Abstract
Staphylococcus aureus is a medically important bacterial pathogen that is a common cause of superficial and deep-seated abscesses in humans. Most S. aureus isolates produce either a serotype 5 or 8 capsular polysaccharide (CP) that has been shown to enhance bacterial virulence. We investigated the role of S. aureus CPs in modulating abscess formation in an experimental animal model of intraabdominal infection. Structural studies of CP8 revealed that it has a zwitterionic charge motif conferred by the negatively charged carboxyl group of N-acetylmannosaminuronic acid and free amino groups available on partially N-acetylated fucosamine residues. We report that purified CP5 and CP8 facilitated intraabdominal abscess formation in animals when given i.p. with a sterile cecal contents adjuvant. Chemical modifications that neutralized the positively or negatively charged groups on CP8 abrogated its ability to provoke abscesses. Rats prophylactically treated with CP8 s.c. were protected against abscess formation induced by homologous or heterologous zwitterionic polysaccharides. Likewise, treatment with CP8 protected against challenge with viable S. aureus strains PS80 (a capsule type 8 strain) or COL (a methicillin-resistant capsule type 5 strain). Purified CP8 was a potent activator of rat and human CD4+ T cells in vitro. When transferred to naïve rats, these activated T cells modulated the development of intraabdominal abscess formation. These results provide a structure/function rationale for abscess formation by S. aureus and expand the sphere of encapsulated organisms that interact directly with T cells to regulate this host response to bacterial infection.
Staphylococcus aureus is a major bacterial pathogen that is responsible for a broad and divergent range of human and animal infections. These infections range from cutaneous infections, such as impetigo, folliculitis, and boils, to infections of wounds and infections originating from prosthetic devices, to severe life-threatening infections, such as osteomyelitis, endocarditis, and bacteremia with metastatic complications. Toxin-mediated diseases caused by S. aureus include food poisoning, toxic shock syndrome, and scalded skin syndrome. The bacterial components and secreted products that affect the pathogenesis of S. aureus infections are numerous and include surface-associated adhesins, exoenzymes, exotoxins, and capsular polysaccharide (CP). This constellation of bacterial products allows staphylococci to adhere to eukaryotic membranes, resist opsonophagocytosis, lyse mammalian cells, invade deep tissues, and trigger the production of a cascade of host immunomodulating molecules (1).
Bacterial CPs are well-characterized virulence factors that potentiate microbial infections. Serotype 5 or 8 CPs are expressed by most isolates of S. aureus (2, 3), and these polysaccharides enhance virulence in a number of animal models of staphylococcal infection (4–6). In contrast, CP5 and CP8 attenuate staphylococcal virulence in a catheter-induced model of endocarditis (7). The S. aureus capsule protects the bacterium against in vitro opsonophagocytic killing by human polymorphonuclear leukocytes (5, 8).
The majority of bacterial CPs whose structures have been deduced are uncharged or possess negative charges. However, certain bacterial CPs possess a zwitterionic charge motif that not only provides resistance to phagocytosis but also directly modulates the host immune response to bacterial infection. The system best characterized is that of Bacteroides fragilis, an anaerobe that produces at least two distinct CPs, polysaccharides A and B (PS A and PS B), with free amino, carboxyl, and/or phosphonate groups (9). Each capsule (in purified form) provokes abscess formation when given i.p. with a sterile cecal contents adjuvant (SCCA) in a rat model of intraabdominal sepsis (10). In contrast, s.c. administration of these polymers in the absence of adjuvant and before bacterial challenge protects against abscess formation induced by live bacteria (11). Chemical neutralization or removal of free amino or negatively charged groups abrogates the modulation of abscess formation (10). A heterologous polysaccharide, such as the type 1 capsule from Streptococcus pneumoniae, with a different repeating sugar subunit structure but similar charged structural groups, also exhibits these biologic activities (10).
Because S. aureus is an encapsulated pathogen that is highly associated with abscess formation in humans and animals, we sought to determine whether purified staphylococcal cell wall-associated polysaccharides modulate abscess formation in rats and to investigate the structural basis for this activity. We report that purified CP5 and CP8, as well as ribitol teichoic acid from S. aureus, modulate abscess formation in the rat model of intraabdominal abscess formation. Structural analyses revealed that CP8 possesses a partially N-acetylated trisaccharide repeating unit with a zwitterionic charge motif that may contribute to the predominance of this organism in clinical cases of abscess formation.
Materials and Methods
Bacterial Strains.
S. aureus strains Reynolds and Becker are the prototype strains for capsular serotypes 5 and 8, respectively (12). Serotype 8 strain PS80 was obtained from the American Type Culture collection (no. 27700). S. aureus strain O13 (13), a serotype 8 isolate from a cystic fibrosis patient, was provided by Ali Fattom (Nabi, Rockville, MD). S. aureus strain COL, a methicillin-resistant serotype 5 strain, was obtained from John Iandolo (University of Oklahoma Health Sciences Center, Oklahoma City, OK). Mutant JL243 is an acapsular mutant of strain Reynolds (5). Staphylococci were cultivated for 24 h at 37°C on Columbia agar (Difco) supplemented with 2% NaCl. B. fragilis National Collection of Type Cultures 9343 is a reference strain used in our previous studies (14).
Purification of Bacterial Polysaccharides.
B. fragilis PS A.
PS A was isolated from B. fragilis NCTC 9343, as previously described (11). Its composition and purity were assessed by 1H NMR spectroscopy, UV spectroscopy (260 and 280 nm), and by reducing PAGE on gradient gels with subsequent silver staining.
S. aureus CP5 and CP8.
Staphylococci were cultivated on solid medium that was overlaid with a sheet of Spectra/Por molecularporous dialysis membrane (Spectrum Laboratories, Houston; 12,000–14,000 molecular weight cutoff). CP5 and CP8 were purified from capsular extracts of the bacteria by methods modified from those published previously (15, 16). Briefly, autoclaved bacterial extracts were treated with DNase and RNase (100 μg/ml each) for 6 h at 37°C, followed by overnight treatment with pronase (4 units/ml). Samples were dialyzed and treated with 0.05 M NaIO4 to oxidize teichoic acid polymers. The dialyzed material was passed over a column (2.6 × 30 cm) of DEAE-Sephacel (Amersham Pharmacia Fine Chemicals) equilibrated with 0.05 M Na acetate/0.05 M NaCl, pH 6.0. Fractions that eluted in a 2-liter gradient (0.05 M Na acetate with 0.05–0.5 M NaCl) were analyzed for serologic activity with antibodies to CP5 or CP8. Relevant fractions were pooled, dialyzed, and lyophilized. The polysaccharide was chromatographed on an S-300 (Amersham Pharmacia) column equilibrated in 0.2 M NaCl. Both CP5 and CP8, detected by absorbance at 206 nm, eluted near the void volume of the column, with Kav values of 0.01. Serologically active fractions were pooled, dialyzed, and lyophilized. The purity of the polysaccharides was assessed by UV (260 and 280 nm) and NMR spectroscopy.
Other Polysaccharides.
Isolation and purification of S. aureus wall teichoic acid was accomplished by methods described by Peterson et al. (17). Purified group B streptococcus type III CP was generously provided by Lawrence Paoletti (Channing Laboratory, Boston, MA). S. pneumoniae type 1 and 3 CPs were purchased from ATCC and purified as previously described (18).
Chemical Modifications to CP8.
Three distinct chemical modifications to CP8 were performed as described previously (10). In brief, free amino groups on CP8 were converted to N-acetyl moieties by treating the sample with acetic anhydride in 5% (wt/vol) NaHCO3 (9). Carboxyl groups were converted to hydroxymethyl groups by carbodiimide-mediated reduction (19). Last, the polymer was O-deacetylated by treatment with 0.1 N NaOH overnight at room temperature (20). A portion of this material was then N-acetylated, as described above. All modifications to polysaccharide structures were confirmed by NMR spectroscopy.
NMR Spectroscopy and Analytical Methods.
NMR experiments were performed on a Varian Unity 500 spectrometer (Varian) with a proton resonance frequency of 500.13 MHz. Proton spectra were recorded at 70°C in D2O, and chemical shifts were referenced to the water resonance at 4.35 ppm as calibrated externally. Free amino groups on zwitterionic polysaccharides were measured by fluorometric analysis after derivatization with fluorescamine (21).
Animal Model for Intraabdominal Abscess Formation.
The animal model of intraabdominal abscess formation was described previously (11). For experiments in which bacterial inocula were used, 10-fold serial dilutions [ranging from 109 to 101 colony-forming units (cfu)] of S. aureus were tested. For protection experiments, animals were challenged with 1 × 107 cfu S. aureus or 1 × 108 cfu B. fragilis NCTC 9343. In studies to assess abscess induction by various S. aureus polysaccharides, appropriate 10-fold dilutions of the test polymer (ranging from 200 to 0.02 μg) were mixed with SCCA. A control group challenged with SCCA alone was included in each experiment. The animals were killed 6 days after challenge and examined in an observer-blinded fashion for intraabdominal abscesses. A logistic regression model (10) was used to (i) calculate the dose required to induce abscesses in 50% of the animals (termed AD50) for each bacterial strain or polysaccharide tested and (ii) compare the biologic activities of modified and unmodified polysaccharides over a range of doses. For protection studies, animals were treated s.c. with 20 μg of the test polysaccharide at −24, 0, and +24 h relative to challenge. These animals were examined as described above for the development of abscess formation. Evaluation of differences between groups of animals in protection studies was performed by χ2 analysis (instat, GraphPad, San Diego). The results shown are a compilation of at least two separate experiments.
T Cell Proliferation Assays.
Human leukocytes were obtained from human leukopacs (discarded white cells from anonymous platelet donors), as previously described (22, 23). Rat CD4+ T cell proliferation assays were performed as described previously (24). For both human and rat T cell assays, cells were cultured with 20 μg/ml of either S. aureus PS80 CP8, S. pneumoniae type 1 CP, or group B streptococcus type III CP. Staphylococcal enterotoxin A (5 ng/ml, Sigma) was included as the positive control. Proliferation was measured at 6 and 9 days postculture for human CD4+ T cells and at 4 and 6 days postculture for rat CD4+ T cells. Data were expressed as the average cpm of triplicate wells ± the standard deviation. For all proliferation experiments, the data presented are representative of experiments performed at least three times.
T Cell Transfer Experiments.
CD4+ T cells were cultured for 5 days in vitro with irradiated antigen presenting cells and CP8 (20 μg/ml), as described above. After passage over a nylon wool column, T cells were washed extensively to remove the polysaccharide. The resulting population was >95% T cells (18). To assess their ability to induce abscess formation, 3 × 106 T cells were surgically implanted into the peritoneal cavities of rats along with SCCA, as previously described (25). Alternatively, T cells (3 × 106 cells/animal) were transferred intracardially (i.c.) to naïve rats 24 h before peritoneal challenge with ≈1 × 107 cfu S. aureus PS80 and SCCA. Animals were scored for abscess formation 6 days after challenge.
Results
Intraabdominal Abscess Induction by S. aureus.
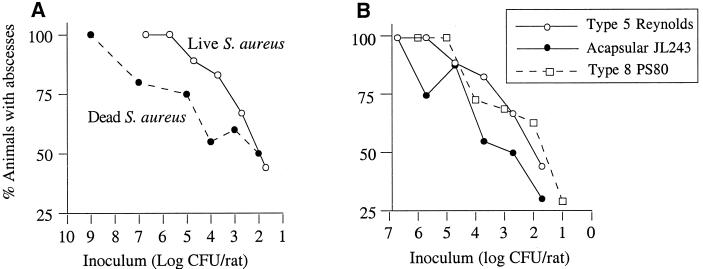
The rat model of intraabdominal abscess induction has been used most frequently with bacterial members of the human colonic flora. Whereas the AD50 of B. fragilis in this model is ≈105 cfu/rat (unpublished observations), facultative organisms such as Enterococcus faecalis and Escherichia coli do not induce abscesses at challenge doses as high as 5 × 107 cfu (26). Our results with S. aureus indicate that this organism is a potent inducer of abscess formation in rats. Rats were challenged with doses of the serotype 5 strain Reynolds that ranged from 107 to 101 cfu, and the AD50 for strain Reynolds was calculated to be 60 cfu (Fig. 1A). Bacterial viability was not an essential component of abscess induction, because formalin-killed Reynolds cells also provoked abscess formation (AD50 = 667 cfu; P = 0.05 compared with live Reynolds cells). The contribution of CP5 expression to the induction of abscess formation was evaluated by challenging additional groups of rats with serial dilutions of acapsular mutant JL243. The mutant also induced abscesses in the rats (Fig. 1B), although the calculated AD50 of 1.2 × 103 cfu was significantly greater than that of the parent strain (P = 0.02). This finding suggests that bacterial products other than capsule may also promote abscess induction. Experiments with the serotype 8 strain PS80 (Fig. 1B) revealed that its AD50 was 51 cfu, similar to that of strain Reynolds.
Figure 1.
Abscess induction by S. aureus. (A) Rats were challenged with live or formalin-killed S. aureus Reynolds cells. (B) Dose titration curves for serotype 5 strain Reynolds, the acapsular mutant JL243, and serotype 8 strain PS80. The calculated AD50 values for three strains were 60, 1.2 × 103, and 51 cfu, respectively.
Structural Composition of S. aureus CP8.
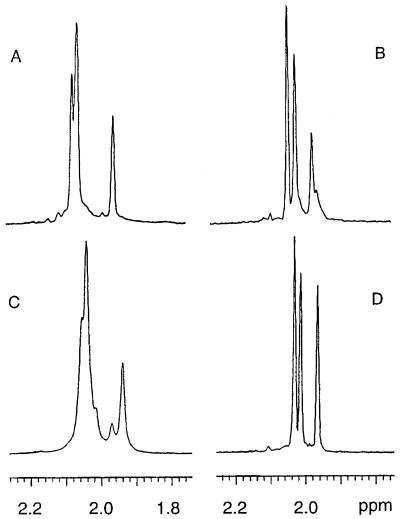
Previous studies have shown that the structure of CP8 is [→3)-4-O-Ac-β-ManNAcA-(1→3)-α-FucNAc-(1→3)-β-FucNAc-(1→]n (27, 28). CP8 was extracted and purified from three different S. aureus type 8 strains and analyzed by NMR spectroscopy. CP8 from strains Becker, PS80, and O13 were structurally similar, but each showed different degrees of N-acetylation. Proton NMR spectroscopy of native CP8 revealed resonance signals around 2 ppm corresponding to methyl protons from both -OAc and -NAc groups (Fig. 2 A and C). Mild alkali treatment of CP8 to remove O-acetyl groups associated with ManNAcA resulted in better resolution of the signals around 2 ppm (Fig. 2 B and D). For Becker CP8, the resonance signals at 2.032, 2.011, and 1.963 ppm were from the methyl protons of the N-acetyl groups of the three aminosugars comprising the repeating unit of this polymer (Fig. 2B). The ratio of these signals was 1:0.87:0.51, which indicates that at least two of the aminosugars were not completely N-acetylated (87 and 51%, respectively). For strain O13, the NMR spectrum of the O-deacetylated polymer indicates that two of the sugar components are ≈85% N-acetylated (Fig. 2D). Free amino groups are most likely present on the two FucNAc residues, because different isomers of the FucNAc were detected by two-dimensional NMR analysis (data not shown). A fluorometric analysis of CP8 after treatment with fluorescamine confirmed the presence of free amino groups on this polysaccharide.
Figure 2.
Partial 1H NMR spectra of native and O-deacetylated CP8 showing the NAc and OAc methyl protons. (A) Native CP8 from Becker; (B) O-deacetylated Becker CP8; (C) native CP8 from strain O13; (D) O-deacetylated CP8 from strain O13. The spectra in B and D indicate that CP8 from strains Becker and O13 have different degree of N-acetylation. CP8 from strains O13 and PS80 have nearly identical NMR spectra.
Abscess Induction by S. aureus Polysaccharides.
On the basis of our analysis, the CP8 repeating unit has positively charged free amino groups (on the partially N-acetylated FucNAc residues) and a negatively charged ManNAcA residue, conferring a zwitterionic charge motif to this polymer. The abscess-inducing potential of the PS80 CP8 was evaluated by challenging rats i.p. with doses ranging from 20 to 0.02 μg per rat. As shown in Table 1, purified CP8 was a potent inducer of abscess formation with an AD50 of 0.33 μg. This activity is comparable to that of B. fragilis PS A (AD50 = 0.67 μg) (10) and greater than that of B. fragilis PS B (AD50 = 25 μg) or S. pneumoniae type 1 CP (AD50 = 31 μg) (10). CP8 purified from strains Becker and O13 were also tested in the animal model and demonstrated biologic activity similar to that of CP8 purified from PS80 (data not shown).
Table 1.
Abscess induction by modified S. aureus CP8 polysaccharides
| Polysaccharide | No. of animals with abscesses/total challenged with
|
AD50, μg | P value | ||||
|---|---|---|---|---|---|---|---|
| 200 μg | 20 μg | 2 μg | 0.2 μg | 0.02 μg | |||
| CP8 | ND | 40/43 (93%) | 27/40 (68%) | 15/37 (41%) | 1/9 (11%) | 0.33 | — |
| CP5 | ND | 14/17 (82%) | 14/17 (82%) | 3/9 (33%) | ND | 0.30 | — |
| Teichoic acid | ND | 14/18 (78%) | 11/17 (65%) | 3/10 (30%) | ND | 0.88 | — |
| CP8 (N-acetylated) | 10/19 (53%) | 4/20 (20%) | 3/20 (15%) | ND | ND | 184 | <0.0001* |
| CP8 (reduced) | 3/10 (30%) | 3/10 (30%) | 2/9 (22%) | ND | ND | 240 | <0.0001* |
| CP8 (de-O-acetylated) | ND | 8/8 (100%) | 6/7 (86%) | 2/8 (25%) | ND | 0.25 | NS |
| CP8 (de-O-/N-acetylated) | 2/8 (25%) | 3/10 (30%) | 2/9 (22%) | ND | ND | 245 | <0.0001† |
NS, not significant. ND, not done.
Compared with CP8.
Compared with de-O-acetylated CP8.
S. aureus CP5 is structurally similar to CP8; the two polysaccharides differ only in the linkages between the sugars and the sites of O-acetylation of the ManNAcA residues. As shown in Table 1, CP5 was also a potent inducer of abscess formation with an AD50 of 0.30 μg in rats. Cell wall-associated teichoic acid is another soluble polysaccharide from S. aureus with a zwitterionic charge motif. It is composed of a linear backbone structure of 4-O-β- and 4-O-α-N-acetyl-d-glucosaminyl-d-ribitol bridged by 1,5-phosphodiester linkages. Approximately 50% of the ribitol residues are esterified at the C-2 position with d-alanine (29). Because this structure contains negatively charged phosphate groups and positively charged free amino groups (on the alanines), it fits the zwitterionic charge motif described above. Purified teichoic acid showed potency similar to that of CP5 and CP8, with an AD50 of 0.88 μg (Table 1).
Role of Charged Substituent Groups in Abscess Formation by S. aureus CP8.
We chemically modified CP8 to neutralize the charged groups associated with the CP8 repeating unit, and the resultant modified polymers were tested in the animal model. N-acetylation of the free amino groups on the CP8 repeating unit resulted in a significant loss of abscess-inducing activity. The AD50 for the modified saccharide over the range of doses tested was 184 μg (Table 1; P < 0.0001 compared with native CP8). Similarly, carbodiimide-mediated reduction of the carboxyl groups on CP8 to hydroxymethyl groups resulted in a similar loss of biologic activity, yielding a polymer with an AD50 of 240 μg (P < 0.0001).
The ManNAcA residue of CP produced by different S. aureus isolates is variably O-acetylated (15, 30). Although the presence or absence of O-acetyl groups does not confer a positive or negative charge to the polymer, we O-deacetylated CP8 with mild alkali to rule out the contribution of this group to abscess formation. As predicted, the AD50 of this modified polymer was 0.25 μg, comparable to that of native CP8 (Table 1). In contrast, N-acetylation of the O-deacetylated CP8 resulted in a significant loss of activity (AD50 >245 μg; P < 0.0001 compared with the O-deacetylated CP8). These results demonstrate that O-acetyl groups did not influence abscess formation, whereas neutralization of the free amino groups on CP8 by N-acetylation abrogated its biologic activity.
Protection Against Abscess Formation by Treatment of Rats with S. aureus or B. fragilis Polysaccharides.
Groups of animals were treated with saline, 20 μg S. aureus CP8, or 20 μg B. fragilis PS A, as described in Material and Methods section and challenged i.p. with 20 μg CP8 or PS A mixed with SCCA. The results are shown in Table 2. Animals treated with saline before challenge with CP8 had an 89% abscess rate, whereas prior treatment with CP8 or PS A resulted in a significant reduction in abscess formation in each group (37 and 5%, respectively, P < 0.0005). In addition, treatment with CP8 or PS A protected against challenge with PS A, reducing abscess formation in the saline-treated control from 85% to 15% in each case (P < 0.0001).
Table 2.
Protection against abscess formation by S. aureus CP8
| Treatment (20 μg) | Challenge | No. rats with abscesses/total | P value* |
|---|---|---|---|
| Protection against heterologous polysaccharide challenge | |||
| Saline | CP8 | 24/27 (89%) | — |
| CP8 | CP8 | 7/19 (37%) | 0.0003 |
| PS A | CP8 | 1/20 (5%) | <0.0001 |
| Saline | PS A | 17/20 (85%) | — |
| CP8 | PS A | 3/20 (15%) | <0.0001 |
| PS A | PS A | 3/20 (15%) | <0.0001 |
| Protection against heterologous S. aureus challenge | |||
| Saline | PS80 | 17/20 (85%) | — |
| CP8 | PS80 | 6/18 (33%) | 0.002 |
| PS A | PS80 | 5/19 (26%) | <0.005 |
| S. pneumoniae type 3 CP | PS80 | 6/8 (75%) | NS† |
| Saline | COL | 12/14 (86%) | — |
| CP8 | COL | 4/15 (27%) | 0.003 |
Compared with saline-treated control group.
NS, not significant.
To determine whether treatment with these polysaccharides could protect against abscesses induced by live bacteria, animals were treated s.c. with saline, CP8, or PS A and then challenged with 107 cfu S. aureus PS80. Treatment with saline resulted in abscesses detected in 85% of animals, whereas treatment with CP8 or PS A reduced the incidence of abscesses to 33 and 26%, respectively (P < 0.005; Table 2). Similar treatment with a negatively charged polysaccharide (S. pneumoniae type 3 CP) did not confer protection against strain PS80, resulting in a 75% abscess rate. In a separate experiment, animals were treated with saline or CP8 and challenged with the serotype 5 methicillin-resistant S. aureus COL. Only 27% of CP8-treated rats developed abscesses compared with 86% in the saline-treated control group (Table 2, P = 0.003).
In Vitro T Cell Activation by S. aureus CP8.
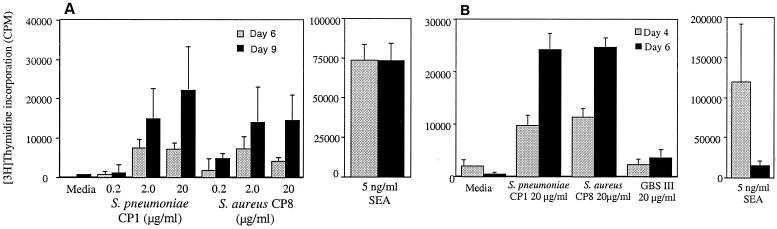
We tested CP8 purified from S. aureus PS80 in these studies because this bacterial strain does not produce superantigens (enterotoxins A, B, C, D, E, H, toxic shock syndrome toxin-1, or exfoliative toxin A). CD4+ T cells were cultured with irradiated antigen-presenting cells and either S. aureus CP8 or control polysaccharides. Cellular proliferation was measured at 6 and 9 days postculture for human CD4+ T cells and at 4 and 6 days postculture for rat CD4+ T cells. CP8 elicited a potent proliferative response in both human and rat T cells (Fig. 3). This response occurred in a dose-dependent manner in human cells and peaked at day 9 postculture (Fig. 3A). The T cell response was comparable to that seen with the S. pneumoniae type 1 CP over the same dose range and was significantly higher than that of media alone. The group B Streptococcus type III capsule, a negatively charged polysaccharide, failed to activate human T cells in this assay (data not shown). The S. aureus superantigen SEA (5 ng/ml) induced a T cell proliferative response that was significantly higher than proliferation induced by either of the polysaccharide antigens.
Figure 3.
Purified CP8 elicited a potent proliferative response in both human (A) and rat (B) T cells. Activation of human T cells by S. pneumoniae CP1 and S. aureus CP8 peaked at day 9 postculture (A), whereas the rat T cell response peaked on day 6 (B).
CP8 also yielded a potent proliferative response in rat CD4+ T cells after coculture (Fig. 3B). The T cell response peaked at day 6 postculture and was comparable to the response elicited by S. pneumoniae type 1 CP at 20 μg/ml. The negatively charged group B Streptococcus type III polysaccharide failed to stimulate rat T cells in vitro.
CD4+ T Cells Activated by CP8 in Vitro Modulate Abscess Formation in Vivo.
The role of T cell activation by CP8 in the modulation of abscess formation was determined in T cell transfer experiments. Purified rat CD4+ T cells were cultured in vitro with CP8 in the presence of irradiated antigen presenting cells for 5 days, split into two aliquots, and transferred to different groups of animals by two separate routes of administration. The first group of animals received stimulated T cells i.p. along with SCCA. The second group of animals was given the stimulated T cells i.c. 24 h before i.p. challenge with ≈107 cfu S. aureus PS80 and SCCA. Animals were scored for the development of abscesses 6 days later, and the results are shown in Table 3. CD4+ T cells cultured with medium or an irrelevant control polysaccharide (group B Streptococcus type III capsule) that were implanted into the peritoneal cavity with SCCA failed to induce abscesses. However, animals challenged with SCCA and CD4+ T cells cultured with CP8 yielded an abscess rate of 80%. i.p. challenge with CD4+ T cells in the absence of SCCA did not induce abscess formation.
Table 3.
CP8-activated T cells can facilitate or protect against abscess formation
| Abscess induction
|
Abscess protection
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| T cell stimulus | T cell transfer | SCCA | No. rats with abscesses/total | P value* | T cell stimulus | T cell transfer | i.p. Challenge | No. rats with abscesses/total | P value |
| Medium | i.p. | + | 1/10 (10%) | — | Medium | i.c. | S. aureus/SCCA | 9/10 (90%) | — |
| CP8 | i.p. | − | 8/10 (80%) | <0.01 | CP8 | i.c. | S. aureus/SCCA | 2/10 (20%) | <0.01 |
| CP8 | i.p. | − | 0/10 (0%) | — | GBS type III | i.c. | S. aureus/SCCA | 9/10 (90%) | — |
| GBS type III | i.p. | + | 0/10 (0%) | — | |||||
Compared to respective medium control.
Administration of the same CP8-stimulated CD4+ T cells to animals i.c. 24 h before bacterial challenge protected against abscess formation (Table 3). Animals given medium- or GBS type III CP-stimulated CD4+ T cells and challenged with viable S. aureus PS80 showed a 90% abscess rate, whereas only 20% of rats given CP8-stimulated CD4+ T cells developed abscesses (P < 0.01).
Discussion
Zwitterionic polysaccharides are potent abscess-inducing agents when implanted into the peritoneal cavity along with SCCA (10). Conversely, s.c. administration of these polymers (in the absence of adjuvant) before i.p. challenge with different abscess-inducing bacteria prevents subsequent abscess formation (11). Bacterial polysaccharides with dissimilar repeating unit structures that exhibit this zwitterionic charge motif (B. fragilis PS A and PS B and S. pneumoniae type 1 CP) share these biologic properties. On the basis of these data, we hypothesized that an organism such as S. aureus that is highly associated with clinical cases of abscesses may possess one or more cell wall-associated polysaccharides with a zwitterionic charge motif and that these polymers may modulate abscess induction by this organism.
In 1990, Moreau et al. (15) showed that S. aureus CP5 was a partially (50%) O-acetylated polymer with the following repeating unit structure: [→4)-3-O-Ac-β-d-ManpNAcA-(1→4)-α-l-FucpNAc-(1→3)-β-d-FucpNAc-(→]n. Vann et al. (28) reported the structure of CP8 to be [→3)-4-O-Ac-β-d-ManpNAcA-(1→3)-α-l-FucpNAc-(1→3)-β-d-FucpNAc-(→]n, but only 13C data were provided to support this conclusion. Our analysis of the structure of S. aureus CP8 revealed that it is only partially N-acetylated, resulting in a polymer that has free amino groups and negatively charged carboxyl groups. Purified CP8 from three strains (Becker, O13, and PS80) showed varying degrees of N-acetylation. The finding of a zwitterionic charge motif associated with this polymer prompted us to assess its ability to modulate abscess formation in a relevant animal model. Our results demonstrated that viable S. aureus, killed S. aureus, purified CPs, and ribitol teichoic acid from S. aureus induced abscesses in rats. Chemical modifications to the structure of CP8 showed that the free amino and carboxyl groups of this repeating unit are critical to its activity. However, removal of O-acetyl groups did not affect its ability to induce abscesses.
Animals treated s.c. with CP8 24 h before challenge with either CP8 or PS A mixed with SCCA were protected against abscess formation. The 20-μg dose of polysaccharide used to challenge animals in these experiments was ≈60-fold higher than the AD50 values for these polymers. Prophylactic treatment with CP8 also prevented abscesses resulting from bacterial challenge with viable S. aureus PS80 or the type 5 strain COL. The challenge dose of PS80 in this study was ≈1.6 × 105-fold greater than the AD50 value for this organism. The ability to prevent abscesses induced by heterologous antigens indicates that CP8 has broad protective activity.
Demonstration that CP8 from S. aureus activates both human and rat CD4+ T cells in vitro further supports the immunomodulatory properties of this polymer. T cell activation by bacterial polysaccharides is rare, and thus far only polymers with a zwitterionic charge motif have been shown to possess this activity (18). CP8 purified from S. aureus PS80 was used for most of these studies, because strain Becker produces enterotoxin B. Detailed chemical and NMR analysis showed that the polysaccharide preparations were devoid of protein and lipopolysaccharide contamination. A biologic role for CD4+ T cells activated by CP8 was demonstrated in the animal model. Cells from the same in vitro CP8-stimulated culture could facilitate abscess formation when implanted with SCCA into the peritoneal cavities of rats or confer protection when administered i.c. 24 h before challenge with viable S. aureus. These results corroborate our previous studies with T cells activated by B. fragilis PSA or the S. pneumoniae type 1 CP (25) and raise questions concerning the role of CD4+ T cells in the modulation of abscess formation. Demonstration that the same cell type can have seemingly paradoxical activities leading to immunopathology (abscess formation) or immunity (prevention of abscess formation) is similar to the modulation of experimental autoimmune encephalomyelitis (EAE). This disease process can be induced in rodents by injecting an autoantigen, myelin basic protein, in the presence of Freund's complete adjuvant, whereas prophylactic administration of this protein in the absence of Freund's leads to protection (31). These biologic properties are also under the control of CD4+ T cells. In addition, the role of SCCA in our studies is similar to that of Freund's adjuvant in EAE. However, it can be argued that the use of SCCA as adjuvant with our model closely resembles the course of events (i.e., spillage of colonic contents into the peritoneal cavity) that lead to abscess formation in human disease. Currently, we are investigating whether different CD4+ T cell subpopulations are responsible for abscess induction or protection or whether the presence of SCCA in the peritoneal cavity for abscess induction experiments is the determining factor.
Previously, we showed that treatment of rats with zwitterionic polysaccharides before challenge with a cecal contents inoculum prevents abscess formation and lowers the mortality rate in animals (32). These results correlate with previous studies in humans and rats in which the use of fibrinolytic compounds lowered mortality and was accompanied by increased drainage efficacy, absence of fragmentation of abdominal contents, and absence of secondary abscesses (33). This challenges the notion that walling off bacteria within developing abscesses is beneficial to the host and is consistent with more recent experimental and clinical data that support the concept that prevention of intraabdominal abscesses is ultimately beneficial to patients.
In summary, we have demonstrated that S. aureus produces multiple cell-associated zwitterionic polymers that potentiate intraabdominal abscess formation. In addition to CP5, CP8, and teichoic acid, uncrosslinked peptidoglycan (present in growing cells) and lipoteichoic acid also possess a zwitterionic charge motif and may contribute to abscess induction. Interestingly, the staphylococcal polysaccharides tested are more potent than the majority of CPs that have been shown to induce abscess formation (10). S. aureus CP8 activates human and rat CD4+ T cells, and activated T cells show a clear role in modulating abscess formation in an experimental animal model. This work provides a structure/function rationale for the ability of the staphylococcus to induce intraabdominal abscesses and may explain, in part, the prevalence of staphylococcal abscesses at different sites of infection. The role of S. aureus zwitterionic polysaccharides in the induction of abscesses at other body sites is currently under investigation.
Acknowledgments
We thank Drs. Dennis Kasper and Andrew Onderdonk for critical suggestions and Dr. Lawrence Paoletti for the group B streptococcal type III polysaccharide. We acknowledge the technical assistance of Jin-Sir Park, Derek Frederickson, Brian Hyett, and Barbara Reinap. The work was supported by National Institutes of Health Grants AI34073 and AI45563 (to A.T.) and AI29040 (to J.L).
Abbreviations
- CP
capsular polysaccharide
- PS A
Bacterioides fragilis polysaccharide A
- SCCA
sterile cecal contents adjuvant
- cfu
colony-forming units
- AD50
the dose required to induce abscesses in 50% of animals tested
- i.c.
intracardially
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Archer G L. Clin Infect Dis. 1998;26:1179–1181. doi: 10.1086/520289. [DOI] [PubMed] [Google Scholar]
- 2.Arbeit R D, Karakawa W W, Vann W F, Robbins J B. Diagn Microbiol Infect Dis. 1984;2:85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- 3.Hochkeppel H K, Braun D G, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan E L, Boutonnier A, Fournier J M. J Clin Microbiol. 1987;25:526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson I-M, Lee J C, Bremell T, Ryden C, Tarkowski A. Infect Immun. 1997;65:4216–4221. doi: 10.1128/iai.65.10.4216-4221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakker M, Park J-S, Carey V, Lee J C. Infect Immun. 1998;66:5183–5189. doi: 10.1128/iai.66.11.5183-5189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portoles M, Kiser K B, Bhasin N, Chan K H N, Lee J C. Infect Immun. 2001;69:917–923. doi: 10.1128/IAI.69.2.917-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baddour L M, Lowrance C, Albus A, Lowrance J H, Anderson S K, Lee J C. J Infect Dis. 1992;165:749–753. doi: 10.1093/infdis/165.4.749. [DOI] [PubMed] [Google Scholar]
- 8.Karakawa W W, Sutton A, Schneerson R, Karpas A, Vann W F. Infect Immun. 1988;56:1090–1095. doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumann H, Tzianabos A O, Brisson J R, Kasper D L, Jennings H J. Biochemistry. 1992;31:4081–4089. doi: 10.1021/bi00131a026. [DOI] [PubMed] [Google Scholar]
- 10.Tzianabos A O, Onderdonk A B, Rosner B, Cisneros R L, Kasper D L. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 11.Tzianabos A O, Kasper D L, Cisneros R L, Smith R S, Onderdonk A B. J Clin Invest. 1995;96:2727–2731. doi: 10.1172/JCI118340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karakawa W W, Vann W F. Semin Infect Dis. 1982;4:285–293. [Google Scholar]
- 13.Fattom A, R, S, Watson D C, Karakawa W W, Fitzgerald D, Pastan I, Li X, Shiloach J, Bryla D A, Robbins J B. Infect Immun. 1993;61:1023–1032. doi: 10.1128/iai.61.3.1023-1032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pantosti A, Tzianabos A O, Onderdonk A B, Kasper D L. Infect Immun. 1991;59:2075–2082. doi: 10.1128/iai.59.6.2075-2082.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreau M, Richards J C, Fournier J M, Byrd R A, Karakawa W W, Vann W F. Carbohydr Res. 1990;201:285–297. doi: 10.1016/0008-6215(90)84244-o. [DOI] [PubMed] [Google Scholar]
- 16.Lee J C, Michon F, Perez N E, Hopkins C A, Pier G B. Infect Immun. 1987;55:2191–2197. doi: 10.1128/iai.55.9.2191-2197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson P K, Wilkinson B J, Kim Y, Schmeling D, Douglas S D, Quie P G. J Clin Invest. 1978;61:597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzianabos A O, Finberg R W, Wang Y, Chan M, Onderdonk A B, Jennings H J, Kasper D L. J Biol Chem. 2000;275:6733–6740. doi: 10.1074/jbc.275.10.6733. [DOI] [PubMed] [Google Scholar]
- 19.Taylor R L, Conrad H E. Biochemistry. 1972;11:1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- 20.Bhasin N, Albus A, Michon F, Livolsi P J, Park J S, Lee J C. Mol Microbiol. 1998;27:9–21. doi: 10.1046/j.1365-2958.1998.00646.x. [DOI] [PubMed] [Google Scholar]
- 21.Udenfried S, Stein S, Bohlen P, Dairman W, Leingruber W, Weigele M. Science. 1972;178:871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- 22.Haregewoin A, Soman G, Hom R C, Finberg R W. Nature (London) 1989;340:309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- 23.Finberg R W, White W, Nicholson W A. J Immunol. 1992;149:2055–2060. [PubMed] [Google Scholar]
- 24.Brubaker J O, Li Q, Tzianabos A O, Kasper D L, Finberg R W. J Immunol. 1999;162:2235–2242. [PubMed] [Google Scholar]
- 25.Tzianabos A O, Chandraker A, Kalka-Moll W, Stingele F, Dong V M, Finberg R W, Peach R, Sayegh M H. Infect Immun. 2000;68:6650–6655. doi: 10.1128/iai.68.12.6650-6655.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onderdonk A B, Bartlett J G, Louie T, Sullivan-Seigler N, Gorbach S L. Infect Immun. 1976;13:22–26. doi: 10.1128/iai.13.1.22-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fournier J M, Vann W F, Karakawa W W. Infect Immun. 1984;45:87–93. doi: 10.1128/iai.45.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vann W F, Moreau M, Sutton A, Byrd R A, Karakawa W W. In: Bacterial Host–Cell Interaction. Horwitz M A, editor. Vol. 64. New York: Liss; 1988. pp. 187–198. [Google Scholar]
- 29.Archibald A R, Baddiley J. Adv Carbohydr Chem Biochem. 1966;21:323–375. doi: 10.1016/s0096-5332(08)60320-3. [DOI] [PubMed] [Google Scholar]
- 30.Fattom A I, Sarwar J, Basham L, Ennifar S, Naso R. Infect Immun. 1998;66:4588–4592. doi: 10.1128/iai.66.10.4588-4592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teitelbaum D, Arnon R, Sela M. Proc Natl Acad Sci USA. 1999;96:3842–3847. doi: 10.1073/pnas.96.7.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzianabos A O, Gibson F C, 3rd, Cisneros R L, Kasper D L. J Infect Dis. 1998;178:200–206. doi: 10.1086/515594. [DOI] [PubMed] [Google Scholar]
- 33.Grigoryev E G, Kogan A S, Kolmakov S A, Nechaev E V, Usov S A, Fadeeva T V. Int Surg. 1998;83:245–249. [PubMed] [Google Scholar]