmune encephalomyelitis (EAE). These studies identify a new strategy to manipulate Th17/Th1 cells through TLR7 signaling, with important implications for successful immunotherapy against autoimmune and inflammatory diseases.
Keywords: Th17 cells, Th1 cells, Toll-like receptor, Experimental autoimmune encephalomyelitis, Imiquimod
Introduction
Th17 cells are an important cell subset involved in the pathogenesis of a broad array of inflammatory and autoimmune diseases such as rheumatoid arthritis, psoriasis and inflammatory bowel disease, and also play an important role in host defense against microbial infections (1–3). In addition, Th17 cells are a key cell component in the tumor microenvironment involved in the pathogenesis and tumorigenesis of cancer development (4–7). Differentiation and development of Th17 cells is dependent on the specific cytokine combinations of IL-1, IL-6, IL-21, IL-23 and TGF-β both in mice and in humans (8–11). Furthermore, molecular programming of transcription regulation is also a determinant for Th17 development, including signal transducer and activator of transcription 3 (Stat3), retinoid-related orphan receptor γt (RORγt), RORα, IFN regulatory factor-4 (IRF4), BATF, and hypoxia-inducible factor 1 alpha (HIF1)(12–14). Improved understanding of the molecular mechanisms involved in regulation of Th17 cell development and functions will facilitate the development of novel strategies specifically targeting Th17 cells for clinical interventions.
TLRs have recently been recognized as critical components of the innate immune system, acting as a link between innate and adaptive immunity (15). TLRs are also very important for regulating T cell subset development and functions, including Th1, regulatory T (Treg) and Th17 cells (16, 17). TLR2 signaling can directly enhance both the proliferation and IL-17 production by Th17 cells and promote the pathogenesis of EAE (16). TLR3 ligand Poly (I:C) and TLR9 ligand CpG can stimulate Th1 responses and mediate potent anti-tumor activity (17). TLR4 activation in CD4+ T cells is essential for the proliferation and survival of Th1 and Th17 cells and stimulates IL-17 production (17, 18). We have demonstrated that human TLR8 signaling directly reverses the suppressive functions of naturally occurring CD4+CD25+ Treg cells, as well as tumor-derived CD4+, CD8+ and γδ Treg cells (19–23). Furthermore, we found that TLR signaling promotes chemoattraction and generation of Th17 cells in the tumor microenvironment through tumor cells and tumor-derived fibroblasts (5). Understanding the unique signaling pathways involved in the precise regulation of T cell subsets by different TLRs will be important for the development of TLR-based immunotherapy against various diseases. In fact, several TLR ligands are now being developed as immunotherapeutic drugs or vaccine adjuvants for cancer treatment (24, 25).
Besides the promotion of T cell immune responses, limited information is known about whether TLR signaling can inhibit Th17 and/or Th1 cells, which is important for controlling excessive inflammation and autoimmune responses. EAE is the most extensively studied animal model for multiple sclerosis (MS), an inflammatory demyelinating disease of the central nervous system (CNS) (26). Both Th1 and Th17 myelin reactive CD4+ T cells are now known to be involved in MS and EAE (27). Regulation of EAE development and pathogenesis through innate TLR signaling has been investigated (28). Recent reports demonstrated that agonists of certain TLRs, including TLR2, TLR4, and TLR9, promoted pathogenesis of EAE (16, 29, 30); while TLR3 signaling protected against disease (31). The role of TLR7 signaling in the regulation of EAE has also been explored but remains controversial. On the one hand, TLR7 signaling was reported to protect against EAE through increased IFN-β production and elicitation of IL-10 and IL-10–inducing cytokines (32–34). In contrast, several studies have shown that stimulation of TLR7 can also promote EAE pathogenesis by direct regulation of DCs and downstream effects on adaptive T cell responses (35–38). Therefore, whether and/or how TLR7 signaling influences Th17 and/or Th1 cells, two key players for EAE remains unknown. Furthermore, whether DCs are required for TLR7 regulation of autoimmune EAE and T cell responses in the disease progression is also unclear and needs to be investigated. Improved understanding of these molecular mechanisms and regulatory events will open new avenues to develop novel therapeutic strategies for the treatment of T cell-mediated immune diseases.
To better understand the regulation of Th17 cells mediated by TLR signaling, we screened a panel of TLR ligands on both murine and human Th17 cells. We demonstrated that activation of TLR7 signaling in T cells can inhibit both Th17 cell differentiation from naïve T cells and IL-17 production in established Th17 cells. We further revealed that downregulated STAT3 signaling is responsible for the TLR7-mediated inhibition of Th17 cells, dependent on the induction of SOCS3 and SOCS5. In addition, we also observed that TLR7 signaling could suppress Th1 cell development and function, which may through a different mechanism from that of Th17 cells. Importantly, our complementary in vivo studies demonstrated that activation of TLR7 signaling prevents the development of EAE and reduces the disease severity in vivo. These studies provide a new strategy to specifically target Th17/Th1 cells via TLR7 signaling for successful immunotherapy against T cell-related inflammatory and autoimmune diseases.
Materials and Methods
Human samples and cell lines
Tumor samples were obtained from hospitalized patients in the Department of Surgery at St. Louis University from 2004 to 2014 who have given informed consents for enrollment in a prospective tumor procurement protocol approved by the Saint Louis University Institutional Review Board. Buffy coats from healthy donors were obtained from the Gulf Coast Regional Blood Center at Houston. Peripheral blood mononuclear cells (PBMCs) were purified from buffy coats using Ficoll-Paque. Human naïve CD4+ and CD8+ T cells were purified by EasySep enrichment kits (StemCell Technologies). Jurkat T and 293T cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA), and maintained in RPMI 1640 medium containing 10% FCS.
Mice
C57BL/6 and FoxP3EGFP transgenic mice were purchased from The Jackson Laboratory. STAT3fl/flCD4cre− and STAT3fl/flCD4cre+ mice were kindly provided by Dr. Daniel Hawiger (Department of Molecular Microbiology & Immunology at Saint Louis University School of Medicine). MyD88−/− mice were provided by Dr. Richard Flavell (Yale University School of Medicine). All mice were maintained in the institutional animal facility and all animal studies have been approved by the Institutional Animal Care Committee of Saint Louis University.
T cell subset differentiation
Mouse CD4+ T cell in vitro differentiation was performed as previously described (8, 10). Briefly, naïve CD4+ T cells were purified from spleens and peripheral lymph nodes of 6–8 week mice of C57BL/6, FoxP3EGFP, STAT3fl/fl CD4cre−, STAT3fl/flCD4cre+, or MyD88−/− mice, with CD4+ T-cell enrichment kit (Stem cell Technologies) and then cultured with plate-bound anti-CD3 (2 μg/ml) and anti-CD28 (2 μg/ml) (Bio X Cell) plus polarization condition medium at 37°C for 6 days. For Th1 differentiation, naïve T cells were cultured in the presence of anti-IL-4 neutralizing antibody (10 μg/ml, 11B11, Bio X Cell), and recombinant mouse IL-12 (rmIL-12; 5 ng/ml, R & D). For Th2 differentiation, naïve T cells were cultured in the presence of anti-IFN-γ neutralizing antibody (10 μg/ml, XMG1.2, Bio X Cell) and rmIL-4 (4 ng/ml, R & D). For Th17 differentiation, naïve T cells were cultured in the presence of anti-IL-4 and anti-IFN-γ neutralizing antibodies (10 μg/ml), rmIL-6 (50 ng/ml, Peprotech) and rmTGF-β (1 ng/ml, R & D). For Treg differentiation, naïve T cells from FoxP3EGFP mice were culture in the presence of rmIL-2 (100 U/ml, R & D) and rmTGF-β (5 ng/ml, R & D). Human Th17 cell differentiation was induced as we described previously (4, 5). Naïve T cells purified from PBMCs of healthy donors were cultured in T cell medium (RPMI-1640 medium containing 10% human serum supplemented with L-glutamine, 2-mercaptethanol, and 50 U/ml IL-2) in the presence of IL-1β (20 ng/ml), IL-6 (20 ng/ml), and IL-23 (10 ng/ml) (R & D) for 6 days. In some experiments, T cell differentiation was induced in the presence or absence of TLR ligands, including Pam3CSK4 (200 ng/ml), Poly (I:C) (25 μg/ml), LPS (100 ng/ml), flagellin (10 μg/ml), loxoribine (Lox, 500 μm), imiquimod (Imiq, 10 μg/ml), and CpG-B (3 μg/ml) (Invivogen ).
Mouse DC preparation and in vitro polarization of Th17 cells with DCs
Mouse DCs were generated with the B16-Flt3L injection strategy (39) (B16-Flt3L was kindly provided by Dr. John T. Harty at University of Iowa). CD11c+ cells were isolated from the spleen cells of tumor-bearing mice using anti-CD11c microbeads (Miltenyi Biotec). The purity and activation status of DCs were determined by expression of CD11c, CD86, and MHC-class II. For Th17 differentiation with DCs, mouse naïve CD4+ T cells were cultured with DCs at a ratio of 10:1 with 1 μg/ml soluble anti-CD3 antibody and Th17 polarization condition medium, in the presence or absence of various TLR ligands for 6 days. In some experiments, DCs were pretreated with/without various TLR ligands for 1 day, and then co-cultured with CD4+ T cells to generate Th17 cells, or DCs were separated with CD4+ T cells by a transwell insert (0.4 um) in the Transwell systerm, as we described before (5, 21, 23).
Generation of human tumor-infiltrating Th17 cell clones
Tumor-infiltrating lymphocytes (TIL) were generated from different tumor tissues derived from cancer patients, as we previously described (4, 5). Briefly, tissues were minced into small pieces followed by digestion with collagenase type IV, hyaluronidase, and deoxyribonuclease. After digestion, cells were washed in RPMI1640, and then cultured in RPMI1640 containing 10% human serum supplemented with L-glutamine, 2-mercaptethanol and 50 U/ml of IL-2 for the generation of T cells. Th17 cell clones were generated from TILs by a limiting dilution cloning method and confirmed by FACS analysis after intracellular staining for IL-17, as we previously described (4, 21).
Flow cytometry analysis
Expression markers on T cells were determined by FACS analysis after surface staining or intracellular staining with specific anti-human or anti-mouse antibodies conjugated with PE, FITC, APC, PerCP-Cy5.5, or V450. Anti-human antibodies included: anti-IL4, anti-IFN-γ, anti-IL-17A, and anti-FoxP3. Anti-mouse antibodies included: anti-IL4, anti-IFN-γ, anti-IL-17A, anti-CD25, anti-FoxP3, anti-CD19, anti-CD11c, anti-CD11b, anti-Ly6G/Ly6C (Gr-1), and anti-TCRγδ (purchased from BD Biosciences, BioLegend or eBioscience). Intracellular staining for cytokine-producing T cells was performed on T cells stimulated with PMA and ionomycin (Sigma-Aldrich) in the presence of GolgiStop (BD Bioscience) for 5 hours. All stained cells were analyzed on a FACSCalibur flow cytometer (BD Bioscience) and data analyzed with FlowJo software (Tree Star).
Luciferase Assays
Jurkat T cells or 293T cells were transfected with plasmid constructs of empty vector pGL3-B, control h17C-Luc, or h17P-Luc containing IL-17 promoter with the Gene Pulser II electroporator (Bio-Rad) at 250 V, 950 microfarads (Constructs were kindly provided by Dr. Sarah Gaffen, University of Pittsburgh) (40). Each reporter experiment included 20 ng of Renilla luciferase construct as an internal control. Cells were collected at 24 hours after transfection, and then lysed, and luciferase activity was quantified using the Dual-Luciferase assay system (Promega) according to the manufacturer’s instructions. All experiments were performed in triplicate.
Reverse-transcription PCR analysis
Total RNA was extracted from T cells using Trizol reagent (Invitrogen), and cDNA was transcribed using a SuperScript II RT kit (Invitrogen), both according to manufacturers’ instructions. Expression levels of transcription factors, cytokines and cytokine receptors were determined by reverse-transcription PCR using specific primers, and mRNA levels in each sample were normalized to the relative quantity of Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression. All experiments were performed in triplicate. The specific primers used for human T cells are as described previously (4, 5). The specific primers used for mouse T cells are listed in Table 1.
Table 1.
The specific primers used for mouse T cells
| Primer Name | Sequence (5′--3′) |
|---|---|
| Mouse IL-4 forward | TGAACGAGGTCACAGGAGAAG |
| Mouse IL-4 reverse | GAGCTCACTCTCTGTGGTGTT |
| Mouse IL-10 forward | TGAATTCCCTGGGTGAGAAGCT |
| Mouse IL-10 reverse | TGCTCCACTGCCTTGCTCTTAT |
| Mouse IL-17A-forward | CTCCAGAAGGCCCTCAGACTAC |
| Mouse IL-17A-reverse | GGGTCTTCATTGCGGTGG |
| Mouse IL-21-forward | ATCCTGAACTTCTATCAGCTCCAC |
| Mouse IL-21-reverse | GCATTTAGCTATGTGCTTCTGTTTC |
| Mouse IFNγ-forward | ATGAACGCTACACACTGCATC |
| Mouse IFNγ-reverse | CCATCCTTTTGCCAGTTCCTC |
| Mouse TGFβ-1-forward | GCCCGAAGCGGACTACTATG |
| Mouse TGFβ-1-reverse | TGCTTCCCGAATGTCTGACG |
| Mouse IL6Ra Forward | CCACATAGTGTCACTGTGCG |
| Mouse IL6Ra Reverse | GGTATCGAAGCTGGAACTGC |
| Mouse IL23R-forward | GCCAAGAAGACCATTCCCGA |
| Mouse IL23R-reverse | TCAGTGCTACAATCTTCAGAGGAC |
| Mouse RORc-Forward | TTTGGAACTGGCTTTCCATC |
| Mouse RORc-Reverse | AAGATCTGCAGCTTTTCCACA |
| Mouse IRF4-forward | TGAAAATGGTTGCCAGGTGACAGG |
| Mouse IRF4-reverse | GCAGCCTTCAGGGCTCGTCG |
| Mouse T-bet-forward | TTTCCAAGAGACCCAGTTCATTG |
| Mouse T-bet-reverse | ATGCGTACATGGACTCAAAGTT |
| Mice Gata-3-forward | CTCGGCCATTCGTACATGGAA |
| Mice Gata-3-reverse | GGATACCTCTGCACCGTAGC |
| Mouse FOXP3-Forward | CCCATCCCCAGGAGTCTTG |
| Mouse FOXP3-Reverse | ACCATGACTAGGGGCACTGTA |
| Mouse socs-1-forward | GTGGGCACCTTCTTGGTG |
| Mouse socs-1-reverse | AAAGGCAGTCGAAGGTCTCG |
| Mouse SOCS3-forward | GAGATTTCGCTTCGGGACTA |
| Mouse SOCS3-reverse | GGAAACTTGCTGTGGGTGAC |
| Mice Socs5-forward | GCGTAGTGGGAGCTTACTCG |
| Mice Socs5-reverse | TGATTAGAAGAGCTCCACGGC |
Western-blotting analysis
T cells were cultured in anti-CD3 (2 μg/ml) and anti-CD28 (2 μg/ml) pre-coated 24-well plates with Th17 polarization condition medium in the absence or presence of different TLR ligands for various time points. Co-cultured T cells were purified and then lysates prepared for western blot analyses. The antibodies used in western blotting are as following: anti–STAT1, anti-phospho-STAT1 (Y701), anti–STAT2, anti- phospho-STAT2 (Y609), anti-STAT3, anti–phospho-STAT3 (Y705), anti-phospho-STAT3 (S727), anti-STAT5, anti–phospho-STAT5 (Y694), anti–STAT6, anti-phospho-STAT6 (Y641), and anti-GAPDH. All antibodies are purchased from Cell Signaling Technology.
Gene knockdown with siRNA in T cells
For SOCS3 and SOCS5 gene knockdown experiments, anti-CD3 and anti-CD28-preactivated CD4+ T cells were transfected with siRNAs targeting SOCS3 and SOCS5 (NM_007707 and NM_019654, Sigma), or nonspecific scrambled control siRNAs at a final concentration of 100 nmol/L using the standard transfection reagents (TransIT-siQUEST®, Mirus Bio LLC). The transfected T cells were then cultured in T cell polarization medium in the presence or absence of various TLR ligands for 5 days to generate different T cell subsets.
EAE Model
Eight- to ten-week-old female C57BL/6 mice were injected with synthetic Myelin Oligodendrocyte Glycoprotein peptide (MOG35–55, 200 μg/mouse) in Complete Freund’s Adjuvant (CFA, containing 4 mg/ml Mycobacterium tuberculosis, Sigma) subcutaneously in each flank (41). Pertussis Toxin (200 ng/mouse, List Biological Laboratories Inc.) was injected intraperitoneally at days 0 and 2 post MOG injection. Clinical score of EAE was graded on a scale of 1–4: 0, no clinical signs; 1, flaccid tail; 2, hind limb weakness, abnormal gait; 3, complete hind limb paralysis; 4, complete hind limb paralysis and forelimb weakness or paralysis. For early treatment experiments, imiquimod (invivo gene) (50 μg in 200 μl PBS/mouse) or PBS (200 μl) was administered intraperitoneally on every second day from Day 3 after MOG immunization, and then continued for a total of 6 time injections throughout the experiment. For late treatment experiments, imiquimod (80 μg in 200 μl PBS/mouse) or PBS (200 μl) was administered intraperitoneally on every second day from day 9 after MOG immunization (mice start EAE symptoms), and then continued for a total of 4 time injections throughout the experiment. Mice were scored daily. Each experimental group was scored in a blinded fashion. Blood, spleens, lymph nodes, and spinal cords were isolated from the early treatment EAE mice sacrificed on day 12, 16, 19 and 24 of EAE development. Cell populations and functions were determined by flow cytometer analysis and MOG recall assay.
MOG recall assay of T cell proliferation
Splenocytes from the EAE mice were incubated with 1, 5, or 25 μg/ml MOG for 3 days. At 16 hours prior to the 3-day culture, 3H-thymidine (Amersham/GE Healthcare) was added at a final concentration of 1 μCi/well. The incorporation of 3H-thymidine was measured with a liquid scintillation counter (22, 42).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism5 software. Unless indicated otherwise, data are expressed as mean ± standard deviation (SD). For multiple group comparison in vivo studies, the one-way analysis of variance (ANOVA) was used, followed by the Dunnett’s test for comparing experimental groups against a single control. For single comparison between two groups, paired Student’s t test was used. Nonparametric t-test was chosen if the sample size was too small and not fit Gaussian distribution.
Results
TLR7 signaling directly inhibits both murine and human Th17 differentiation and development
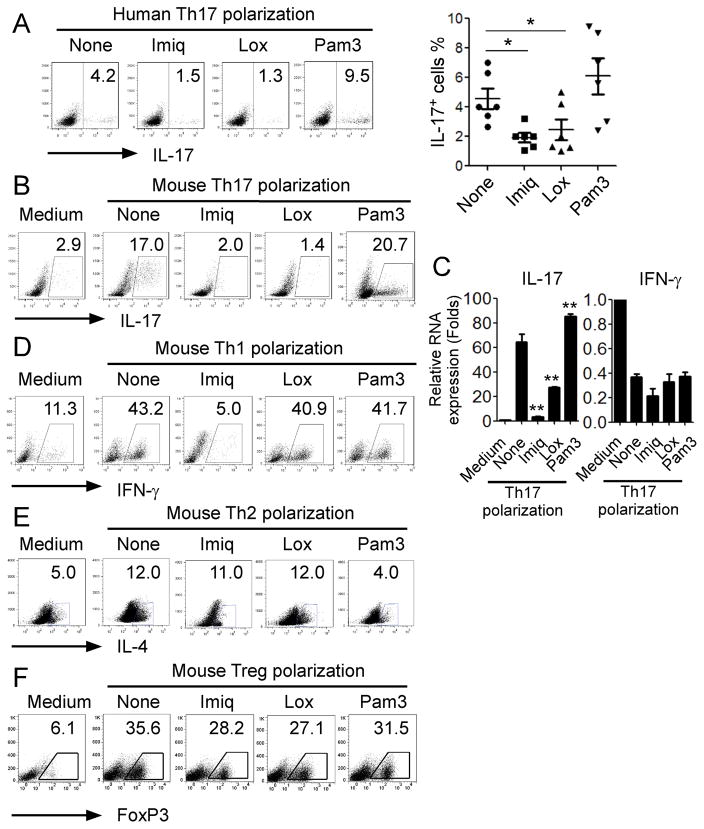
Recent studies have shown that TLRs directly modulate differentiation, proliferation and function of T lymphocytes. TLR2 signaling in T cells could directly promote Th17 generation and expansion, while TLR4 signaling in T cells enhanced proliferation and survival of T cells (16, 18). We also showed that TLR and NOD2 signaling promote the attraction and generation of Th17 cells induced by tumor cells and tumor-derived fibroblasts (4, 5). To determine the direct effects of different TLR signaling on Th17 cell differentiation and development, we screened a panel of TLR ligands for effects on human Th17 cells during the course of Th17 polarization from naïve T cells purified from healthy donors. Consistent with the previous findings, we showed that the TLR2 ligand Pam3CSK4 could promote Th17 cell differentiation in naïve CD4+ T cells (Figure 1A) (16). However, TLR7 ligands, imiquimod (Imiq) and loxoribine (Lox) significantly inhibited human Th17 differentiation from naïve CD4+ T cells. We further confirmed this effect and the generality of TLR7 ligand-mediated inhibition of human Th17 cell development from naïve T cells in six of additional healthy donors (Figure 1A). In addition to the suppression of human Th17 cell differentiation, we observed that imiquimod and loxoribine strongly inhibited mouse Th17 differentiation from naïve CD4+ T cells, decreasing IL-17-producing cell populations and IL17 mRNA expression levels in the cultured cells (Figure 1B and Figure 1C). We next determined whether TLR7 ligands specifically influenced Th17 differentiation rather than the other T cell subsets. Mouse naïve CD4+ T cells were cultured with Th1, Th2, or Treg polarization condition medium in the presence or absence of various TLR ligands. Unexpectedly, we found that imiquimod but not loxoribine dramatically inhibited Th1 cell differentiation (Figure 1D). Furthermore, neither imiquimod nor loxoribine affected Th2 and Treg development from naïve CD4+ T cells (Figure 1E & 1F).
Figure 1. Direct inhibitory effect of TLR7 ligands on Th17 differentiation from naïve T cells.
(A) TLR7 ligands inhibited human Th17 cell differentiation from naïve CD4+ T cells in vitro. Human naïve CD4+ T cells were cultured with Th17 polarization condition medium in the presence of TLR7 ligands (Imiq and Lox) or TLR2 ligand (Pam3) for 6 days. Results shown in left panel are representative intracellular staining of IL-17-producing T cells in differentiated Th17 cells with different TLR ligand treatments using flow cytometry analyses after stimulation with PMA and ionomycin for 5 hours. Results shown in right panel are the summary of IL-17-producing cells in differentiated Th17 cells with indicated TLR ligand treatments in human naïve CD4+ T cells isolated from different donors. Result of each dot shown is derived from an individual donor and data shown are mean ± SD. *p<0.05, compared with the medium only group determined by paired t-test. (B) Imiq and Lox, but not Pam3 inhibited mouse Th17 cell differentiation from naïve CD4+ T cells in vitro. Intracellular staining of IL-17-producing cells was performed using flow cytometry analyses following Th17 differentiation from mouse naïve CD4+ T cells in the presence of imiq, lox or Pam3 for 6 days. (C) Imiq and Lox, but not Pam3 suppressed IL-17 mRNA expression in mouse CD4+ T cells during Th17 polarization. Mouse CD4+ T cells were cultured in Th17 polarization condition medium in the presence of Imiq and Lox and Pam3 for 6 days. IL-17 and INF-γ mRNA expression levels were determined by real-time PCR. The expression level of each gene was normalized to GAPDH expression and adjusted to the levels in naïve CD4+ T cells. Data are means ± SD from 3 representative naïve CD4+ T cells. ** p< 0.01, compared with the medium only group determined by paired t-test. (D) Imiq, but not Lox and Pam3 inhibited mouse Th1 polarization from naïve CD4+ T cells. Mouse CD4+ T cells were cultured in Th1 differentiation medium in the presence of indicated TLR ligands for 6 days. INF-γ-producing T cells were analyzed using flow cytometry after stimulation with PMA and ionomycin for 5 hours. (E) No inhibitory effect of TLR7 ligands on mouse Th2 polarization from naive CD4+ T cells. Mouse CD4+ T cells were cultured in Th2 differentiation medium in the presence of indicated TLR ligands for 6 days. IL-4-producing T cells were analyzed using flow cytometry after stimulation with PMA and ionomycin for 5 hours. (F) No inhibitory effect of TLR7 ligands on mouse Treg polarization from naive CD4+ T cells. Naïve CD4+ T cells from FoxP3-GFP transgenic mice were cultured in Treg differentiation medium in the presence of indicated TLR ligands for 6 days. GFP+ T cells were analyzed using flow cytometry.
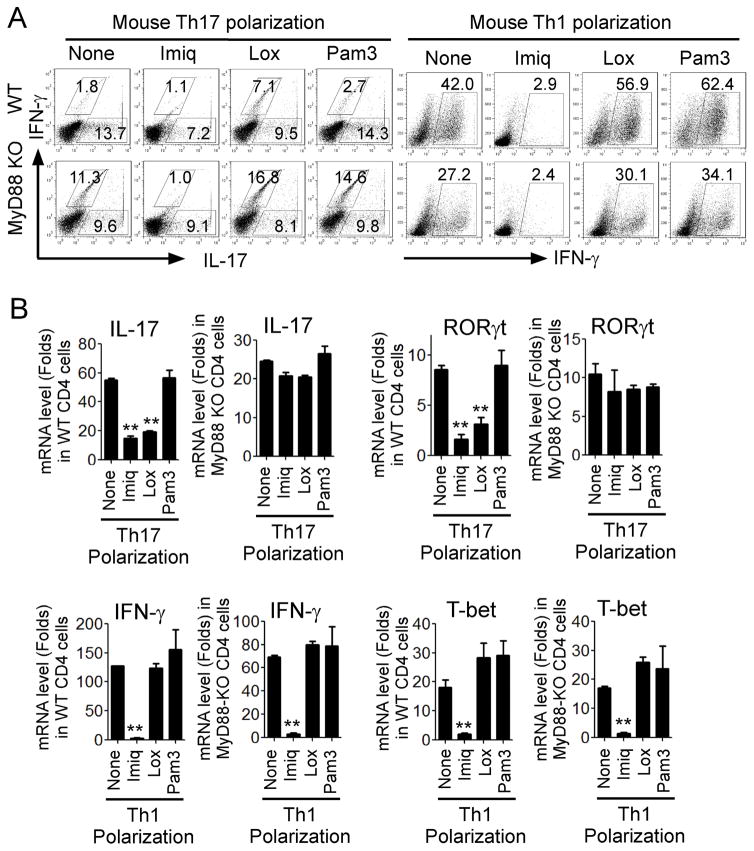
We then attempted to confirm that the effects on Th17 differentiation mediated by imiquimod and loxoribine were TLR7 signaling dependent. Given that MyD88 is a key adaptor molecule shared by different TLRs pathways including TLR7 signaling (15), we utilized MyD88-deficient mouse CD4+ cells to develop Th17 cells in the presence of TLR7 ligands. Mouse naïve CD4+ T cells derived from wild-type or MyD88−/− mice were cultured with Th17 or Th1 polarization medium in the presence of TLR ligands for 6 days and T cell subsets were determined. As shown in Figure 2A, both imiquimod and loxoribine inhibited Th17 cell differentiation from naïve T cells derived from wild-type mice. In contrast, there was no suppressive effect on Th17 cell differentiation from naïve T cells derived from MyD88−/− mice. In addition, we confirmed that both imiquimod and loxoribine exhibited strong suppression of RoRγt and IL-17 mRNA expression in Th17 cells differentiated from wild-type CD4+ T cells but not from MyD88-deficient CD4+ T cells (Figure 2B). These results clearly suggested that suppression of Th17 differentiation and development mediated by imiquimod and loxoribine is TLR7 signaling-specific and MyD88 is required for the effect. We also investigated whether imiquimod-mediated suppression of Th1 differentiation was through TLR7 signaling. We observed that decreased numbers of Th17 or Th1 cells were generated from MyD88−/−CD4+ T cells, compared with that of wild-type CD4+ T cells. These results were consistent with previous reports showing that MyD88−/−CD4+ cells exhibited impaired ability to produce IL-2, IL-17 or IFN-γ (43, 44). Furthermore, imiquimod and loxoribine had similar suppressive effects on Th1 polarization and both IFN-γ and T-bet mRNA expression comparing wild-type and MyD88-deficient CD4+ T cells (Figure 2A and 2B). These results suggest that imiquimod-mediated suppression of Th1 differentiation does not involve TLR7-MyD88 signaling, different from the mechanism responsible for suppression of Th17 cells.
Figure 2. Suppression of Th17 differentiation mediated by Imiq and Lox is TLR7-signaling dependent.
(A) Left panel: Both Imiq and Lox inhibited Th17 cell differentiation from naïve T cells derived from wild-type mice. However, there was no suppressive effect on Th17 cell differentiation from naïve T cells derived from MyD88−/− mice. Right panel: Only Imiq inhibited Th1 cell polarization and had similar suppressive effects in both wild-type and MyD88-deficient CD4+ T cells. Mouse CD4+ T cells purified from wild-type or MyD88-deficient KO mice were cultured in the Th17 or Th1 polarization medium in the presence or absence of the indicated TLR ligands for 6 days. IFN-γ– and IL-17-producing T cells were determined using flow cytometry analyses after stimulation with PMA and ionomycin. Data shown are a representative of three individual experiments with similar results. (B) Upper panel: Both Imiq and Lox exhibited strong suppression of RoRγt and IL-17 mRNA expression in Th17 cells differentiated from wild-type CD4+ T cells but not from MyD88-deficient CD4+ T cells. Lower panel: Imiq had a similar suppressive effect on IFN-γ and T-bet mRNA expression in Th1 cells differentiated from both wild-type and MyD88-deficient CD4+ T cells. Cell culture and treatment were identical to the description in (A). mRNA expression of each gene was determined by real-time PCR and expression level was normalized to GAPDH expression. Data shown are mean ± SD from three independent experiments, and paired t-test was performed. **p<0.01, compared with the medium only group.
TLR7 signaling decreases IL-17 production by established Th17 cells
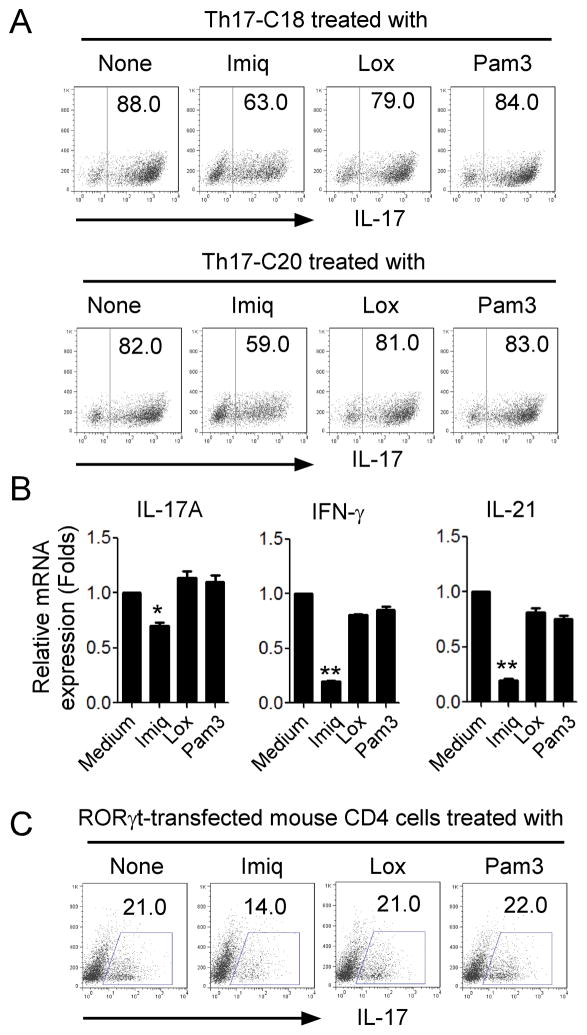
We further examined whether TLR7 ligands also inhibited already established Th17 cells. We previously established Th17 clones derived from ovarian cancer, colon cancer and melanoma cancer patients (4, 5). Two human colon cancer-derived Th17 cell clones were cultured in the presence of TLR ligands and then IL-17-producing cell populations determined. We found that imiquimod but not loxoribine partially decreased IL17-producing cell populations in both established Th17 cell clones (from 88% to 63% in Th17-C18 cells and from 82% to 59% in Th17-C20 cells, respectively) (Figure 3A). In addition, imiquimod treatment also dramatically reduced IL17A, IFN-γ, and IL-21 mRNA expression levels in human tumor-derived Th17 cells (Figure 3B). Over-expression of RoRγt could convert CD4+ T cells into Th17 cells (45, 46). Therefore, we utilized murine Th17 cells generated from RoRγt-transfected mouse CD4+ T cells as established Th17 cells. Consistent with the effects on human established Th17 cells, we observed that imiquimod could also decrease the IL-17 and RoRγt expression in mouse Th17 cells (Figure 3C and Data not shown). These results collectively suggest that activation of TLR7 signaling can not only inhibit the Th17 cell differentiation but also suppress IL-17 production in established Th17 cells.
Figure 3. TLR7 signaling decreases IL-17 production by established Th17 cells.
(A) Decreased IL-17-producing cell population in the established human Th17 cell clones after treatment with Imiq. Human colon cancer-derived Th17 cell clones, Th17-C18 and Th17-C20, were treated with Imiq (10 μg/ml), Lox (500 μM) or Pam3 (200 ng/ml) for 48 hours. IL-17-procuing cell population was analyzed using flow cytometry after intracellular staining. (B) Decreased mRNA expression levels of IL-17, IFN-γ, and IL-21 in human Th17 clones after treatment with Imiq. Th17 clones were treated with the indicated TLR ligands for 48 hours and mRNA expression was determined by real-time PCR. The expression level of each gene was normalized to GAPDH expression and then adjusted to the level in Th17 cells cultured in medium. Data shown are mean ± SD from three independent experiments, and paired t-test was performed. *p<0.05, compared with the medium only group. (C) Decreased IL-17+ T cells in mouse RORγt- transfected Th17 cells after treatment with Imiq. RORγt-transfected mouse CD4+ T cells were treated with Imiq (10 μg/ml), Lox (500μM) or Pam3 (200ng/ml) for 48 hours. IL-17-procuing cells were determined using flow cytometry analysis after intracellular staining.
TLR7 signaling inhibits lineage-specific transcription factors in Th17 cells
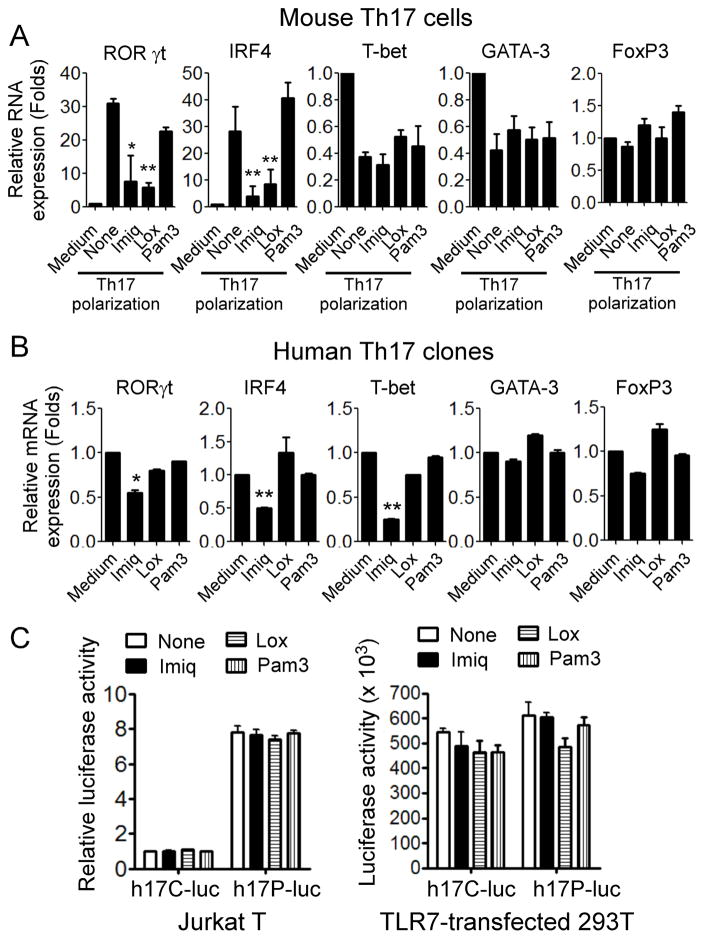
Based on our observations that TLR7 signaling significantly inhibited Th17 cell differentiation and development, we reasoned that these phenotypic alterations could be the result of changed expression of lineage-restricted transcription regulators that control and direct T cell programming (45, 46). To test this possibility, we first determined gene expression of the key CD4+ T cell subset differentiation transcription factors, including RORγt and IRF-4 (Th17), as well as T-bet (Th1), GATA-3 (Th2) and FoxP3 (Treg), in polarized Th17 cells and in established Th17 cells in the presence of various TLR ligands using real-time PCR (4, 6). As expected, mouse Th17 cells expressed higher levels of the Th17-specific transcription factors RORγt and IRF-4 but only low levels of T-bet, GATA-3 and FoxP3 during the course of Th17 polarization. However, expression levels of RORγt and IRF-4 were dramatically decreased following the treatment with TLR7 ligands imiquimod and loxoribine (Figure 4A). In addition, in human tumor-derived Th17 clones, imiquimod but not loxoribine dramatically inhibited Th17-related transcription factors RORγt and IRF4, but also suppressed Th1-related transcription factor T-bet (Figure 4B).
Figure 4. Inhibitory effect of TLR7 ligands on lineage-specific transcription factors in Th17 cells.
(A) TLR7 ligand Imiq and Lox inhibited the gene expression of RORγt and IRF4, but not T-bet, GATA-3 and FoxP3 in mouse CD4+ T cells cultured in Th17 polarization condition medium for 6 days. Relative mRNA expression level of each gene was determined by real-time PCR with specific primers, normalized to GAPDH expression and then adjusted to the level in naïve CD4+ T cells. Data shown are mean ± SD from three independent experiments, and paired t-test was performed. *p<0.05 and ** p<0.01, compared with none treatment group. (B) Imiq treatment inhibited RORγt, IRF4, and T-bet gene expression in human Th17 cells. Human Th17 cell clones were cultured in the presence of indicated TLR ligands for 24 hours, and gene expression levels of transcription factors were determined by real-time PCR. Relative mRNA expression level of each gene was normalized to GAPDH expression and then adjusted to the level in Th17 cells cultured in medium only. Data shown are mean ± SD from triplicate experiments, and paired t-test was performed. *p<0.05 and ** p<0.01, compared with the medium only group. (C) Treatment with TLR7 ligands did not affect IL-17 promoter activity. Jurkat T or 293T cells were transfected with either human IL-17 promoter-luciferase plasmid (h17P-Luc) or control luciferase vector (hp17C-Luc) for 24 hours and then treated with Imiq, Lox, or Pam3 for additional 24 hours. Luciferase activities were measured in cell lysates and normalized to the activity in cells only transfected with the empty vector. Results shown are mean ± SD from three independent experiments.
To explore the molecular mechanisms of TLR7-mediated suppression of Th17 cell development, we determined whether TLR7 ligand-induced IL-17 inhibition was due to the transcriptional regulation of IL-17 gene. We transfected a human IL-17 promotor-luciferase construct (h17P-Luc) into Jurkat T cells and then stimulated the cells with TLR ligands (40). However, we did not find any suppressive effects on IL-17 promoter activity (luciferase activity) induced by TLR7 ligands (Figure 4C). These results were further confirmed in 293T cells cotransfected with h17p-Luc and TLR7 genes (Figure 4C). We then investigated whether TLR7 ligands inhibited Th17 cells by posttranscriptional regulation of IL-17 and/or key Th17-related transcription factors. Human Th17 clones were pre-treated with the transcriptional inhibitor actinomycin D (ACD) for 24 hours to block de novo RNA synthesis. The pretreated Th17 cells were further cultured in the presence of TLR ligands and IL-17, IRF4 and RORγt mRNA expression was determined by Real-time PCR. However, TLR7 ligand treatment did not affect IL-17, IRF4 and RORγt mRNA stability measured at 3 or 5 hours after ACD treatment in Th17 cells (Data not shown). Collectively, our data indicate that TLR7 signaling had strong suppressive effects on both Th17 differentiation and established Th17 cell maintenance, which were not through transcriptional and posttranscriptional regulation of the IL-17 gene. Given that lineage-specific cytokines instruct T cell subset specific differentiation programs, we thus investigated possible changes of interactions between cytokine and cytokine receptor in Th17 cells mediated by TLR7 signaling. We determined the expression levels of cytokine receptors for IL-23, IL-6 and IL-21 in T cells, which are important for Th17 differentiation. We did not observe any significant decreases in expression of these cytokine receptors in Th17 cells induced by TLR7 ligands (Supplemental Figure 1A), suggesting that TLR7-mediated inhibition of Th17 cells does not involve down-regulation of these key cytokine-cytokine receptors.
Transcription factors STAT3 and STAT5 are critical for Th17 inhibition mediated by TLR7 signaling
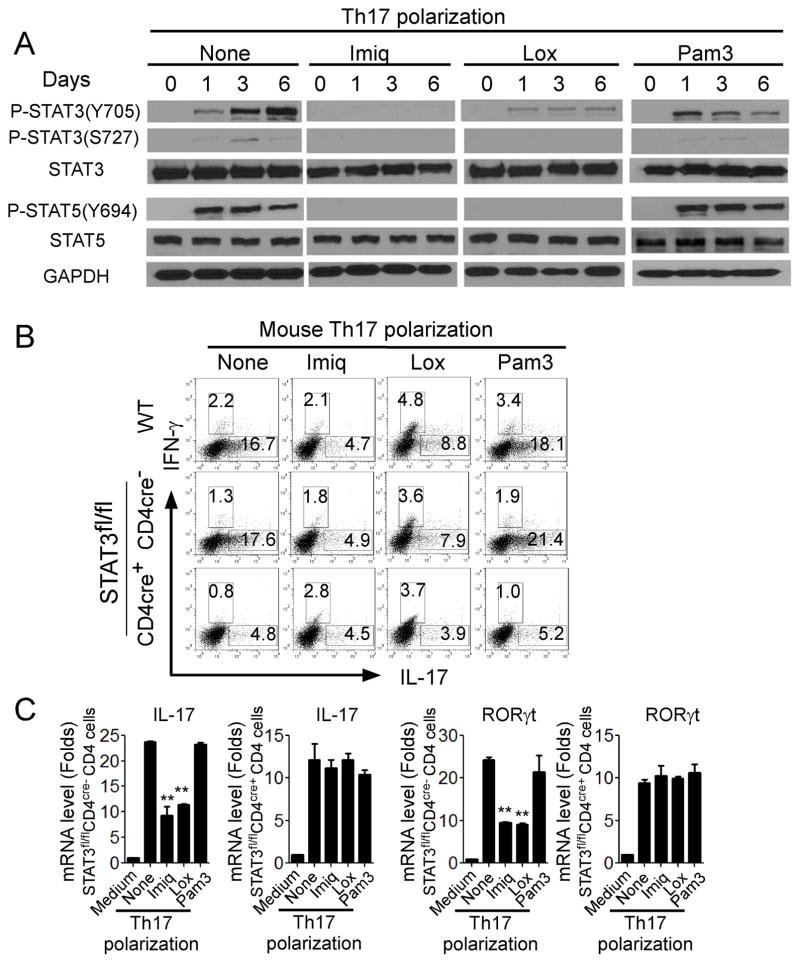
It is well established that signal transducer and activator of transcription (STAT) family proteins are critical in mediating T cell development and differentiation (47, 48). STAT1 and STAT4 are required for the polarization of Th1 cells, while Th2 cell differentiation requires STAT6 (45, 46). It is now recognized that STAT3 is required for commitment of naive T cells toward the Th17 developmental pathway (12, 49, 50). We thus reasoned that TLR7 signaling may inhibit STAT3 signaling in T cells resulting in inhibition of Th17 differentiation. We first determined the expression levels and phosphorylated activation patterns of the STAT members in T cells during Th17 differentiation in the presence of TLR ligands. STAT1, STAT2 and STAT6 were not activated (phosphorylated) in CD4+ T cells cultured in Th17 polarization medium (Data not shown). Furthermore, addition of Pam3CSK4, loxoribine, and imiquimod did not alter their activation status. However, STAT3 (both Y705 and S727 sites) and STAT5 (Y694 site) were dramatically phosphorylated in CD4+ T cells during Th17 polarization in the absence of TLR signaling (Figure 5A). Moreover, loxoribine and imiquimod treatment significantly prevented the phosphorylated activation of STAT3 and STAT5, while Pam3CSK4 did not have suppressive effects on both STAT3 and STAT5 activation (Figure 5A). In addition, the suppression on STAT3 phosphorylation induced by TLR7 signaling was further confirmed in already established human Th17 cells (Data not shown). Notably, studies from other group have shown that STAT5 is a negative regulator of Th17 differentiation and IL-2 signaling (51). Therefore, activation of STAT5 during Th17 differentiation in our results might be a negative feedback response detectable in the co-culture system.
Figure 5. STAT3 signaling down-regulation is involved in TLR7-induced inhibition of Th17 cells.
(A) TLR7 ligands inhibited phosphorylation of STAT3 (both Y705 and S727 sites) and STAT5 (Y694 site) in mouse CD4+ T cells during Th17 polarization. Mouse naïve CD4+ T cells were cultured in Th17 differentiation medium for various time points in the absence or presence of Imiq, Lox or Pam3. Co-cultured CD4+ T cells were purified and lysates performed western blot analyses. (B) STAT3 deficiency abolished TLR7-mediated suppression of Th17 cell differentiation in CD4+ T cells. Furthermore, CD4+ T cells derived from STAT3fl/flCD4cre+ mice had lower ability to develop Th17 cells compare with those from wild-type and STAT3fl/fl CD4cre− mice. CD4+ T cells derived from wild-type, STAT3fl/flCD4cre−, and STAT3fl/flCD4cre+ were culture in the Th17 polarization medium in the presence or absence of TLR ligands for 6 days, and IL-17- and IFN-γ-producing cells were evaluated using flow cytometry after stimulation with PMA and ionomycin for 5 hours. (C) TLR7 ligands Lox and Imiq treatment markedly inhibited IL-17 and RORγt mRNA expression in polarized Th17 cells derived from STAT3fl/fl CD4cre− mice, but not from STAT3fl/flCD4cre+ mice. CD4+ T cells derived from wild-type, STAT3fl/fl CD4cre−, and STAT3fl/flCD4cre+ were cultured in the Th17 polarization medium in the presence or absence of TLR ligands for 6 days. mRNA expression of IL-17 and RORγt was determined by real-time PCR. Relative mRNA expression level of each gene was normalized to GAPDH expression and then adjusted to the level in naïve CD4+ T cells. Data shown are mean ± SD from the triplicate experiments, and paired t-test was performed. ** p<0.01, compared with the Th17 polarization medium only group.
To further confirm that transcription factor STAT3 is the key player controlling the molecular process of Th17 inhibition induced by TLR7 signaling, we generated STAT3fl/flCD4cre+ mice by crossing CD4 Cre mice with STAT3fl/fl mice. We determined whether we could abolish TLR7 signaling-mediated suppression of Th17 cell differentiation in CD4+ T cells without STAT3 signaling. CD4+ T cells derived from wild-type, STAT3fl/flCD4cre−, and STAT3fl/flCD4cre+ mice were cultured under Th17 polarization condition in the presence or absence of TLR ligands for 5 days, and the numbers of IL-17-producing cells generated were evaluated. Consistent with published results from other groups, CD4+ T cells derived from STAT3fl/flCD4cre+ mice had a lower ability to develop into Th17 cells compared with those from wild-type and STAT3fl/fl CD4cre− mice, demonstrating the importance of STAT3 in Th17 cell polarization (Figure 5B)(49, 50). Furthermore, consistent with the results shown in Figure 1, we found that loxoribine and imiquimod treatment significantly inhibited Th17 development; while addition of Pam3CSK4 increased IL-17-producing T cells in the co-cultured CD4+ T cells derived from wild-type and STAT3fl/fl CD4cre− mice (Figure 5B). In contrast, loxoribine and imiquimod treatment did not have any suppressive activity on Th17 polarization in CD4+ T cells derived from STAT3fl/flCD4cre+ mice. In addition, loxoribine and imiquimod treatment markedly inhibited levels of IL-17 and RORγt mRNA expression in polarized Th17 cells in CD4+ T cells derived from STAT3fl/fl CD4cre− mice, but not from STAT3fl/flCD4cre+ mice (Figure 5C). These results clearly confirm that inhibition of Th17 development mediated by TLR7 signaling is controlled by the transcription factor STAT3.
Since imiquimod can inhibit Th1 differentiation (Figure 1), we therefore determined whether STAT3 signaling is required for imiquimod-mediated suppression of Th1 cells. CD4+ T cells derived from wild-type, STAT3fl/flCD4cre−, and STAT3fl/flCD4cre+ mice were cultured under Th1 polarization condition in the presence or absence of TLR ligands for 5 days. Unexpectedly, we found that development of Th1 cells in CD4+ T cells derived from STAT3fl/flCD4cre+mice was significantly impaired compared with CD4+ T cells from wild-type and STAT3fl/flCD4cre− mice, suggesting that STAT3 is also critical for Th1 cell polarization (Supplemental Figure 2A). Importantly, imiquimod treatment significantly inhibited Th1 differentiation in co-cultured CD4+ T cells derived from all three types of mice, and there was no difference of the suppressive effects on CD4+ T cells comparing STAT3fl/flCD4cre− mice and STAT3fl/flCD4cre+ mice (Supplemental Figure 2A). Furthermore, imiquimod treatment significantly inhibited mRNA expression levels of IFN-γ and T-bet in polarized Th1 cells in CD4+ T cells derived from both STAT3fl/flCD4cre− and STAT3fl/flCD4cre+mice with a similar level, further suggesting that STAT3 is not involved in imiquimod-mediated suppression of Th1 polarization (Supplemental Figure 2B). These results further indicate that suppression of Th17 cells and Th1 cells mediated by imiquimod involves different mechanisms and molecular processes.
SOCS3 and SOCS5 are involved in the reciprocal regulation of STAT3 during TLR7-mediated Th17 suppression
We next explored the potential mechanisms involved in the reciprocal regulation of STAT3 during inhibition of Th17 development mediated by TLR7 signaling. Proinflammatory cytokines, such as IL-6, IL-8, IFN-γ and TNF-α have been shown to be critical inducers for activation of STAT1/STAT3 signaling or to be their targets (47, 48). We thus first tested whether these cytokines were involved in the STAT3 regulation loop and required for the inhibition of Th17 development by TLR7 signaling. However, loxoribine and imiquimod treatment did not suppress the cytokine expression levels, including IL-4, IL-8, IL-10 and TNF-α, compared with those in Th17 condition medium group (Supplemental Figure 1B). In contrast, imiquimod treatment promoted IL-4 gene expression, and loxoribine treatment increased IL-8 and TNF-α expression (Supplemental Figure 1B). These results suggested that proinflammatory cytokines were not responsible for the down-regulation of STAT3 signaling in TLR7 signaling-mediated Th17 suppression.
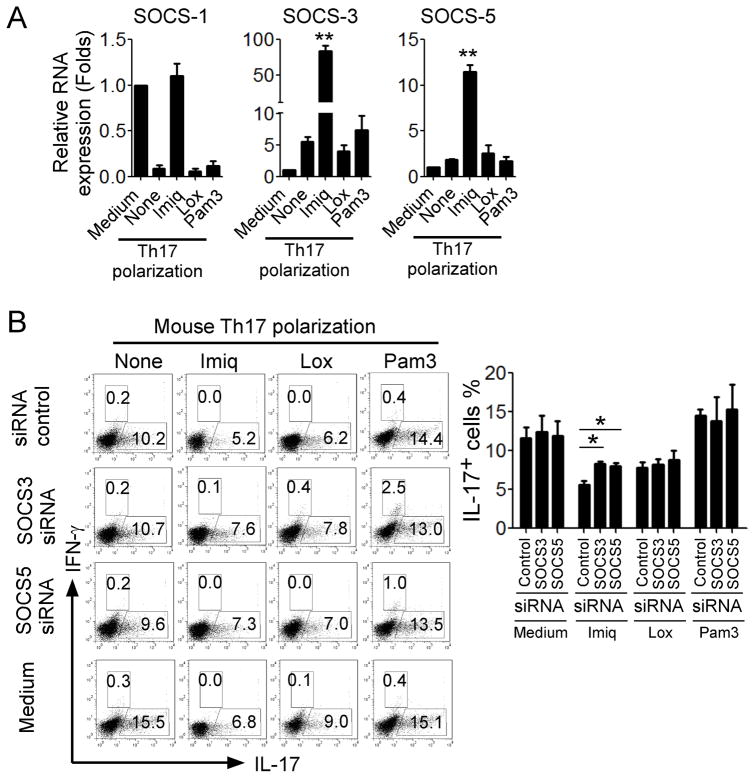
Regulation of STAT activation is also dictated by negative regulators termed suppressors of cytokine signaling (SOCS). SOCS3 has been shown to block STAT3 signaling and its deletion results in enhanced Th17 differentiation (52, 53). A recent study demonstrated that SOCS proteins are induced not only via the JAK/STAT pathway, but also through TLR and Dectin-1 (54). We thus hypothesized that TLR7 signaling may induce SOCS in CD4+ T cells, resulting in the inhibition of STAT3 signaling and Th17 development. We first determined expression levels of SOCSs induced by TLR7 ligands. We observed imiquimod treatment significantly promoted expression of SOCS3 and SOCS5 in the polarized Th17 cells, compared with those in the medium group (Figure 6A). Surprisingly, we did not notice significant effect on SOCS expression mediated by loxoribine treatment. To verify the importance of SOCS3 and SOCS5 in TLR7 signaling-mediated suppression of Th17 cells, we utilized a loss-of-function strategy with specific siRNAs to knockdown gene expression of SOCS3 and SOCS5 in T cells, as we have done in previously studies (19, 21). Knockdown of SOCS3 and SOCS5 genes in CD4+ T cells dramatically alleviated the imiquimod-mediated suppression of Th17 development (Figure 6B). However, transfection of both siRNA-SOCS3 and siRNA-SOCS5 in CD4+ T cells could not reverse the suppressive effect of imiquimod on Th1 polarization (Supplemental Figure 2C), supporting a specific role of SOC3 and SOCS5 only for imiquimod-mediated inhibition on Th17 polarization. These studies collectively suggest that induction of SOCS3 and SOCS5 is responsible for the inhibition of STAT3 signaling and Th17 differentiation mediated by TLR7 signaling.
Figure 6. Induction of SOCS3 and SOCS5 is responsible for the STAT3 signaling inhibition and Th17 differentiation mediated by TLR7.
(A) Imiq but not Lox treatment significantly promoted SOCS3 and SOCS5 expression in CD4+ T cells in the presence of Th17 polarization condition. Mouse naïve CD4+ T cells were cultured in Th17 differentiation medium for 6 days in the presence or absence of Imiq, Lox or Pam3. mRNA expression levels of SOCS-1, SOCS3 and SOCS5 were determined by real-time PCR. Relative mRNA expression level of each gene was normalized to GAPDH expression and then adjusted to the level in naïve CD4+ T cells. Data shown are mean ± SD from triplicate experiments, and paired t-test was performed. ** p<0.01, compared with Th17 polarization medium only group. (B) Knockdown of SOCS3 and SOCS5 genes in CD4+ T cells dramatically alleviated the imiquimod-mediated suppression of Th17 development. Mouse CD4+ T cells were transfected with specific siRNAs against SOCS3 or SOCS5, as well as related scrambled siRNA controls, and then cultured in Th17 polarization medium in the presence or absence indicated TLR ligands for 6 days. IL-17- and IFN-γ-producing cells were evaluated using flow cytometry analysis after stimulation with PMA and ionomycin for 5 hours. Results shown in the right panel are the summary of IL-17-producing cells in differentiated Th17 cells with indicated TLR ligand and siRNA treatments obtained from three different individual experiments. Data shown are mean ± SD, and *p<0.05, compared with the scrambled siRNA control group determined by paired t-test.
DCs are not required for TLR7-mediated suppression of Th17 cells
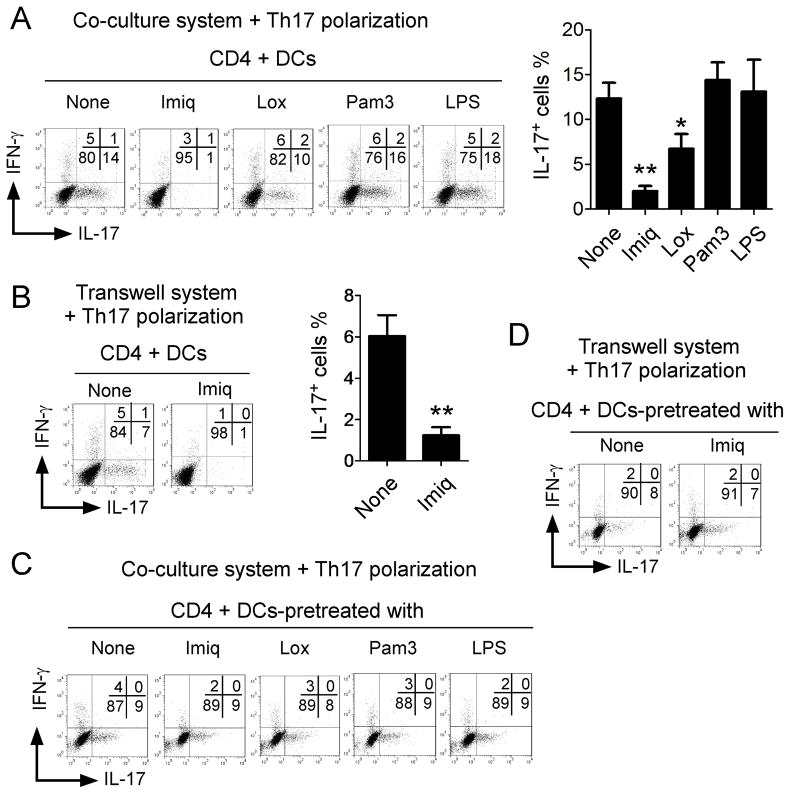
Recent studies suggested that TLR7 ligands could affect Th17 differentiation or IL-17 responses by acting on DCs, although these results remain controversial (34, 35). We therefore explored whether TLR7 signaling may also regulate DCs and then indirectly affect Th17 differentiation and development. We observed that IL-17-producing cell populations were significantly induced in a DC co-culture system with Th17 polarization conditions. Addition of loxoribine and imiquimod significantly inhibited Th17 cell generation in the DC co-culture system (Figure 7A). In contrast, LPS and Pam3CSK4 promoted generation of Th17 cells in this system (Figure 7A). We then separated DCs from CD4+ T cells by transwell inserts to determine the DC requirement for Th17 polarization. We observed a significant decrease of Th17 polarization when DCs and T cells were separated in the Transwell system (from 13% to 6%), indicating that DC-T cell direct interaction is required for Th17 polarization in this DC co-culture system (Figure 7B). However, the DC-T cell separation did not alleviate the suppression of Th17 cell differentiation mediated by imiquimod (Figure 7B). To exclude the possibility that TLR7 signaling may first affect DCs and then TLR7-treated DCs can induce suppression of Th17 differentiation and development, DCs were pre-treated with TLR ligands and then utilized for Th17 development. We found that pretreatment of DCs with TLR7 ligands did not influence Th17 differentiation either in the coculture or in the transwell systems (Figure 7C and 7D), suggesting that TLR7 signaling plays an inhibitory role on Th17 differentiation by acting directly on CD4+ T cells, rather than on DCs.
Figure 7. TLR7-mediated inhibitory effect of Th17 differentiation does not require DC involvement.
(A) Imiq and Lox significantly inhibited Th17 cell generation in the DC co-culture system. In contrast, LPS and Pam3 promoted the generation of Th17 cells in this system. Mouse naïve CD4+ T cells were co-cultured with DCs at a ratio of 10:1 with 1μg/ml soluble anti-CD3 in the absence or presence of indicated TLR ligands. IL-17- and IFN-γ-producing CD4+ T cells were evaluated using flow cytometry after stimulation with PMA and ionomycin for 5 hours. Results shown in the right panel are the summary (mean ± SD) of IL-17-producing cells in the DC co-culture system in the presence of indicated TLR ligands from 3 repeated experiments with similar results. *p<0.05 and **p<0.01, compared with the medium only group determined by paired t-test. (B) Separation of DCs from CD4+ T cells by a transwell insert dramatically decreased Th17 polarization in the Transwell system, but did not affect the suppression effect of Th17 cell differentiation mediated by Imiq. Naïve CD4+ T cells were separated from DCs using transwell inserts, and cultured in the Th17 differentiation medium in the absence or presence of Imiq. Results shown in the right panel are mean ± SD of IL-17-producing cells in the Transwell system from 3 repeated experiments with similar results. **p<0.01, compared with the medium only group determined by paired t-test. (C) and (D) Pre-treatment of DCs with TLR7 ligands did not influence Th17 differentiation either in coculture (in C) or in transwell systems (in D). Mouse DCs were pretreated with or without Imiq, Lox, Pam3 and LPS for 1 day, and then cocultured with CD4+ T cells (in C), or separated with naïve CD4+ T cells by a transwell insert (in D), in the presence of soluble anti-CD3 (1μg/ml) and Th17 polarization cytokines for 6 days. IL-17- and IFN-γ-producing CD4+ T cells were evaluated using flow cytometry after stimulation with PMA and ionomycin for 5 hours. Data shown in (A) to (D) are a representative from at least three individual experiments.
Activation of TLR7 signaling delays the development of EAE and reduces the disease severity in vivo
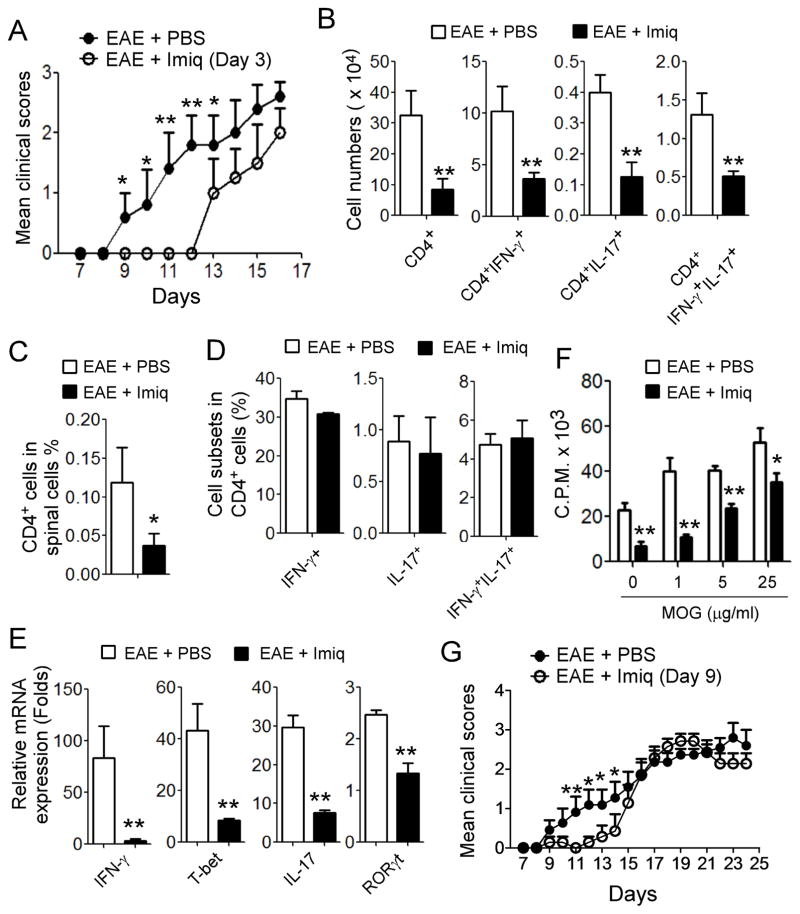
Our in vitro studies provided us important information showing that TLR7 signaling activation in T cells can significantly suppress both Th17 and Th1 cell development, although the molecular actions on these two cell subsets may be through different mechanisms. We next performed complementary in vivo studies to explore whether TLR7 signaling is critical for controlling Th17 and Th1 cells in vivo in pathological conditions. Since both Th1 and Th17 cells are important regulators of CNS inflammation in the EAE mouse model (27), we therefore selected EAE as our experimental model to determine whether TLR7 signaling activation can prevent EAE development and/or alleviate disease severity. Furthermore, due to the strong and consistent suppressive effects of imiquimod on Th1 and Th17 cells in our in vitro studies, we selected imiquimod as a potential immunotherapy in our in vivo EAE studies. EAE was induced by injection of Oligodendrocyte Glycoprotein peptide MOG plus CFA and Pertussis Toxin, and EAE clinical scores were graded during the disease progression (41). In parallel experiments, the mice were administered intraperitoneally with imiquimod or PBS every second day, beginning at day 3 after the MOG immunization, and then continued injection for 6 times total throughout the experiment. The control immunized mice exhibited clinical symptoms on day 9–10 after immunization and then reached peak of disease severity on day 16–18 (Figure 8A). However, treatment with imiquimod dramatically delayed the onset of EAE, and the mice did not display clinical symptoms until day 13 after MOG immunization. Importantly, imiquimod treatment also significantly reduced the disease severity and decreased clinical scores (Figure 8A). These results strongly indicate that activation of TLR7 signaling can prevent the development of EAE. To further validate that the protection of EAE mediated by imiquimod is due to the inhibition of Th1 and Th17 cells, we isolated the blood, spleen, lymph node, and spinal cord from the treated mice sacrificed on day 12, 16 and 19 of EAE development. Cell populations and functions obtained from different organs were analyzed. Since the imiquimod-treated mice had delayed EAE onset of symptoms until day 13 after immunization, we thus first compared the cell components from the two groups on day 12. As expected, we found that absolute numbers of CNS-infiltrating CD4+, CD4+IFN-γ+, CD4+IL-17+ and CD4+IFN-γ+IL-17+ T cell populations in spinal cords were significantly decreased, compared with those in the control group (Figure 8B). Furthermore, imiquimod-treated mice also had reduced percentages of infiltrated CD4+ T cells in the total spinal cord tissues (Figure 8C). We further observed decreased percentages of Th1 (IFNγ+) and Th17 (IL-17+) T cell populations in the total spinal tissue-infiltrating CD4+ T cells, although the reduction was not dramatic, possibly due to the small sample size of the mice utilized (Figure 8D). In addition, we analyzed gene expressions of CNS-infiltrating cells, revealing that significantly decreased mRNA expression levels of IFN-γ, T-bet, IL-17 and RORγt in CNS-infiltrating cells were obtained from imiquimod-treated mice compared with the control group (Figure 8E). These studies strongly suggest that imiquimod treatment can reduce Th1 and Th17 cells in the local inflammatory microenvironment, resulting in the prevention of EAE development.
Figure 8. Treatment with TLR7 ligand Imiq prevents the development of EAE and reduces the disease severity in vivo.
(A) Treatment with Imiq dramatically delayed the onset of EAE and decreased the clinical scores. Mice were administered intraperitoneally with Imiq or PBS on every second day beginning at day 3 after the MOG immunization, and then continued injection for 6 times total throughout the experiment. Each experimental group was scored daily in a blinded fashion. Results shown are mean ± SD (n= 5 mice per group). *p<0.05 and ** p<0.01, compared with the clinical scores from PBS administration group in EAE mice using unpaired t-test. (B) Imiq treatment decreased absolute cell numbers of infiltrated CD4+, CD4+IFN-γ+, CD4+IL-17+, CD4+IFN-γ+IL-17+ cells in spinal cords of EAE mice. Cells were isolated from the spinal cords of two treatment groups sacrificed on day 12 of EAE development. Cytokine-producing CD4+ T cells were evaluated using flow cytometry after stimulation with PMA and ionomycin for 5 hours. Data shown are summary with mean ± SD (n= 5 mice per group) and ** p<0.01, compared with those from PBS administration group in EAE mice using unpaired t-test. (C) and (D) Imiq treatment decreased cell percentages of infiltrated CD4+ cells (in C), but not cell subsets of CD4+IFNγ+, CD4+CD17+ and CD4+IFNγ+IL17+ cells ( in D) in spinal cords from mice sacrificed on day 12 of EAE development. Cell isolation and analyses were identical to the performance in (B). Data shown are mean ± SD (n= 5 mice per group) and * p<0.05, compared with those from PBS administration group in EAE mice using unpaired t-test (in C). (E) Decreased mRNA expression levels of IFN-γ, T-bet, IL-17 and RORγt in CNS-infiltrating cells obtained from Imiq-treated mice compared with those in PBS-treated mice during EAE development. mRNA expression of each gene was determined by real-time PCR and expression level was normalized to GAPDH expression. Data shown are mean ± SD (n= 5 mice per group) and unpaired t-test was performed. ** p<0.01, compared with those from PBS administration group in EAE mice. (F) Imiq treatment mice showed dramatically inhibited MOG-induced specific T cell proliferation from spleen cells in EAE mice. In contrast, stimulation with MOG peptide significantly promoted antigen-specific T cell proliferation with a dose-dependent manner in PBS-treated mice. Splenocytes obtained from the treatment groups in EAE mice were stimulated with the MOG peptide for 3 days, and proliferation of the antigen-specific T cells was determined with the [3H]-thymidine incorporation assay. Results shown are mean ± SD (n= 5 mice per group) and *p<0.05 and ** p<0.01, compared with that of PBS administration group in EAE mice using unpaired t-test. (G) Treatment with imiquimod in the late stage also significantly delayed the progression of EAE and markedly reduced the disease severity and decreased clinical scores. EAE mice were administered intraperitoneally with imiquimod after the immunized mice exhibited clinical symptoms (at day 9), and continued to inject every second day for a total of 4 times. Results shown are mean ± SD (n= 6 mice per group). *p<0.05 and ** p<0.01, compared with the clinical scores from PBS administration group in EAE mice using unpaired t-test.
Besides studying the local spinal cords, we investigated cell population alterations in the periphery mediated by TLR7 signaling activation during EAE development. We found that treatment with imiquimod also markedly decreased the percentages of total CD4+ T cells in blood, spleens and lymph nodes in EAE mice (Supplemental Figure 3). Furthermore, imiquimod treatment reduced the fractions of Th1 and Th17 cells in the blood and lymph nodes but not in the spleens after attempted EAE induction (Supplemental Figure 3). In addition to cell population changes, we next determined whether TLR7 signaling affects the specific immune response during EAE progress. Splenocytes obtained from the treatment groups in EAE mice were stimulated with MOG peptide for 3 days, and the proliferation of the antigen-specific T cells was determined with the 3H-thymidine incorporation assay. We found that stimulation with MOG peptide significantly promoted antigen-specific T cell proliferation in EAE mice in a dose-dependent manner (Figure 8F). However, imiquimod treatment dramatically inhibited MOG-induced specific T cell proliferation in co-cultures in vitro, confirming suppression of antigen-specific immune responses and function. Given that imiquimod treatment significantly reduced the disease severity throughout EAE progression, we thus extended the analyses of cell populations to days 16, 19 and 24 after immunization. We showed significant reductions in both absolute numbers and percentages of infiltrated CD4+IFN-γ+ and CD4+IFN-γ+IL-17+cells in the spinal cords on days 16 and 19 in imiquimod-treated EAE mice (Supplemental Figure 4A & 4B and Data not shown). Furthermore, significant reductions of CD4+IFN-γ+, CD4+IL-17+ and CD4+IFN-γ+IL-17+ T cell populations were also observed in blood and lymph nodes in imiquimod- treated EAE mice (Supplemental Figure 4C). These studies collectively indicate that TLR7 signaling not only decreases the Th1 and Th17 cells in blood and peripheral lymph organs, and in local spinal cords, but also inhibits the MOG-specific T cell responses and function, which result in the prevention of EAE development and reduction of disease severity. In addition to CD4+ T cells, we determined whether imiquimod treatment influences global cell numbers and other cell populations in spinal cords. We observed that imiquimod treatment did not alter the whole cell numbers, as well as both cell numbers and fractions of CD8+ T cells and DCs (CD11b−CD11c+) in spinal cords (Supplemental Figure 4D). However, imiquimod treatment significantly decreased the cell numbers and percentages of B cells, granulocytes (Gr-1/Ly6G/Ly6C) and macrophages (CD11b+CD11c−) in the spinal cords in EAE mice, suggesting that TLR7 may also affect humoral and inflammatory immune responses locally.
To further explore the translational potential of imiquimod as an immunotherapy drug for autoimmune diseases, the EAE mice were administered intraperitoneally with imiquimod when the immunized mice exhibited clinical symptoms of EAE (at day 9), and continued to inject every second day for a total of 4 times. Consistence with the results shown in Figure 8A, the control immunized mice continued to develop clinical symptoms of EAE and then reached peak of disease severity on day 17–19 (Figure 8G). However, treatment with imiquimod in the late stage still can significantly delay the progression of EAE even in the mice already developed EAE, and markedly reduce the disease severity and decrease clinical scores. In addition, the imiquimod-treated mice continued the trend for the reduction of EAE severity and clinical scores after they reached the peak of EAE severity even the imiquimod admistration was stopped (Figure 8G). These results strongly indicate that activation of TLR7 signaling could be an effective therapeutic strategy for autoimmune therapy.
Discussion
Th17 cells are an important player involved in the pathogenesis of inflammatory and autoimmune diseases, and also are critical for protection against some infections and anti-tumor immunity. Improved understanding of the molecular regulation of Th17 cells will facilitate the development of novel strategies specifically targeting Th17 cells for effective immunotherapy against Th17-related diseases. Our current study identified that activation of TLR7 signaling can inhibit both Th17 cell differentiation and maintenance. We further demonstrated that TLR7-mediated suppression of Th17 cells is mechanistically dependent on the induction of SOCS3 and SOCS5, resulting in the inhibition of STAT3 signaling and Th17-related transcriptional factors. In addition to inhibition of Th17 cells, the TLR7 ligand imiquimod also suppressed Th1 cell development and function, which involves a mechanism different from that of Th17 cells. Most importantly, we utilized EAE as a model to further demonstrate that activation of TLR7 signaling can inhibit Th1 and Th17 cells in vivo, resulting in immunotherapeutic prevention of EAE development and reduction of the disease severity. These studies indicate activation of TLR7 signaling may be successful as a new strategy to specifically target Th17/Th1 cells, which should have therapeutic potential in autoimmune and inflammatory diseases and other Th17/Th1-related diseases as well.
TLRs are critical for triggering innate immune responses and priming antigen-specific adaptive immunity (15). However, increasing evidence suggests that TLRs can also directly modulate the differentiation, proliferation and function of T lymphocytes (16, 17). Activation of Th17 cells through different TLRs has recently also been investigated by different groups. TLR2 signaling in T cells could directly promote Th17 generation and expansion, while TLR4 signaling enhances proliferation and survival of Th17 cells (16–18). Both TLR2 and TLR4 signaling are critical for amplification of autoimmune inflammation and promote the pathogenesis of EAE in vivo (16–18). However, little is known about whether other TLR signaling pathways can inhibit Th17 cells and control excessive inflammation and autoimmune responses. Our current study provides strong evidence showing that TLR7 can significantly inhibit Th17 differentiation and development, as well as suppress already established Th17 maintenance and stability in both mice and humans. In support of our observations, previous studies have already shown that imiquimod treatment could reduce the disease severity of asthma and EAE (33, 55), but mechanistic details responsible for these imiquimod-mediated effects have not been reported. Importantly, our current studies significantly improve our molecular understanding of TLR7-mediated suppression of Th17 cells. We confirmed that the ligand-mediated suppression of Th17 cells is TLR-signaling specific using T cells derived from MyD88 knockout mice. Utilizing loss-of-functions strategies with STAT3 knockout T cells and siRNAs, we have further shown that TLR7-induced Th17 suppression involves the reciprocal regulation of SOCS3/SOCS5 and STAT3 signaling. Surprisingly, we also found that the TLR7 ligand imiquimod suppressed Th1 cell development and function. However, the molecular processes of suppression of Th1 cells did not require MyD88 signaling or STAT3-SOCS regulation, distinct from Th17 cells. Furthermore, we did not observe any effects on γδ T cell changes mediated by imiquimod in vivo during the EAE development (data not shown)(18). These diverse results further suggest that TLR mediated regulation of T cell subsets is complex and may vary by cell subset and among different TLR ligands. A better understanding of the function and regulation of TLR signaling pathways in different T cell subsets will not only facilitate the identification of the molecular mechanisms involved in T cell mediated pathogenesis of immune diseases, but also will provide novel strategies for the development of effective clinical treatments.
Our current studies indicate the potential development of TLR7-targeted Th17/Th1 cells for immunotherapy of autoimmune and inflammatory diseases. In our initial screening studies, we used loxoribine and imiquimod, the two well-known ligands for TLR7 signaling. Our studies suggest that imiquimod and loxoribine may have a different activity on T cell development, although they induce similar effects and responses in macrophages, DCs and B cells (56, 57). Both Imiquimod and loxoribine can inhibit Th17 polarization and development, but imiquimod is more potent than loxoribine. Furthermore, loxoribine does not have strong activity on established Th17 cells both in humans and mice, and cannot inhibit Th1 development. The differences of TLR7 ligands imiquimod and loxoribine on T cells are supported by the study from other group showing that imiquimod and loxoribine induce different responses in astrocytes both in vitro and in vivo (58). Our studies clearly show that imiquimod can induce consistent suppression of both Th1 and Th17 cells, suggesting that imiquimod could be a feasible immunotherapeutic agent. In support of this notion, our complementary in vivo studies using the EAE model demonstrated that activation of TLR7 signaling through imiquimod significantly prevented EAE development and reduced EAE disease severity. These in vivo therapeutic effects mediated by imiquimod were associated with inhibition of both Th1 and Th17 cell responses, demonstrated by decreases in both absolute cell numbers and cell fractions of IFN-γ+, IL-17+ and IFN-γ+IL-17+ T cells in the local inflammatory microenvironment in spinal tissue, decreases of cell percentages of these cells in the periphery, as well as downregulation of antigen-specific T cell responses during EAE development. In addition, our novel concept is supported by studies from other groups showing that the TLR7 agonist imiquimod stimulation could reduce severity of EAE, and decrease allergic diseases such as asthma and rhinitis both in mouse models and in humans (33, 55, 59, 60). Actually, the TLR7 agonist imiquimod is now being developed as an immunotherapeutic drug or vaccine adjuvant for cancer treatment in cancer patients (24, 25).
Our studies clearly suggest that TLR7-mediated Th17 inhibition mechanistically involves direct effects on Th17 cells rather than indirectly effects on DCs. Studies from humans have shown that stimulation of plasmacytoid dendritic cells (PDCs), MoDCs or PBMC with TLR7 ligands can modify DC maturation and function, resulting in the promotion of Th17 differentiation and polarization from naïve T cells, as well as IL-17 production (35–38). Interestingly, some previous studies have been reported in mouse models suggesting inhibitory effects of TLR7 signaling on Th17 cells dependent on DC effects in both inflammatory and EAE models (33, 34, 61). We investigated the possibility that TLR7-mediated inhibition of Th17 differentiation and development involves effects on DCs. Using both co-culture and transwell systems, we found that pretreatment of DCs with TLR7 ligands did not influence Th17 differentiation. These studies clearly indicate that TLR7 signaling plays an inhibitory role on Th17 differentiation acting directly on CD4+ T cells, but not DCs. Besides DCs, TLR7 signaling may also regulate B cell responses which could then influence T cells (62). Furthermore, B cells also contribute to both pathogenesis and protection in EAE (63). Our studies demonstrated that imiquimod treatment significantly decreased the cell numbers and percentages of B cells, granulocytes and macrophages in the spinal cords in EAE mice (sFigure 4D). Therefore, our future studies will continue to explore the effects of TLR7 signaling on different immune components involved in humoral and inflammatory immune responses during the processos of inflammatory and autoimmune diseases.
Our studies identified that TLR7-mediated suppression of Th17 cells mechanistically involves the inhibition of STAT3 signaling and Th17-related transcriptional factors. It is now recognized that STAT3 is the key transcription factor controlling Th17 cell development and fate (49, 50). Th17 polarizing cytokines (IL-6, IL-21, and IL-23) preferentially activate STAT3, enhancing expression of the transcription factors RORγt and RORα, which in cooperation with the arylhydrocarbon receptor promote the expression of unique Th17 cytokine products, including IL-17, IL-21 and IL-22 (49, 50). In turn, IL-17 and IL-21 genes are also the direct targets of STAT3 (50). In addition to cytokine regulation, SOCS3 can directly block STAT3 signaling and its deletion results enhanced Th17 differentiation (52, 53). In our efforts to identify the mechanisms responsible for the TLR7-mediated suppression of Th17 cells, we discovered that STAT3 is the key player controlling the molecular process of Th17 inhibition mediated by TLR7 signaling. Using STAT3fl/flCD4cre+ mice, we further demonstrated that STAT3 is very important for the polarization of both Th17 and Th1 cells. Although the TLR7 ligand imiquimod can inhibit both Th17 and Th1 cells, we observed that STAT3 signaling is involved only in TLR7-mediated suppression of Th17 cells, but not Th1 cells. Importantly, using a loss-of-function strategy with siRNA, we further elucidated that induction of SOCS3 and SOCS5 is responsible for TLR7-mediated inhibition of STAT3 signaling and Th17 differentiation. Additionally, our studies show that TLR7 ligand treatment can partially decrease IL-23R expression in T cells. Given that IL-23 is important for Th17 cell maintenance, this effect could also be involved as a causative link for inhibition of both Th17 differentiation and established Th17 cell maintenance. Furthermore, STAT3 is important for IL-23R expression, therefore our study showing decreased expression of IL-23R in TLR7 treated T cells might also be the result of STAT3 inhibition (50). Our future studies will focus on a better understanding of the different molecular mechanisms and unique signaling pathways involved in TLR7-regulated Th1 and Th17 cells. These studies will be critical preludes for the application of TLR7 ligands as clinically therapeutic interventions.
In summary, we have identified that activation of TLR7 signaling in T cells can inhibit both Th17 and Th1 cell differentiation and function, but through different molecular mechanisms. We further reveal that inhibition of STAT3 signaling is responsible for the TLR7-mediated suppression of Th17 cells, which is reciprocally regulated by the induction of SOCS3 and SOCS5. Importantly, our complementary in vivo studies demonstrate that activation of TLR7 signaling can suppress both Th17 and Th1 cells, resulting in the therapeutic prevention of EAE development and reduction of the disease severity in vivo. These studies provide a new strategy focused on the activation of TLR7 signaling to specifically target Th17/Th1 cells for overcoming autoimmune and inflammatory diseases.
Supplementary Material
Acknowledgments
The authors would like to thank Joy Eslick and Sherri Koehm for FACS sorting and analyses. We also thank Dr. Rajeev Aurora (Department of Molecular Microbiology & Immunology at Saint Louis University) for providing FoxP3-GFP mice. This work was partially supported by grants from the American Cancer Society (RSG-10-160-01-LIB, to G. P), the Melanoma Research Alliance (to G.P), and the National Institutes of Health (AI097852 and CA184379 to G. P).
Footnotes
Conflict-of interest disclosure
The authors declare no competing financial interests.
Author contributions
JY and GP: designed research, analyzed data, prepared figures and wrote the paper. JY, YW, XL, LL, AO, and HL: performed experiments. EH: provided tumor samples and clinical information. TW and DH: advised the design of research and prepared MyD88 and STAT3 mice.
References
- 1.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dandekar S, George MD, Baumler AJ. Th17 cells, HIV and the gut mucosal barrier. Curr Opin HIV AIDS. 2010;5:173–178. doi: 10.1097/COH.0b013e328335eda3. [DOI] [PubMed] [Google Scholar]
- 4.Ye J, Su X, Hsueh EC, Zhang Y, Koenig JM, Hoft DF, Peng G. Human tumor-infiltrating Th17 cells have the capacity to differentiate into IFN-gamma+ and FOXP3+ T cells with potent suppressive function. Eur J Immunol. 2011;41:936–951. doi: 10.1002/eji.201040682. [DOI] [PubMed] [Google Scholar]
- 5.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 6.Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol. 2013;182:10–20. doi: 10.1016/j.ajpath.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 9.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 11.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Yang W, Gupta S, Biswas P, Smith P, Bhagat G, Pernis AB. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds JM, Pappu BP, Peng J, Martinez GJ, Zhang Y, Chung Y, Ma L, Yang XO, Nurieva RI, Tian Q, Dong C. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi G, Vistica BP, Nugent LF, Tan C, Wawrousek EF, Klinman DM, Gery I. Differential involvement of Th1 and Th17 in pathogenic autoimmune processes triggered by different TLR ligands. J Immunol. 2013;191:415–423. doi: 10.4049/jimmunol.1201732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci U S A. 2012;109:13064–13069. doi: 10.1073/pnas.1120585109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 20.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 21.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, Varvares MA, Hoft DF, Peng G. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120:2021–2031. doi: 10.1182/blood-2012-03-416040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye J, Ma C, Hsueh EC, Eickhoff CS, Zhang Y, Varvares MA, Hoft DF, Peng G. Tumor-Derived gammadelta Regulatory T Cells Suppress Innate and Adaptive Immunity through the Induction of Immunosenescence. J Immunol. 2013;190:2403–2414. doi: 10.4049/jimmunol.1202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulos CM, Kaiser A, Wrzesinski C, Hinrichs CS, Cassard L, Boni A, Muranski P, Sanchez-Perez L, Palmer DC, Yu Z, Antony PA, Gattinoni L, Rosenberg SA, Restifo NP. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smits EL, Ponsaerts P, Berneman ZN, Van Tendeloo VF. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist. 2008;13:859–875. doi: 10.1634/theoncologist.2008-0097. [DOI] [PubMed] [Google Scholar]
- 26.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 27.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien K, Fitzgerald DC, Naiken K, Alugupalli KR, Rostami AM, Gran B. Role of the innate immune system in autoimmune inflammatory demyelination. Current medicinal chemistry. 2008;15:1105–1115. doi: 10.2174/092986708784221458. [DOI] [PubMed] [Google Scholar]
- 29.Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, Piesche M, Schroers R, Weiss E, Kirschning CJ, Rochford CD, Bruck W, Becher B. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visser L, Jan de Heer H, Boven LA, van Riel D, van Meurs M, Melief MJ, Zahringer U, van Strijp J, Lambrecht BN, Nieuwenhuis EE, Laman JD. Proinflammatory bacterial peptidoglycan as a cofactor for the development of central nervous system autoimmune disease. J Immunol. 2005;174:808–816. doi: 10.4049/jimmunol.174.2.808. [DOI] [PubMed] [Google Scholar]
- 31.Touil T, Fitzgerald D, Zhang GX, Rostami A, Gran B. Cutting Edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-beta. J Immunol. 2006;177:7505–7509. doi: 10.4049/jimmunol.177.11.7505. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi T, Gray CS, Chan M, Tawatao RI, Ronacher L, McGargill MA, Datta SK, Carson DA, Corr M. Prevention of autoimmune disease by induction of tolerance to Toll-like receptor 7. Proc Natl Acad Sci U S A. 2009;106:2764–2769. doi: 10.1073/pnas.0813037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien K, Fitzgerald D, Rostami A, Gran B. The TLR7 agonist, imiquimod, increases IFN-beta production and reduces the severity of experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2010;221:107–111. doi: 10.1016/j.jneuroim.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Vultaggio A, Nencini F, Pratesi S, Maggi L, Guarna A, Annunziato F, Romagnani S, Parronchi P, Maggi E. The TLR7 ligand 9-benzyl-2-butoxy-8-hydroxy adenine inhibits IL-17 response by eliciting IL-10 and IL-10-inducing cytokines. J Immunol. 2011;186:4707–4715. doi: 10.4049/jimmunol.1002398. [DOI] [PubMed] [Google Scholar]
- 35.Yu CF, Peng WM, Oldenburg J, Hoch J, Bieber T, Limmer A, Hartmann G, Barchet W, Eis-Hubinger AM, Novak N. Human plasmacytoid dendritic cells support Th17 cell effector function in response to TLR7 ligation. J Immunol. 2010;184:1159–1167. doi: 10.4049/jimmunol.0901706. [DOI] [PubMed] [Google Scholar]
- 36.Lombardi V, Van Overtvelt L, Horiot S, Moingeon P. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-gamma, and IL-17A by naive CD4+ T cells. J Immunol. 2009;182:3372–3379. doi: 10.4049/jimmunol.0801969. [DOI] [PubMed] [Google Scholar]
- 37.Kattah MG, Wong MT, Yocum MD, Utz PJ. Cytokines secreted in response to Toll-like receptor ligand stimulation modulate differentiation of human Th17 cells. Arthritis and rheumatism. 2008;58:1619–1629. doi: 10.1002/art.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzopalic T, Dragicevic A, Vasilijic S, Vucevic D, Majstorovic I, Bozic B, Balint B, Colic M. Loxoribine, a selective Toll-like receptor 7 agonist, induces maturation of human monocyte-derived dendritic cells and stimulates their Th-1- and Th-17-polarizing capability. International immunopharmacology. 2010;10:1428–1433. doi: 10.1016/j.intimp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Pham NL, V, Badovinac P, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu XK, Lin X, Gaffen SL. Crucial role for nuclear factor of activated T cells in T cell receptor-mediated regulation of human interleukin-17. J Biol Chem. 2004;279:52762–52771. doi: 10.1074/jbc.M405764200. [DOI] [PubMed] [Google Scholar]
- 41.Henderson JG, Opejin A, Jones A, Gross C, Hawiger D. CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens. Immunity. 2015;42:471–483. doi: 10.1016/j.immuni.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Ye J, Ma C, Hsueh EC, Dou J, Mo W, Liu S, Han B, Huang Y, Zhang Y, Varvares MA, Hoft DF, Peng G. TLR8 signaling enhances tumor immunity by preventing tumor-induced T-cell senescence. EMBO molecular medicine. 2014;6:1294–1311. doi: 10.15252/emmm.201403918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukata M, Breglio K, Chen A, Vamadevan AS, Goo T, Hsu D, Conduah D, Xu R, Abreu MT. The myeloid differentiation factor 88 (MyD88) is required for CD4+ T cell effector function in a murine model of inflammatory bowel disease. J Immunol. 2008;180:1886–1894. doi: 10.4049/jimmunol.180.3.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomita T, Kanai T, Fujii T, Nemoto Y, Okamoto R, Tsuchiya K, Totsuka T, Sakamoto N, Akira S, Watanabe M. MyD88-dependent pathway in T cells directly modulates the expansion of colitogenic CD4+ T cells in chronic colitis. J Immunol. 2008;180:5291–5299. doi: 10.4049/jimmunol.180.8.5291. [DOI] [PubMed] [Google Scholar]
- 45.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Wei L, Laurence A, O’Shea JJ. New insights into the roles of Stat5a/b and Stat3 in T cell development and differentiation. Semin Cell Dev Biol. 2008;19:394–400. doi: 10.1016/j.semcdb.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park JS, Lim MA, Cho ML, Ryu JG, Moon YM, Jhun JY, Byun JK, Kim EK, Hwang SY, Ju JH, Kwok SK, Kim HY. p53 controls autoimmune arthritis via STAT-mediated regulation of the Th17 cell/Treg cell balance in mice. Arthritis and rheumatism. 2013;65:949–959. doi: 10.1002/art.37841. [DOI] [PubMed] [Google Scholar]
- 49.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 50.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 51.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, Yoshimura A, Tedgui A, Mallat Z. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eberle ME, Dalpke AH. Dectin-1 stimulation induces suppressor of cytokine signaling 1, thereby modulating TLR signaling and T cell responses. J Immunol. 2012;188:5644–5654. doi: 10.4049/jimmunol.1103068. [DOI] [PubMed] [Google Scholar]
- 55.Drake MG, Scott GD, Proskocil BJ, Fryer AD, Jacoby DB, Kaufman EH. Toll-like receptor 7 rapidly relaxes human airways. American journal of respiratory and critical care medicine. 2013;188:664–672. doi: 10.1164/rccm.201303-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 57.Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, Cottam HB. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butchi NB, Pourciau S, Du M, Morgan TW, Peterson KE. Analysis of the neuroinflammatory response to TLR7 stimulation in the brain: comparison of multiple TLR7 and/or TLR8 agonists. J Immunol. 2008;180:7604–7612. doi: 10.4049/jimmunol.180.11.7604. [DOI] [PubMed] [Google Scholar]
- 59.Adner M, Starkhammar M, Georen SK, Dahlen SE, Cardell LO. Toll-like receptor (TLR) 7 decreases and TLR9 increases the airway responses in mice with established allergic inflammation. European journal of pharmacology. 2013;718:544–551. doi: 10.1016/j.ejphar.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Aryan Z, Holgate ST, Radzioch D, Rezaei N. A new era of targeting the ancient gatekeepers of the immune system: toll-like agonists in the treatment of allergic rhinitis and asthma. International archives of allergy and immunology. 2014;164:46–63. doi: 10.1159/000362553. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi T, Yao S, Crain B, Chan M, Tawatao RI, Gray C, Vuong L, Lao F, Cottam HB, Carson DA, Corr M. Treatment of autoimmune inflammation by a TLR7 ligand regulating the innate immune system. PLoS One. 2012;7:e45860. doi: 10.1371/journal.pone.0045860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bishop GA, Ramirez LM, Baccam M, Busch LK, Pederson LK, Tomai MA. The immune response modifier resiquimod mimics CD40-induced B cell activation. Cellular immunology. 2001;208:9–17. doi: 10.1006/cimm.2001.1769. [DOI] [PubMed] [Google Scholar]
- 63.Mann MK, Ray A, Basu S, Karp CL, Dittel BN. Pathogenic and regulatory roles for B cells in experimental autoimmune encephalomyelitis. Autoimmunity. 2012;45:388–399. doi: 10.3109/08916934.2012.665523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.