Abstract
Background and aim
Little is known about population-based epidemiology and disease burden of autoimmune hepatitis (AIH). The aim of this study was to investigate the prevalence, incidence, comorbidity and direct medical cost of AIH in South Korea.
Methods
The data was from the nationwide, population-based National Health Insurance Service claims database and the Rare Intractable Disease registration program. Age and gender-specific prevalence rates were calculated, and data on comorbidity, diagnostic tests, prescribed drugs, and medical costs were retrieved for patients registered under the disease code K75.4 (AIH) from 2009 to 2013.
Results
A total of 4,085 patients with AIH were identified between 2009 and 2013 with a female-to-male ratio of 6.4. The age-adjusted prevalence rate was 4.82/100,000 persons and gender adjusted prevalence rates were 8.35 in females and 1.30 in males. The age-adjusted calculated incidence rate was 1.07/100,000 persons (gender-adjusted 1.83 in females and 0.31 in males). Ascites, variceal bleeding, and hepatocellular carcinoma were found in 1.4%, 1.3%, and 2.2% of the patients, respectively. Forty-six patients (1.1%) underwent liver transplantation during the study period. Case-fatality was 2.18%. Corticosteroid and azathioprine were prescribed in 44.1% and 38.0% of prevalent patients with AIH in 2013, respectively. The nationwide total direct medical cost was less than 4.0 million USD, and the average cost for each patient was 1,174 USD in 2013.
Conclusion
This is the first report on the nationwide epidemiology of AIH in Korea, and it showed a lower prevalence than that of Western countries with considerable disease burden.
Introduction
Autoimmune hepatitis (AIH) is a rare, chronic liver disease characterized by the presence of auto-antibodies, hypergammaglobulinemia, and interface hepatitis on histological examination. AIH, unless treated properly, can progress to cirrhosis or hepatic failure. Nonetheless, the epidemiology, treatment pattern, and disease burden of AIH are difficult to determine because of diverse clinical presentations and low prevalence.
The disease prevalence and incidence of AIH differ by race and ethnicity. Prevalence estimates range widely from 4 to 42.9 cases per 100,000 persons [1–11], and reported annual incidences range from 0.67 to 2.23 cases per 100,000 persons.[1, 4, 6, 8–14] The variable results may be attributed to differences in genetics, environmental factors, and study population or design, and these heterogeneous factors make it challenging to understand the global epidemiology and disease burden of AIH.
A comprehensive study on the nationwide epidemiology and direct medical costs of AIH may be able to overcome selection biases related to patients and organizations when based on a population-based administrative database. This study used a claims data base from the Korean National Health Insurance (NHI) system, which is a mandatory nationwide insurance system operated by the government. This data set includes almost all of the population’s inpatient and outpatient healthcare utilization data (96.6% in 2010) and contains all medical and prescription drug-claim records. In addition, NHI has an established registry program for rare intractable diseases (RID), which includes AIH or primary biliary cirrhosis (PBC), for copayment reduction since 2009. To be registered to this RID system, physicians must confirm the specific diagnosis by checking the listed diagnostic criteria and then register their patients. The aim of this study was to investigate the nationwide, population-based epidemiology, clinical features (such as complications or comorbidities), and disease burden of AIH in South Korea using these two big databases.
Patients and methods
Data source
The present study used the nationwide NHI claims database and RID database, which contain inpatient and outpatient healthcare utilization information such as patients’ demographics, date of admission and discharge, date of visit, diagnostic procedures such as laboratory tests or imaging studies, surgical procedures, prescription history, principal diagnoses or comorbidities based on the International Classification of Disease, 10th Revision (ICD-10), and RID registry information. Patients with RIDs become eligible for copayment reduction after their diagnoses are confirmed by a physician on the basis of the RID-defined diagnostic criteria provided by the NHI. Diagnoses are reviewed by the corresponding healthcare institution before being submitted to the NHI. All personal identities were encrypted and all data was analyzed anonymously. The cases of death were investigated from the data of Statistics Korea. This study was approved by the Institutional Review Board of the National Cancer Center (NCC2014-0182).
Study population
Patients with the disease code of AIH (K75.4) between January 2009 and December 2013 were retrieved from the claims data of NHI and were matched with the RID registry program. In the RID registry program, AIH was identified through a clinical diagnosis based on the results of auto-antibodies, immunoglobulin G, or histological examination and differential diagnosis from other liver diseases such as viral hepatitis. A prevalent case was defined as a case with AIH as the primary or secondary diagnosis of the year along with records of visits, hospitalizations, or surgeries in the claims database of NHI. An incident case was defined as a newly registered one without any claims data for AIH during the washout period to exclude prevalent cases. A 2-year washout period (2009–2010) was used in this study; therefore, new cases from 2011 were those that had no claims data for AIH in 2009 and 2010.
Statistical analyses
This study calculated the crude and adjusted prevalence of AIH in Korea during 2009 to 2013 using the number of AIH cases in the corresponding year divided by the population and by age- and gender-specific populations of the standard population. The standard population for the age- and gender adjusted prevalence rates was the registered population in 2010 by the Ministry of Government Administration and Home Affairs of the Republic of Korea.[15] The incidence rate was calculated by newly registered cases divided by the registered population. Cases of death from Statistics Korea were used for the case-fatality rate. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA), and a p-value <0.05 was considered to indicate a statistically significant difference.
Results
Prevalence and incidence of AIH in South Korea
From 2009 to 2013, a total of 4,085 AIH cases were registered after excluding duplicate cases. Among them, 3,493 were females (85.5%) and 592 were males (14.5%) with a female-to-male ratio of 6.4. The median case age was 56 years (56 for females and 55 for males), and the mean age was 54.9 years (55.1 for women and 53.2 for men).
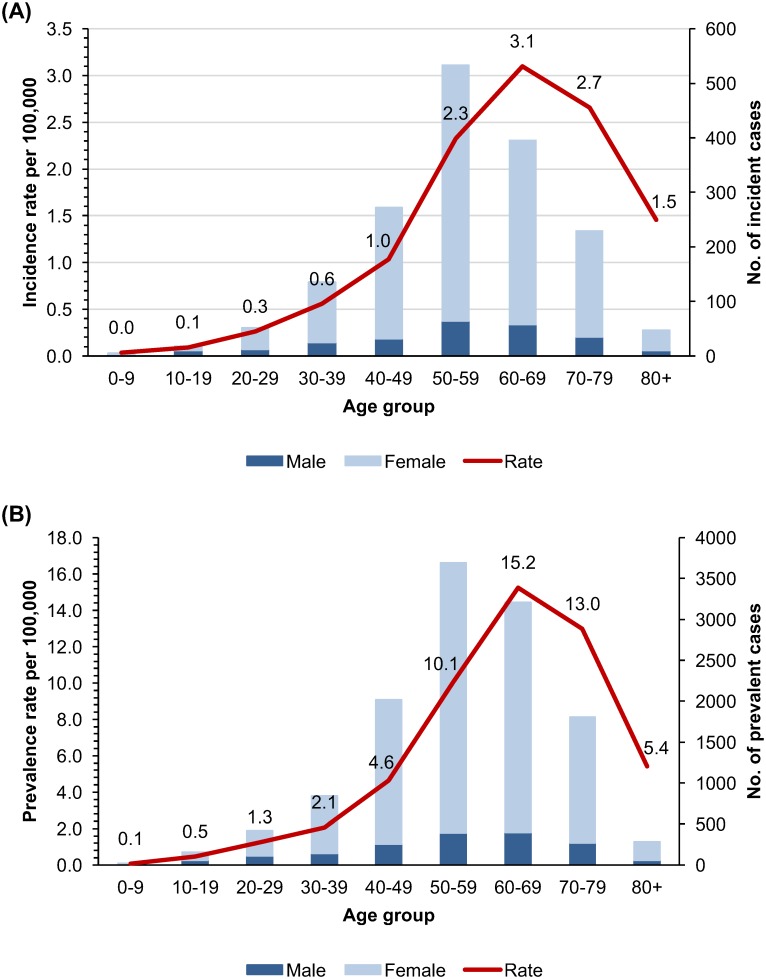
The average age-adjusted prevalence rate of AIH was 4.82/100,000 persons (gender-adjusted: 8.35 in females and 1.30 in males) during 2009–2013 (Table 1). The peak prevalence was in ages 60–69 (15.24/100,000, Fig 1) and was ages 60–69 for females (25.53/100,000) and 70–79 for males (4.62/100,000).
Table 1. Crude and adjusted prevalence and incidence rates per 100,000 of AIH in South Korea.
| Standard population* | Total number of cases | 2009 | 2010 | 2011 | 2012 | 2013 | Average, annual, adjusted rate | |
|---|---|---|---|---|---|---|---|---|
| Prevalence | ||||||||
| Gender | ||||||||
| Male | 25,035,384 | 1686 | 1.12 | 1.13 | 1.28 | 1.47 | 1.51 | 1.30† |
| Female | 24,973,069 | 10,761 | 6.69 | 7.26 | 8.42 | 9.36 | 10.02 | 8.35† |
| Age (years) | ||||||||
| 0–9 | 4757524 | 18 | 0.12 | 0.04 | 0.09 | 0.09 | 0.04 | 0.08‡ |
| 10–19 | 6826875 | 154 | 0.51 | 0.45 | 0.43 | 0.45 | 0.48 | 0.47‡ |
| 20–29 | 6,826,755 | 422 | 1.15 | 1.20 | 1.41 | 1.28 | 1.24 | 1.25‡ |
| 30–39 | 8,278,025 | 842 | 1.80 | 1.74 | 2.23 | 2.30 | 2.25 | 2.06‡ |
| 40–49 | 8,701,782 | 2,021 | 3.84 | 4.26 | 4.67 | 5.09 | 5.27 | 4.63‡ |
| 50–59 | 6,945,354 | 3,689 | 8.69 | 8.68 | 10.00 | 11.26 | 11.65 | 10.06‡ |
| 60–69 | 4,137,957 | 3,210 | 11.87 | 13.22 | 15.03 | 17.06 | 19.04 | 15.24‡ |
| 70–79 | 2,595,847 | 1,810 | 8.63 | 10.67 | 12.89 | 15.54 | 17.14 | 12.97‡ |
| 80+ | 938,334 | 281 | 3.31 | 4.05 | 5.41 | 6.43 | 7.91 | 5.42‡ |
| Total | 50,008,453 | 12,447 | 3.90 | 4.19 | 4.84 | 5.41 | 5.76 | 4.82§ |
| Incidence | ||||||||
| Gender | ||||||||
| Male | 25,035,384 | 249 | 0.32 | 0.36 | 0.27 | 0.31† | ||
| Female | 24,973,069 | 1,438 | 1.91 | 1.87 | 1.70 | 1.83† | ||
| Age (years) | ||||||||
| 0–9 | 4757524 | 5 | 0.04 | 0.06 | 0.00 | 0.04‡ | ||
| 10–19 | 6826875 | 18 | 0.09 | 0.11 | 0.08 | 0.09‡ | ||
| 20–29 | 6,826,755 | 52 | 0.42 | 0.15 | 0.22 | 0.26‡ | ||
| 30–39 | 8,278,025 | 135 | 0.61 | 0.58 | 0.48 | 0.56‡ | ||
| 40–49 | 8,701,782 | 272 | 1.14 | 0.99 | 0.99 | 1.04‡ | ||
| 50–59 | 6,945,354 | 533 | 2.30 | 2.72 | 1.97 | 2.33‡ | ||
| 60–69 | 4,137,957 | 396 | 2.82 | 3.42 | 3.06 | 3.10‡ | ||
| 70–79 | 2,595,847 | 229 | 3.04 | 2.37 | 2.56 | 2.66‡ | ||
| 80+ | 938,334 | 47 | 1.50 | 1.21 | 1.65 | 1.46‡ | ||
| Total | 50,008,453 | 1,687 | 1.11 | 1.12 | 0.98 | 1.07§ |
*Standard population, inhabitants in 2010
†Adjusted for age
‡Adjusted for gender
§Adjusted for age and gender
Fig 1.
(A) Average annual gender-adjusted incidence rate per hundred thousand population and incident cases (2009–2013) of AIH by age in South Korea. (B) Average annual gender-adjusted prevalence rate per hundred thousand population and prevalent cases (2009–2013) of AIH by age in South Korea.
To determine the incidence accurately, the washout period was set for 2 years, and the incidence rate was calculated from 2011 to 2013. The overall age-adjusted AIH incidence rate during 2011–2013 was 1.07/100,000/year (gender-adjusted: 1.83 in females and 0.31 in males). Age-specific incidence rate was highest in ages 60–69 (3.10/100,000/year). By gender, it was the highest in ages 60–69 for women (5.07/100,000/year) and in ages over 80 (1.60/100,000/year) for men.
Complications and case fatality of AIH in South Korea
ICD-10 code identified ascites was present in 1.4% of patients with AIH between 2009 and 2013, while diuretics (spironolactone) was prescribed in 5.8% of those with AIH (Table 2). ICD-10 code identified variceal bleeding was reported in 1.3% of patients with AIH. Endoscopic intervention for variceal bleeding was performed in 1.9% of patients, vasoactive medications such as terlipressin, somatostatin, or octreotide were given in 2.1%, and propranolol in 5.6% of patients during the 5 year study period. ICD-10 code identified hepatic encephalopathy was recorded in 2.2% of patients, and lactulose was prescribed in 12.3%. Hepatocellular carcinoma was diagnosed in 31 patients (0.8%). The median age of the 31 patients was 65 (range, 60–69), and 77.4% were females. Among them, 3 and 9 underwent surgical resection and local ablation, respectively. Liver transplantation was performed in 46 patients, and their median age was 53.5 (range, 43–61) and 89.1% were females.
Table 2. Proportion of AIH patients with complications or comorbidities from 2009 to 2013.
| Complications or comorbidities | Male (n = 592, 14.5%) | Female (n = 3493, 85.5%) | ||||
|---|---|---|---|---|---|---|
| 0–29 65(11.0) |
30–59 288(48.6) |
60+ 239(40.4) |
0–29 163(4.7) |
30–59 1973(56.5) |
60+ 1355(38.8) |
|
| Complications | ||||||
| Ascites* | 0(0.0) | 2(0.7) | 8(3.3) | 1(0.6) | 19(1.0) | 26(1.9) |
| Prescription of spironolactone | 2(3.1) | 19(6.6) | 9(3.8) | 5(3.1) | 114(5.8) | 89(6.6) |
| Prescription of furosemide | 2(3.1) | 26(9.0) | 45(18.8) | 20(12.3) | 184(9.3) | 234(17.3) |
| Variceal bleeding* | 1(1.5) | 3(1.0) | 1(0.4) | 2(1.2) | 25(1.3) | 21(1.5) |
| Endoscopic intervention | 0(0.0) | 4(1.4) | 1(0.4) | 4(2.5) | 33(1.7) | 36(2.7) |
| TIPS† | 0(0.0) | 1(0.3) | 0(0.0) | 0(0.0) | 0(0.0) | 1(0.1) |
| Prescription of vasoactive agents | 1(1.5) | 8(2.8) | 1(0.4) | 4(2.5) | 35(1.8) | 38(2.8) |
| Prescription of propranolol | 2(3.1) | 12(4.2) | 14(5.9) | 10(6.1) | 92(4.7) | 97(7.2) |
| Hepatic encephalopathy* | 0(0.0) | 4(1.4) | 8(3.3) | 5(3.1) | 37(1.9) | 34(2.5) |
| Prescription of lactulose | 4(6.2) | 25(8.7) | 30(12.6) | 14(8.6) | 219(11.1) | 211(15.6) |
| Liver cirrhosis* | 9(13.8) | 69(24.0) | 73(3.1) | 31(19.0) | 545(27.6) | 596(44.0) |
| Hepatocellular carcinoma* | 0(0.0) | 1(0.3) | 6(2.5) | 0(0.0) | 10(0.5) | 14(1) |
| Liver resection | 0(0.0) | 2(0.7) | 0(0.0) | 0(0.0) | 0(0.0) | 1(0.1) |
| Local ablation | 0(0.0) | 0(0.0) | 1(0.4) | 0(0.0) | 3(0.2) | 5(0.4) |
| Transarterial chemoembolization | 0(0.0) | 1(0.3) | 2(0.8) | 1(0.6) | 6(0.3) | 8(0.6) |
| Liver transplantation* | 1(1.5) | 2(0.7) | 2(0.8) | 5(3.1) | 24(1.2) | 12(0.9) |
| Comorbidities | ||||||
| Thyroid disease | ||||||
| Hypothyroidism | 2(3.1) | 4(1.4) | 2(0.8) | 3(1.8) | 68(3.4) | 33(2.4) |
| Hyperthyroidism | 0(0.0) | 3(1) | 0(0.0) | 3(1.8) | 22(1.1) | 9(0.7) |
| Thyroiditis | 0(0.0) | 3(1) | 0(0.0) | 1(0.6) | 22(1.1) | 10(0.7) |
| Prescription of thyroid hormone | 0(0.0) | 13(4.5) | 11(4.6) | 2(1.2) | 69(3.5) | 53(3.9) |
| Prescription of anti-thyroid drugs | 0(0.0) | 1(0.3) | 0(0.0) | 3(1.8) | 8(0.4) | 3(0.2) |
| Autoimmune disease | ||||||
| Primary biliary cirrhosis | 1(1.5) | 20(6.9) | 13(5.4) | 4(2.5) | 173(8.8) | 91(6.7) |
| Systemic lupus erythematosus | 3(4.6) | 2(0.7) | 0(0.0) | 6(3.7) | 46(2.3) | 9(0.7) |
| Systemic sclerosis | 0(0.0) | 0(0.0) | 2(0.8) | 0(0.0) | 5(0.3) | 1(0.1) |
| Rheumatoid arthritis | 0(0.0) | 1(0.3) | 0(0.0) | 0(0.0) | 4(0.2) | 6(0.4) |
| Dyslipidemia | 1(1.5) | 25(8.7) | 38(15.9) | 14(8.6) | 165(8.4) | 228(16.8) |
| Prescription of statin | 3(4.6) | 40(13.9) | 26(10.9) | 9(5.5) | 288(14.6) | 226(16.7) |
| Osteoporosis | 0(0.0) | 0(0.0) | 0(0.0) | 1(0.6) | 4(0.2) | 4(0.3) |
| Prescription of bisphosphonate | 0(0.0) | 0(0.0) | 13(5.4) | 0(0.0) | 38(1.9) | 100(7.4) |
*Complications or comorbidities were identified with ICD-10 codes verified by physicians.
†TIPS denotes transjugular intrahepatic portosystemic shunt surgery.
N = 4085
During the 5 years of study, 271 of 4,085 patients died. The overall case-fatality ratio was 6.63% (6.38% for females and 8.11% for males). The average annual case fatality ratio was 2.18% (2.07% for females and 2.85% for males) as shown in Table 3.
Table 3. Case-fatality ratio (CFR, %)* for patients with AIH by gender and year in South Korea.
| 2009 (n = 1899) |
2010 (n = 2095) |
2011 (n = 2487) |
2012 (n = 2853) |
2013 (n = 3113) |
Five year CFR† (n = 4085) |
|
|---|---|---|---|---|---|---|
| Number (%) | ||||||
| Female | 21 (1.29) | 40 (2.21) | 53 (2.45) | 54 (2.19) | 55 (2.04) | 223 (2.07) |
| Male | 5 (1.82) | 6 (2.13) | 9 (2.74) | 15 (3.85) | 13 (3.16) | 48 (2.85) |
| Total | 26 (1.37) | 46 (2.20) | 62 (2.49) | 69 (2.42) | 68 (2.18) | 271 (2.18) |
* The case-fatality ratio was calculated by dividing the number of death cases in the corresponding year by the treated AIH cases.
†During 5 years (2009–2013), 271 of 4085 patients died, resulting in an overall case-fatality ratio of 2.18%
Diagnosis and treatment for AIH in Korea
Of the 507 patients newly diagnosed with AIH in 2013, liver biopsy was performed in 54.2% of them. Tests for anti-nuclear antibody, anti-smooth muscle antibody, anti-mitochondrial antibody, and anti-liver kidney microsome type 1 antibody were done in 93.9%, 81.7%, 88.4% and 67.5% of patients, respectively. Among 3,113 prevalent AIH cases in year 2013, corticosteroid and azathioprine were prescribed in 44.1% and 38.0%, respectively.
Comorbidities in patients with AIH
The most frequently encountered comorbid disease identified by ICD-10 codes and prescribed drugs was dyslipidemia (11.5%), which was followed by thyroid diseases (Table 2). Prevalence of comorbid PBC was found to be 7.4% in patients with AIH, suggesting an overlap syndrome. Systemic lupus erythematosus was also identified in 1.6% of AIH patients. Osteoporosis estimated by the prescription of bisphosphonate was present in 3.7% of patients.
Out of 302 patients diagnosed with AIH and PBC, 88.7% were females, and the median age was 54 (Table 4). Ascites and variceal bleeding identified by the ICD-10 disease code were more frequently found in patients with AIH-PBC compared to those diagnosed with AIH only. Liver transplantation was also performed more often in patients with AIH-PBC. However, reported frequencies of other comorbidities or HCC were comparable (Table 4).
Table 4. Comparison between patients with only AIH and AIH-PBC.
| Only AIH (n = 3783, 92.6%) |
AIH & PBC (n = 302, 7.4%) |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Gender | ||||
| Male | 558 | 14.8 | 34 | 11.3 |
| Female | 3225 | 85.2 | 268 | 88.7 |
| Age (years) | ||||
| 0–9 | 12 | 0.3 | 0 | 0.0 |
| 10–19 | 60 | 1.6 | 0 | 0.0 |
| 20–29 | 150 | 4.0 | 6 | 2.0 |
| 30–39 | 304 | 8.0 | 24 | 7.9 |
| 40–49 | 643 | 17.0 | 75 | 24.8 |
| 50–59 | 1123 | 29.7 | 94 | 31.1 |
| 60–69 | 921 | 24.3 | 74 | 24.5 |
| 70–79 | 490 | 13.0 | 24 | 7.9 |
| 80+ | 80 | 2.1 | 5 | 1.7 |
| Average | 54.9 | 54.2 | ||
| Median (Q1, Q3)‡ | 56 (47, 65) | 54 (46, 62) | ||
| Fatality in 2009–2013 | ||||
| Gender | ||||
| Male | 45 | 8.1 | 3 | 8.8 |
| Female | 197 | 6.1 | 26 | 9.7 |
| Age (years) | ||||
| Average | 64.5 | 59.9 | ||
| Median (Q1, Q3)‡ | 67 (56, 75) | 61 (55, 67) | ||
| Complications | ||||
| Ascites* | 65 | 1.7 | 12 | 4.0 |
| Prescription of spironolactone | 266 | 7.0 | 45 | 14.9 |
| Prescription of furosemide | 571 | 15.1 | 62 | 20.5 |
| Variceal bleeding* | 59 | 1.6 | 19 | 6.3 |
| Endoscopic intervention | 75 | 2.0 | 21 | 7.0 |
| TIPS† | 2 | 0.1 | 0 | 0.0 |
| Prescription of vasoactive agents | 100 | 2.6 | 15 | 5.0 |
| Prescription of propranolol | 225 | 5.9 | 32 | 10.6 |
| Hepatic encephalopathy* | 94 | 2.5 | 6 | 2.0 |
| Prescription of lactulose | 552 | 14.6 | 50 | 16.6 |
| Liver cirrhosis* | 1268 | 33.5 | 101 | 33.4 |
| Hepatocellular carcinoma* | 45 | 1.2 | 4 | 1.3 |
| Liver resection | 5 | 0.1 | 0 | 0.0 |
| Local ablation | 11 | 0.3 | 0 | 0.0 |
| Transarterial chemoembolization | 29 | 0.8 | 1 | 0.3 |
| Liver transplantation* | 50 | 1.3 | 7 | 2.3 |
| Comorbidities | ||||
| Thyroid disease | ||||
| Hypothyroidism | 140 | 3.7 | 8 | 2.6 |
| Hyperthyroidism | 44 | 1.2 | 8 | 2.6 |
| Thyroiditis | 48 | 1.3 | 5 | 1.7 |
| Prescription of thyroid hormone | 149 | 3.9 | 19 | 6.3 |
| Prescription of anti-thyroid drugs | 15 | 0.4 | 1 | 0.3 |
| Autoimmune disease | ||||
| Systemic lupus erythematosus | 85 | 2.2 | 9 | 3.0 |
| Systemic sclerosis | 12 | 0.3 | 5 | 1.7 |
| Rheumatoid arthritis | 17 | 0.4 | 1 | 0.3 |
| Dyslipidemia | 471 | 12.5 | 54 | 17.9 |
| Prescription of statin | 593 | 15.7 | 90 | 29.8 |
| Osteoporosis | 12 | 0.3 | 2 | 0.7 |
| Prescription of bisphosphonate | 166 | 4.4 | 13 | 4.3 |
*Complications or comorbidities were identified with ICD-10 codes verified by physicians.
†TIPS denotes transjugular intrahepatic portosystemic shunt surgery.
‡Q denotes quartile.
Economic burden of AIH in Korea
The total national direct medical cost for AIH increased from 1,872,872 USD in 2009 to 3,654,099 USD in 2013 (Table 5). The annual direct medical cost per capita was 1,174 USD in 2013. Direct medical cost per capita was higher in men compared to that in women. All national medical costs for health services not covered by the NHI were excluded.
Table 5. Annual direct medical cost* of patients with AIH in 2013.
| Age | Male | Female | ||||
|---|---|---|---|---|---|---|
| N | Total direct medical cost (USD) † | Direct medical cost per capita (USD) | N | Total direct medical cost (USD) | Direct medical cost per capita (USD) | |
| <20 | 14 | 15,859 | 1,133 | 18 | 35,897 | 1,994 |
| 20–29 | 24 | 17,180 | 716 | 57 | 61,167 | 1,073 |
| 30–39 | 27 | 41,899 | 1,552 | 152 | 154,718 | 1,994 |
| 40–49 | 60 | 44,017 | 734 | 405 | 394,693 | 975 |
| 50–59 | 93 | 230,780 | 2,482 | 825 | 884,611 | 1,072 |
| 60–69 | 99 | 169,394 | 1,711 | 734 | 854,979 | 1,165 |
| 70–79 | 79 | 99,628 | 1,261 | 435 | 520,100 | 1,196 |
| 80+ | 16 | 7,110 | 444 | 75 | 122,066 | 1,628 |
| Total | 412 | 625,868 | 1,519 | 2701 | 3,028,231 | 1,121 |
*Direct medical cost includes only cost reimbursed by the National Health Insurance.
†1 USD = 1150 KRW
Discussion
The present study is the first nationwide epidemiology report on AIH in South Korea. The average age- adjusted prevalence of AIH between 2009 and 2013 was 4.8 per 100,000 persons, and the gender-adjusted prevalence rates were 8.4/100,000 for females and 1.3/100,000 for males. The average age-adjusted incidence of AIH was 1.1/100,000/year, and the gender- adjusted prevalence rates were 1.8/100,000/year for females and 0.3/100,000/year for males.
Compared to other populations, the incidence and prevalence of AIH in South Korea found in this study was quite low. The incidence and prevalence rates of AIH vary widely across different populations (Table 6).[1–14, 16] The prevalence was highest in an Alaskan native population (42.9/100,000), modest in European and New Zealand populations (mainly Caucasians, 10.7–24.5/100,000), and low in populations from Singapore and Brunei (4–5.6/100,000).[1–6, 9, 10] The annual incidence rate was low in Taiwan and Israel (0.52–0.67/100,000) and relatively high in Europe (0.85-3/100,000).[1, 4, 8–10, 12–14] Recently, a Japanese study reported a high prevalence and incidence of AIH (23.4/100,000 and 2.23/100,000/year, respectively).[11]
Table 6. Prevalence and incidence of AIH.
| Year | Region | Cases (no) | Prevalence period | Prevalence/100,000 | Incidence period | Annual incidence/100,000 | Diagnostic method | |
|---|---|---|---|---|---|---|---|---|
| Boberg KM et al.[1] | 1998 | Norway (Oslo) | 25 | 1995 | 16.9 | 1986–1995 | 1.9 | Original criteria[16] |
| Lee YM et al.[2] | 2001 | Singapore | 24 (11 definite/13 probable) | 1990–1996 | 4 | NA | NA | Original criteria |
| Hurlburt KJ et al.[3] | 2002 | Alaska natives | 49 (42 definite/7 probable) | 1983–2000 | 42.9 | NA | NA | Revised criteria |
| Koay LB et al.[12] | 2006 | Taiwan | 48 (29 definite/19 probable) | NA | NA | 2000–2004 | 0.52 | Revised criteria |
| Wally S et al.[13] | 2007 | England (West Suffolk) | 6 | NA | NA | 2003–2004 | 3 | Clinical diagnosis |
| Werner M et al.[4] | 2008 | Sweden | 110 | 2003 | 10.7 | 1990–2003 | 0.85 | Clinical diagnosis |
| Primo J et al.[14] | 2009 | Spain (Valencia) | 19 | NA | NA | 2003 | 1.07 | Revised criteria |
| Jalihal A et al.[5] | 2009 | Brunei | 19 | 2008 | 5.6 | NA | NA | Revised criteria |
| Ngu JH et al.[6] | 2010 | New Zealand (Canterbury) | 138 (123 definite/ 15 probable) | 2008 | 24.5 (Age-standardized 18.9) | 2008 | 2.0 (Age-standardized1.7) | Revised/simplified criteria |
| Haider AS et al.[7] | 2010 | Australia (Canberra) | 42 | Unknown | 8 | NA | NA | Revised criteria |
| Delgado JS et al.[8] | 2013 | Israel | 100 (73 definite/27 probable) | 1995–2010 | 11.0 | 1995–2010 | 0.67 | Simplified criteria |
| van Gerven et al.[9] | 2014 | Netherland (Amsterdam) | 146 | 1967–2011 | 18.3 | 2000–2010 | 1.1 | Revised/simplified criteria |
| Gronbaek L et al.[10] | 2014 | Denmark | 1,721 | 2012 | 23.9 | 1994–2012 | 1.68 | Clinical diagnosis |
| Yoshizawa K et al.[11] | 2016 | Japan (Ueda) | 48 | 2014 | 23.4 | 2004–2014 | 2.23 | Revised criteria |
| Kim et al | 2017 | Korea | 4,085 | 2009–2013 | 4.8 | 2011–2013 | 1.1 | Clinical diagnosis |
The wide variation seems attributable to differences between study populations as well as in the identification and definition of cases. In this population-based epidemiological study of AIH in South Korea, cases were identified by ICD-10 code and RID registry information, which are nationwide, comprehensive data sets. The government-run RID registry program is a system for copayment where if a physician diagnoses a patient with AIH based on NHI-provided criteria and enters the ICD code-10 of K75.4, he or she can register the patient for the RID program, which would then provide copayment reduction for the patient. Therefore, we believe the identification of our patients with AIH is reliable.
In other Asian countries, such as Japan and Hong Kong, the frequency of AIH is less than 5% of all chronic active hepatitis cases, whereas in Western Europe and North America, AIH comprises about 20% of these cases.[17] In South Korea, autoimmune liver diseases including AIH have been reported to account for 1% of chronic liver diseases.[18]
The presence of complications or comorbidities was primarily investigated through ICD-10 codes. However, we also examined whether a patient got therapeutic procedures or any relevant medications as ICD-10 codes for complications such as ascites, variceal bleeding or hepatic encephalopathy are often not entered into the claims data system. For example, according to the ICD-10 codes, ascites was present in 3.9% of patients with AIH. However, spironolactone, a drug of choice in the initial treatment of ascites, was prescribed in 11.6%. This range of prevalence of decompensated cirrhosis reflects the limitation of claims data. Hepatocellular carcinoma was diagnosed in 0.8% (31/4,085) of patients. Previously reported frequencies of hepatocellular carcinoma in patients with AIH are 1.1% (8/730 patients),[9] 1.9% (6/322 patients),[19] 3.6% (7/193 patients),[20] 4.0% (10/248 patients)[21] and 6.2% (15/243 patients).[22] Hepatocellular carcinoma frequency mainly depends on the presence of cirrhosis and the duration of the disease.[23] Liver transplantation was performed in 1.1% of our patients. As we could only identify cases of AIH diagnosed with hepatocellular carcinoma or followed by liver transplantation within the 5 year study period, these values may be underreported due to the relatively short follow up period.
A previous nationwide Korean study reported autoimmune thyroiditis as the most common concurrent autoimmune disease, followed by systemic lupus erythematosus.[24] In this study, we could not confirm the diagnosis of autoimmune thyroiditis; however, thyroid hormone was prescribed in 3.6% of patients with AIH. Therefore, it is conceivable that autoimmune thyroiditis was present in 3.6% or less. Out of the 4,085 patients, systemic lupus erythematosus, rheumatoid arthritis, and systemic sclerosis was present in 1.6%, 0.2% and 0.2% of patients, respectively. These were lower compared to previous reports.[25] Corticosteroid or azathioprine was given to approximately 40% of patients, respectively. It seemed to be low; however, analysis was performed for prevalent cases. Prevalent AIH cases in year 2013 possibly include patients who were on remission after treatment for AIH. Previous study also reported that 25% of newly diagnosed patients were not treated.[24]
The strength of the present study is its population-based design. The NHI claims data covers > 95% of the entire Korean population. However, we had some limitations. First, this study was based on insurance claims data. The Korean NHI claims data includes all records of surgery, procedures, tests and medications; however, detailed clinical information such as exact results of tests could not be obtained for each individual patient. Therefore, we were not able to confirm which diagnostic criteria were used, nor could we evaluate clinical features and treatment responses. Some disease codes not responsible for the medical billing could also have been underreported. To complement this limitation, we investigated the prescription rate of concurrent medication or related procedures for complications or comorbidities. Second, the incidence and prevalence of AIH may be underestimated as the actual registration rate of AIH for RID program is not known yet. However, the disease code for AIH (K75.4) is the primary code responsible for the payment and financial benefit and most healthcare institutions and doctors are well aware of the RID program and try to register patients in the program as it offers financial benefits for patients. Third, medical costs may be underestimated because records of some uninsured health services could not be collected. Lastly, the RID registry system has been established since 2009 and this study collected information during a 5 year period, which is limited when evaluating long-term prognoses such as mortality and HCC risk.
In conclusion, this is the first nationwide study on the incidence, prevalence, complications, comorbidities and medical cost of AIH in South Korea. In Korea, the incidence and prevalence were low compared to those of other Western countries with considerable disease burden.
Abbreviations
- AIH
autoimmune hepatitis
- ICD
International Classification of Disease
- NHI
National Health Insurance
- PBC
primary biliary cirrhosis
- RID
rare intractable disease
Data Availability
The data are available from the Korean National Health Insurance Service (NHIS); however, the access to data is limited to researchers who meet the required criteria. Any researchers who propose a study subject and plans with standardized proposal form can access the raw data after being approved by NHIS review committee of research support. Detailed process and a provision guide is now available at (http://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do).
Funding Statement
This work was supported in part by a research grant from Korean Association for the Study of the Liver (KASL academic research fund 2013), and from the National Cancer Center, Korea (NCC-1410860). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33(1):99–103. Epub 1998/03/07. . [DOI] [PubMed] [Google Scholar]
- 2.Lee YM, Teo EK, Ng TM, Khor C, Fock KM. Autoimmune hepatitis in Singapore: a rare syndrome affecting middle-aged women. J Gastroenterol Hepatol. 2001;16(12):1384–9. Epub 2002/02/20. . [DOI] [PubMed] [Google Scholar]
- 3.Hurlburt KJ, McMahon BJ, Deubner H, Hsu-Trawinski B, Williams JL, Kowdley KV. Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterol. 2002;97(9):2402–7. Epub 2002/10/03. doi: 10.1111/j.1572-0241.2002.06019.x . [DOI] [PubMed] [Google Scholar]
- 4.Werner M, Prytz H, Ohlsson B, Almer S, Bjornsson E, Bergquist A, et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol. 2008;43(10):1232–40. Epub 2008/07/09 doi: 10.1080/00365520802130183 . [DOI] [PubMed] [Google Scholar]
- 5.Jalihal A, Telisinghe PU, Chong VH. Profiles of autoimmune hepatitis in Brunei Darussalam. Hepatobiliary Pancreat Dis Int. 2009;8(6):602–7. Epub 2009/12/17. . [PubMed] [Google Scholar]
- 6.Ngu JH, Bechly K, Chapman BA, Burt MJ, Barclay ML, Gearry RB, et al. Population-based epidemiology study of autoimmune hepatitis: a disease of older women? J Gastroenterol Hepatol. 2010;25(10):1681–6. Epub 2010/10/01. doi: 10.1111/j.1440-1746.2010.06384.x . [DOI] [PubMed] [Google Scholar]
- 7.Haider AS, Kaye G, Thomson A. Autoimmune hepatitis in a demographically isolated area of Australia. Internal medicine journal. 2010;40(4):281–5. doi: 10.1111/j.1445-5994.2009.02041.x . [DOI] [PubMed] [Google Scholar]
- 8.Delgado JS, Vodonos A, Malnick S, Kriger O, Wilkof-Segev R, Delgado B, et al. Autoimmune hepatitis in southern Israel: a 15-year multicenter study. J Dig Dis. 2013;14(11):611–8. doi: 10.1111/1751-2980.12085 . [DOI] [PubMed] [Google Scholar]
- 9.van Gerven NM, Verwer BJ, Witte BI, van Erpecum KJ, van Buuren HR, Maijers I, et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand J Gastroenterol. 2014;49(10):1245–54. doi: 10.3109/00365521.2014.946083 . [DOI] [PubMed] [Google Scholar]
- 10.Gronbaek L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60(3):612–7. doi: 10.1016/j.jhep.2013.10.020 . [DOI] [PubMed] [Google Scholar]
- 11.Yoshizawa K, Joshita S, Matsumoto A, Umemura T, Tanaka E, Morita S, et al. Incidence and prevalence of autoimmune hepatitis in the Ueda area, Japan. Hepatol Res. 2016;46(9):878–83. doi: 10.1111/hepr.12639 . [DOI] [PubMed] [Google Scholar]
- 12.Koay LB, Lin CY, Tsai SL, Lee C, Lin CN, Sheu MJ, et al. Type 1 autoimmune hepatitis in Taiwan: diagnosis using the revised criteria of the International Autoimmune Hepatitis Group. Dig Dis Sci. 2006;51(11):1978–84. doi: 10.1007/s10620-005-9068-y . [DOI] [PubMed] [Google Scholar]
- 13.Whalley S, Puvanachandra P, Desai A, Kennedy H. Hepatology outpatient service provision in secondary care: a study of liver disease incidence and resource costs. Clin Med (Lond). 2007;7(2):119–24. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Primo J, Maroto N, Martinez M, Anton MD, Zaragoza A, Giner R, et al. Incidence of adult form of autoimmune hepatitis in Valencia (Spain). Acta gastro-enterologica Belgica. 2009;72(4):402–6. . [PubMed] [Google Scholar]
- 15.Ministry of the Interior (MOI). Population statistics 2015 [cited 2015 30 September]. http://rcps.egov.go.kr:8081/jsp/stat/ppl_stat_jf.jsp.
- 16.Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18(4):998–1005. Epub 1993/10/01. . [DOI] [PubMed] [Google Scholar]
- 17.Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635–47. Epub 2002/10/05. . [DOI] [PubMed] [Google Scholar]
- 18.Lee SS, Byoun YS, Jeong SH, Kim YM, Gil H, Min BY, et al. Type and cause of liver disease in Korea: single-center experience, 2005–2010. Clin Mol Hepatol. 2012;18(3):309–15. doi: 10.3350/cmh.2012.18.3.309 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong RJ, Gish R, Frederick T, Bzowej N, Frenette C. Development of hepatocellular carcinoma in autoimmune hepatitis patients: a case series. Dig Dis Sci. 2011;56(2):578–85. doi: 10.1007/s10620-010-1444-6 . [DOI] [PubMed] [Google Scholar]
- 20.Migita K, Watanabe Y, Jiuchi Y, Nakamura Y, Saito A, Yagura M, et al. Hepatocellular carcinoma and survival in patients with autoimmune hepatitis (Japanese National Hospital Organization-autoimmune hepatitis prospective study). Liver Int. 2012;32(5):837–44. doi: 10.1111/j.1478-3231.2011.02734.x . [DOI] [PubMed] [Google Scholar]
- 21.Danielsson Borssen A, Almer S, Prytz H, Wallerstedt S, Friis-Liby IL, Bergquist A, et al. Hepatocellular and extrahepatic cancer in patients with autoimmune hepatitis—a long-term follow-up study in 634 Swedish patients. Scand J Gastroenterol. 2015;50(2):217–23. doi: 10.3109/00365521.2014.983154 . [DOI] [PubMed] [Google Scholar]
- 22.Yeoman AD, Al-Chalabi T, Karani JB, Quaglia A, Devlin J, Mieli-Vergani G, et al. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: Implications for follow-up and screening. Hepatology. 2008;48(3):863–70. doi: 10.1002/hep.22432 . [DOI] [PubMed] [Google Scholar]
- 23.Czaja AJ. Hepatocellular carcinoma and other malignancies in autoimmune hepatitis. Dig Dis Sci. 2013;58(6):1459–76. doi: 10.1007/s10620-012-2525-5 . [DOI] [PubMed] [Google Scholar]
- 24.Kim BH, Kim YJ, Jeong SH, Tak WY, Ahn SH, Lee YJ, et al. Clinical features of autoimmune hepatitis and comparison of two diagnostic criteria in Korea: a nationwide, multicenter study. J Gastroenterol Hepatol. 2013;28(1):128–34. doi: 10.1111/j.1440-1746.2012.07292.x . [DOI] [PubMed] [Google Scholar]
- 25.Wong GW, Heneghan MA. Association of Extrahepatic Manifestations with Autoimmune Hepatitis. Dig Dis. 2015;33 Suppl 2:25–35. doi: 10.1159/000440707 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the Korean National Health Insurance Service (NHIS); however, the access to data is limited to researchers who meet the required criteria. Any researchers who propose a study subject and plans with standardized proposal form can access the raw data after being approved by NHIS review committee of research support. Detailed process and a provision guide is now available at (http://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do).