Abstract
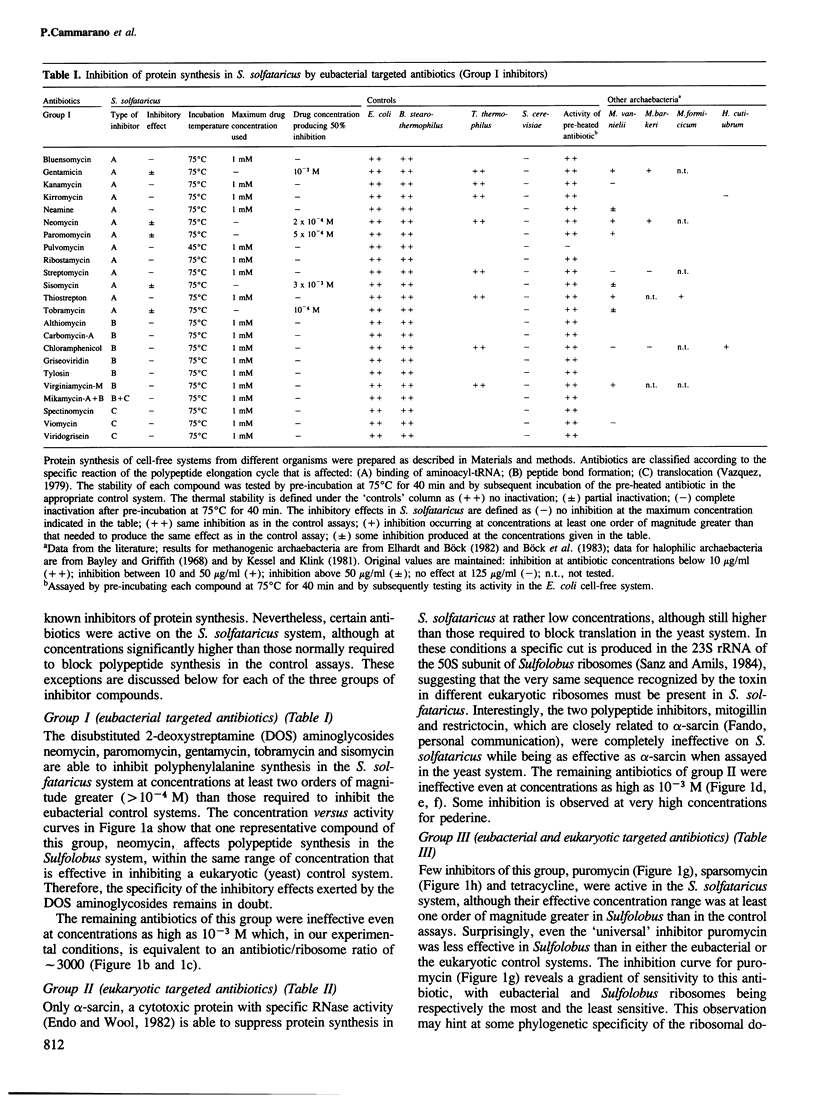
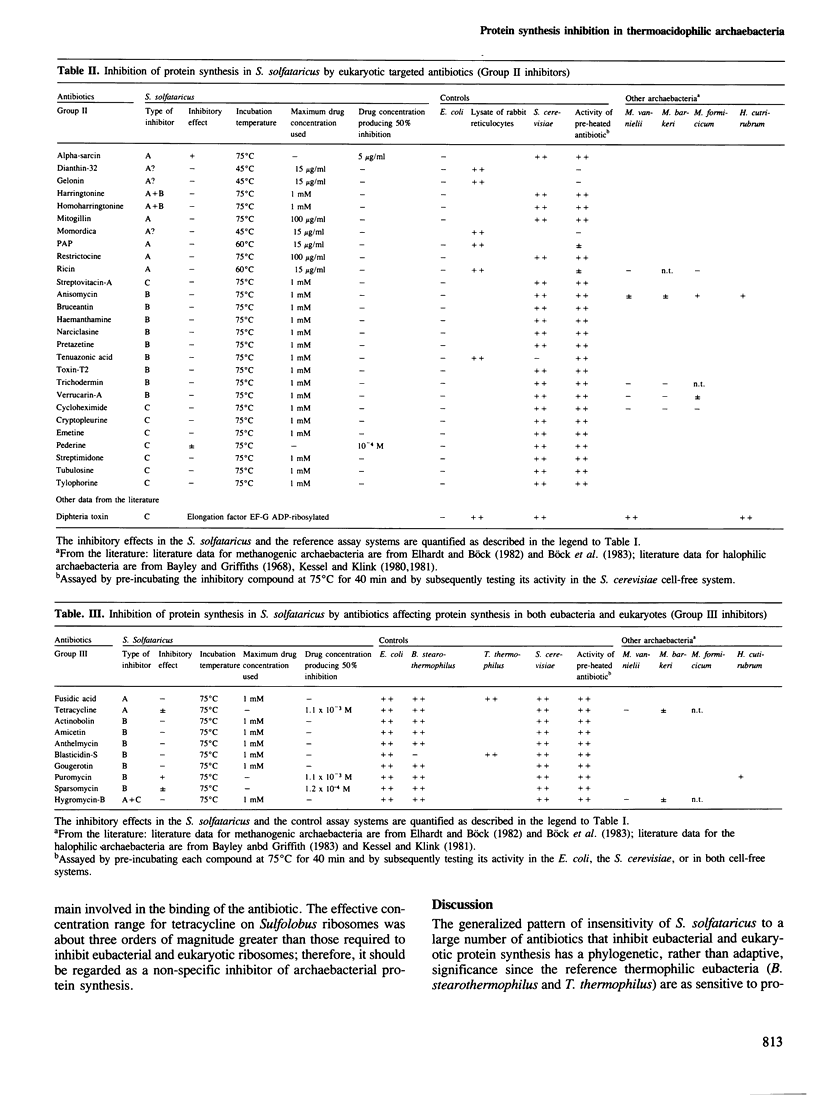
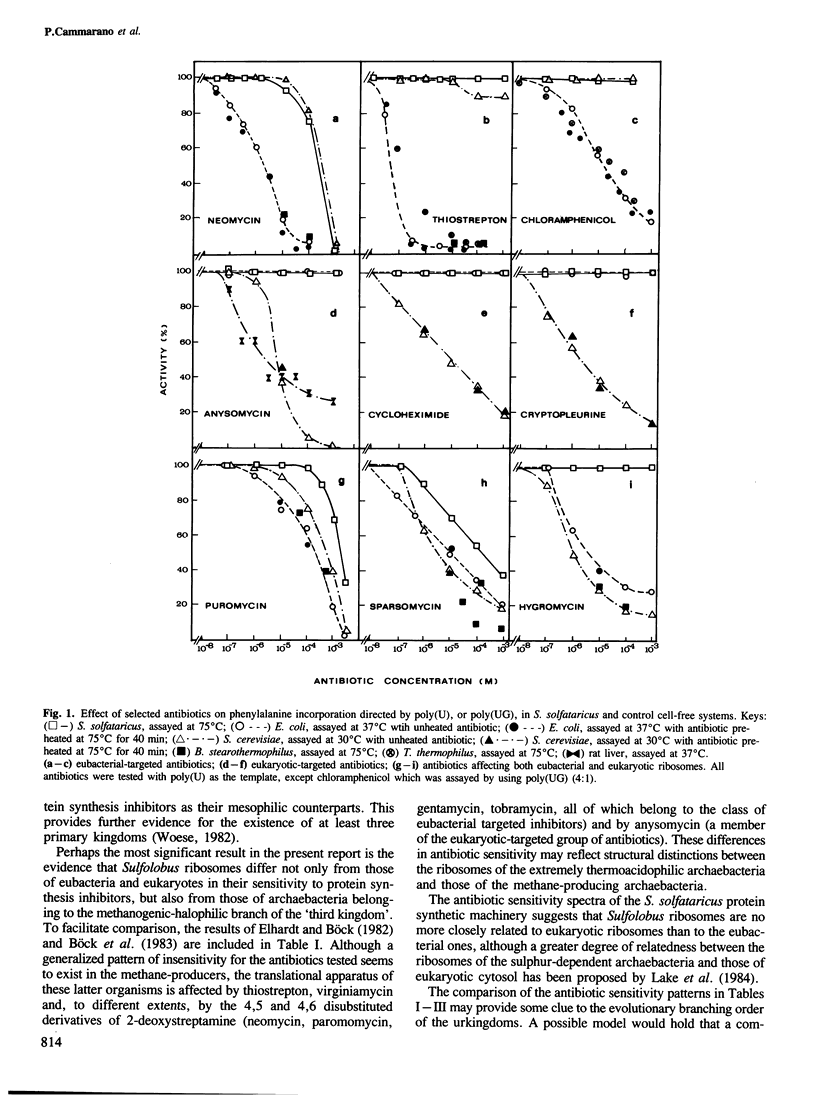
The effect on Sulfolobus solfataricus (an extremely thermoacidophilic archaebacterium) of selected inhibitors affecting reactions of the polypeptide elongation cycle has been tested by using poly(U) and poly(UG) directed cell-free systems. The results reveal a unique pattern of antibiotic sensitivity of Sulfolobus ribosomes with an inhibitory effect observed for only three of 60 compounds tested. Through comparison with suitable eubacterial and eukaryotic cell-free systems the insensitivity of Sulfolobus ribosomes to most inhibitors of protein synthesis appears to reflect a phylogenetic distinction of ribosome structure, rather than the high temperature conditions of the Sulfolobus assay system. In this respect ribosomes of thermoacidophilic archaebacteria differ not only from their eubacterial and eukaryotic counterparts, but also from ribosomes of archaebacteria belonging to the methanogenic-halophilic branch of the 'third' kingdom. The evolutionary implications of these findings are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Algranati I. D., Lengyel P. Polynucleotide-dependent incorporation of amino acids in a cell-free system from thermophilic bacteria. J Biol Chem. 1966 Apr 25;241(8):1778–1783. [PubMed] [Google Scholar]
- Bayley S. T., Griffiths E. A cell-free amino acid incorporating system from an extremely halophilic bacterium. Biochemistry. 1968 Jun;7(6):2249–2256. doi: 10.1021/bi00846a030. [DOI] [PubMed] [Google Scholar]
- Cammarano P., Teichner A., Chinali G., Londei P., de Rosa M., Gambacorta A., Nicolaus B. Archaebacterial elongation factor Tu insensitive to pulvomycin and kirromycin. FEBS Lett. 1982 Nov 8;148(2):255–259. doi: 10.1016/0014-5793(82)80819-3. [DOI] [PubMed] [Google Scholar]
- Endo Y., Wool I. G. The site of action of alpha-sarcin on eukaryotic ribosomes. The sequence at the alpha-sarcin cleavage site in 28 S ribosomal ribonucleic acid. J Biol Chem. 1982 Aug 10;257(15):9054–9060. [PubMed] [Google Scholar]
- Kessel M., Klink F. Archaebacterial elongation factor is ADP-ribosylated by diphtheria toxin. Nature. 1980 Sep 18;287(5779):250–251. doi: 10.1038/287250a0. [DOI] [PubMed] [Google Scholar]
- Kessel M., Klink F. Two elongation factors from the extremely halophilic archaebacterium Halobacterium cutirubrum. Assay systems and purification at high salt concentrations. Eur J Biochem. 1981 Mar;114(3):481–486. doi: 10.1111/j.1432-1033.1981.tb05170.x. [DOI] [PubMed] [Google Scholar]
- Lake J. A., Henderson E., Oakes M., Clark M. W. Eocytes: a new ribosome structure indicates a kingdom with a close relationship to eukaryotes. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3786–3790. doi: 10.1073/pnas.81.12.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz J. L., Amils R. Sensitivity of thermoacidophilic archaebacteria to alpha-sarcin. FEBS Lett. 1984 Jun 4;171(1):63–66. doi: 10.1016/0014-5793(84)80460-3. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Legg R. F., Onyon L. J., Ziska P., Franz H. Inhibition of protein synthesis by a toxic lectin from Viscum album L. (mistletoe). Biochem J. 1980 Sep 15;190(3):843–845. doi: 10.1042/bj1900843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Madrid F., Reyes R., Conde P., Ballesta J. P. Acidic ribosomal proteins from eukaryotic cells. Effect on ribosomal functions. Eur J Biochem. 1979 Aug 1;98(2):409–416. doi: 10.1111/j.1432-1033.1979.tb13200.x. [DOI] [PubMed] [Google Scholar]
- Zillig W., Tu J., Holz I. Thermoproteales--a third order of thermoacidophilic archaebacteria. Nature. 1981 Sep 3;293(5827):85–86. doi: 10.1038/293085a0. [DOI] [PubMed] [Google Scholar]



