Abstract
In barley endosperm arabinoxylan (AX) is the second most abundant cell wall polysaccharide and in wheat it is the most abundant polysaccharide in the starchy endosperm walls of the grain. AX is one of the main contributors to grain dietary fibre content providing several health benefits including cholesterol and glucose lowering effects, and antioxidant activities. Due to its complex structural features, AX might also affect the downstream applications of barley grain in malting and brewing. Using a high pressure liquid chromatography (HPLC) method we quantified AX amounts in mature grain in 128 spring 2-row barley accessions. Amounts ranged from ~ 5.2 μg/g to ~ 9 μg/g. We used this data for a Genome Wide Association Study (GWAS) that revealed three significant quantitative trait loci (QTL) associated with grain AX levels which passed a false discovery threshold (FDR) and are located on two of the seven barley chromosomes. Regions underlying the QTLs were scanned for genes likely to be involved in AX biosynthesis or turnover, and strong candidates, including glycosyltransferases from the GT43 and GT61 families and glycoside hydrolases from the GH10 family, were identified. Phylogenetic trees of selected gene families were built based on protein translations and were used to examine the relationship of the barley candidate genes to those in other species. Our data reaffirms the roles of existing genes thought to contribute to AX content, and identifies novel QTL (and candidate genes associated with them) potentially influencing the AX content of barley grain. One potential outcome of this work is the deployment of highly associated single nucleotide polymorphisms markers in breeding programs to guide the modification of AX abundance in barley grain.
Introduction
In cereals, the arabinoxylan (AX) backbone consists of (1→ 4)-β-linked xylopyranosyl residues [1]. Glucuronic acid residues (sometimes 4-O-methylated) can be attached to the O-2 position on these backbone residues and α-L-arabinofuranosyl moieties are mainly attached to the O-3 position, making glucuronoarabinoxylans (GAX) [2]. GAX from the cell walls of barley aleurone and barley malt is highly substituted and carries arabinofuranosyl residues which can be attached at O-2, doubly linked to O-2 and O-3 or, as found most commonly, singly on the O-3 position [3,4]. While glucuronic acid and 4-O-methylated side chains are reported to be missing from the AX found in barley flour [5], barley husk contains AX with both 4-O-methylated glucuronic acid side chains at the O-2 position as well as arabinofuranosyl units linked to O-3 on the xylan backbone [6]. Acetyl subunits attached to O-2 and/or O-3 of the xylan backbone have been described in AX from wheat straw [7]. The presence of galactose and glucuronic acid substitutions on the AX extracted from Brewers' spent grain has been confirmed by methylation analysis [8] and suggests a more complex structure for barley grain AX than previously thought. Esterification of ferulic acid (FA) to the arabinofuranosyl side chains is considered to be a unique feature of cereal cell walls [9,10]. Cell walls of the aleurone from barley and wheat grain contain high levels of feruloylated AX causing a blue autofluorescence that can be easily detected under the microscope [11].
Cereals are the most widely cultivated crops globally, and the composition and structure of their cell walls have a significant effect on the end use of the grain. Plant cell walls are a major source of dietary fibre and antioxidants, they provide positive effects in human health and nutrition [12,13] and their structure and composition impact the use of grain in brewing, baking, in processed foods and for animal feed [14,15]. AX is the dominant non-cellulosic polysaccharide in the thick aleurone cell walls in barley grain [5], and is the second-most abundant component in the starchy endosperm cell walls after (1,3;1,4)-β-glucan [6]. (1,3;1,4)-β-Glucan constitutes around 75% of the barley starchy endosperm cell walls whilst AX contributes the majority of the remaining 25% of the cell wall matrix [7], while in wheat the converse is true [16]. The effects of higher or lower AX on downstream uses has attracted much less attention than the other major non-cellulosic polysaccharide (1,3;1,4)-β-glucan. However, evidence exists that AX might limit the extractability of (1,3;1,4)-β-glucan from barley grain [17]. This could be due to the fact that ferulic acid residues attached to the arabinosyl side chains can connect AX polysaccharides to each other, and potentially to other polymers through the formation of insoluble dehydrodimers [10,18]. There is evidence for interactions between alkali-extractable AX and (1,3;1,4)-β-glucan [19] whilst the loss of glucuronyltransferase activity in Arabidopsis gux1 and gux2 mutants which led to the absence of glucuronic or methylated glucuronic acid actually increased the extractability of xylan from cell walls [20]. Such reports support the hypothesis that the AX network can significantly influence the inter-molecular interactions and ultimate release of cell wall polysaccharides in cereal grain. This could be relevant to the germination process essential for seedling vigour and plant growth, to industrial processes such as malting, brewing and baking and to events in the human digestive tract where the availability of polysaccharides for microbial fermentation to short chain fatty acids is a key health determinant [21]. Additionally, AX is a major component of grain dietary fibre in cereals such as wheat and barley, and has the potential to provide health benefits which could reduce the chance of developing chronic conditions such as cardiovascular disease, diabetes and colon cancer [12, 13, 14, 21]. Also, the bioactive compounds, ferulic and p-coumaric acids, which are found esterified to the AX polymer have potential antioxidant activities [12, 13].
The biosynthetic machinery required for the synthesis of AX is complex and although there has been significant progress recently in gene identification and characterisation the function of many genes linked to the pathway remain to be definitively established. Members of the glycosyltransferase (GT) family 43 (GT43) in Arabidopsis have been shown to be involved in biosynthesis of the xylan backbone [1,22]. The Arabidopsis Irregular Xylem (IRX) mutants 9 and 14 (irx9 and irx14) are members of the GT43 family exhibiting a dwarf phenotype with a reduction in xylosyltransferase activity [22–24]. The irx10 mutant, a member of the GT47 family in Arabidopsis also exhibited a reduction in xylan content and xylosyltransferase activity similar to that of irx9 and irx14 [25]. The homologous genes IRX9-Like (IRX9-L), IRX14-L and IRX10-L also seem to be involved in xylan biosynthesis [22,23,25] whilst members of the GT8 family are implicated in the addition of glucuronic acid and methylated glucuronic acid residues to the xylan backbone [26]; Arabidopsis gux1 and gux2 mutants showed loss of xylan glucuronyltransferase activity[20]. Members of the DUF579 gene family have been associated with the methylation of glucuronic acid units [27] and mutations in some DUF579 genes, known as IRX15 and IRX15-L in Arabidopsis, resulted in a decrease in xylan content and an increase in the degree of methylation [28,29]. UDP-xylose epimerases are involved in the interconversion of UDP-xylose and UDP-arabinose [30,31] whilst genes from the UDP-arabinose mutase family (also known as GT75) have been shown to be capable of converting UDP-arabinopyranose to the UDP-arabinofuranose form required for the biosynthesis of arabinosyl side chains [32–34]. Transfer of the arabinosyl units is believed to be mediated by GT61 genes [35–37] which comprises a very large gene family and a clade of acetyltransferases from the BAHD superfamily have been suggested to be involved in the feruloylation of arabinosyl side chains [38,39]. Acetylation of the xylan backbone is likely to be mediated by proteins from the DUF231 family [40,41]. Glycoside hydrolases (GH) with β-xylanase and arabinofuranohydrolase activities could also potentially be involved in the modification or turnover of AX [42,43].
Xylan synthesizing complexes (XSC) containing a number of protein types have been identified in wheat [33] and Populous [44] and most recently it was shown that three proteins from Asparagus officinalis, IRX9, 10 and 14 are required in a Golgi-localised XSC for xylan xylosyltransferase activity [45]. It is highly likely that other as yet unrecognised proteins are associated with such complexes, as has been found for cellulose [46,47].
Given the large number of structural genes required for the biosynthesis and modification of AX, the regulatory network is also expected to be complex. The activity of several key transcription factors and regulatory genes associated with secondary cell wall development and xylan biosynthesis in Arabidopsis have been described in the literature [48–50] but there is less information available for cereals in general. There has been less effort to identify genes associated with AX content of barley grain than in wheat starchy endosperm. Given the hexaploid nature of the wheat genome, gene identification in barley, which is closely related to wheat but diploid, is likely to be more straightforward, particularly with the availability of the barley genome [51]. Here a collection of 2-row spring barley cultivars was used to perform a Genome Wide Association Study (GWAS) in order to identify genes significantly influencing AX biosynthesis in whole barley grain. Ten genomic regions were found to be significantly associated with the AX content of barley grain and candidate genes for this trait were identified in these regions.
Results and discussion
Arabinoxylan content of barley grain
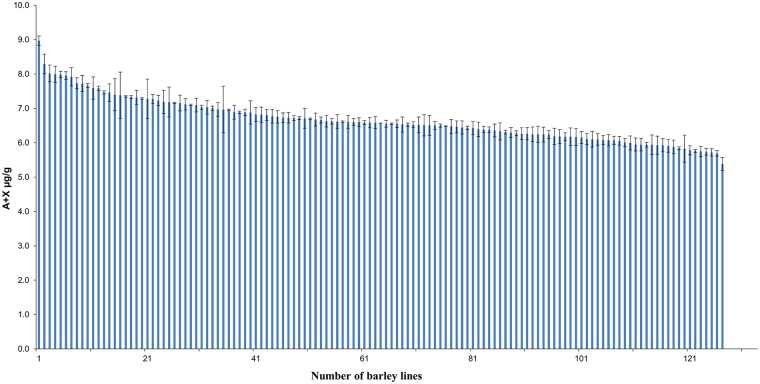
The total grain arabinose plus xylose (A+X) content was quantified in 128 glasshouse grown 2-row spring barley accessions using HPLC analysis across two technical replicates. The large population size used in this study gave us the opportunity to explore natural variation in AX content of 2-row spring barley. An appreciable variation in AX content was observed in the barley grain where AX values expressed as weight/weight (w/w) ranged from 5.3 to ~ 9.0 μg/g (Fig 1) at an average value of 6.7 μg/g. Although these values are similar to those previously reported in the literature for barley grain (4.2–5.4% of dry weight) [52], the current study describes a wider range in barley grain AX content. Grains of other cereals exhibit such a dynamic range, including oat (4.1–14.5% of dry weight) and rye grains (8.0–12.1% of dry weight) [53,54], whilst wheat grain is reported to have an AX content of 5.5–7.8 (% of dry weight) [55]. A study on spring and winter wheat varieties also reported similar values for wheat grain AX content (4.4–6.9% of dry weight) [56]. In the current experiment we observed a higher grain AX content in our collection of barley accessions than that previously recorded for wheat. One reason for this comparably higher level of AX in barley could be the presence of the husk comprising the outer layers of the barley grain. Using four different chemical and enzymatic methods to study the monosaccharide composition of barley husk, it was shown that AX content of isolated fractions ranged from 50–83% [57]. Similar studies also showed that AX is the major polysaccharide found in barley husk, contributing to 45% of the total husk polysaccharide content [58]. A list of the germplasm used with corresponding AX levels is provided in S1 File.
Fig 1. Arabinoxylan levels in wholegrain 2-row spring barley.
Wholegrains of 128 glasshouse-grown lines were chemically analysed using monosaccharide analysis. Values represent the mean of the sum of arabinose (A) and xylose (X) expressed as w/w. Error bars represent standard deviation of the replicates.
GWAS analysis
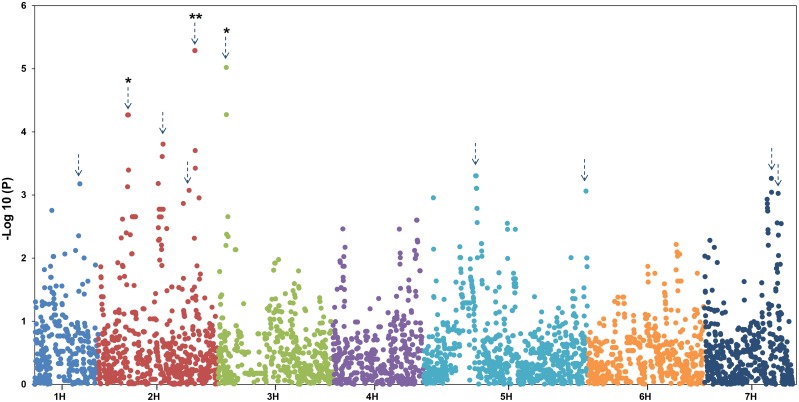
Genome Wide Association Studies have become a common approach for gene identification in cereals. Recently, several studies successfully identified associations for cell wall polysaccharides including the (1,3;1,4)-β-glucan content of barley grain [59] and the AX content of tetraploid wheat [60]. In this study, we performed a GWAS on 2-row spring barley in an attempt to find regions significantly associated with the AX content of the grain. A total of 5182 SNP markers with a minimum allele frequency of >5% and less than 5% missing data were used to conduct the GWAS. We used an Eigenstrat model to account for population structure and to reduce the risk of false positive associations. Ten genomic regions were found to be significantly associated with barley grain AX content (Fig 2).
Fig 2. Manhattan plots of the GWAS of the wholegrain 2-row spring barley using the Eigenstrat model.
The–Log 10 (P-value) is shown on the Y axis. The X axis shows the 7 barley chromosomes. Total AX content of wholegrain expressed as w/w was used for marker-trait association analysis.
Three of the ten regions with a–Log10 (P)>3 (QAX2.S-2H1* (P> 0.05), QAX2.S-2H4** (P>0.01), and QAX2.S-3H1* (P> 0.05)) passed the more stringent FDR significance level (Table 1, Fig 2). The strongest QTL, QAX2.S-2H4**, is located on chromosome 2H (121–125 cM) with a–Log10–(P) value of 5.3 and an adjusted p value (q value) of 0.009. To search for genes within all intervals, we extended the intervals by 2.5 cM either side of the SNP with the highest LOD score. QAX2.S-2H1* and QAX2.S-2H4** contain members of gene families such as GT47 and GT61 (Table 1), for which there is now strong evidence for their role in contributing to AX content [25, 35]. For the 3 QTLS which passed the FDR we assessed the effect of the most significant SNP on grain AX content in the same collection of lines used to carry out the GWAS (S1 Fig). At QAX2.S-2H1, the average grain AX content varied by 0.86 μg/g depending on the allele of SCRI_RS_175065 present (t (4.28), p = 0.0085), where the average grain AX of accessions containing the adenine at this SNP was 6.59 μg/g compared to 7.45 μg/g in the accessions containing the alternate allele, a guanine.
Table 1. Significant associations correlated with barley grain AX content which passed the FDR test.
Number of asterisks indicates the significance level for the adjusted P value (q value). Barley Gene id/ transcript (MLOC), Morex Contig, Developing grain without bracts 5 days post anthesis (CAR 5 DPA FPKM) and CAR 15 DPA FPKM (fragments per kilobase of exon per million fragments mapped) from ics.hutton.ac.uk/morexGenes.
| chr | Peak | QTL position cM | Marker | i_select 9K (cM) | IBSC 2012 (cM) | MxBk POPSEQ 2013 (cM) | LOD | Gene | MxBk POPSEQ 2013 (cM) | contig | CAZY | PFAM | CAR 5 | CAR 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2H | QAX2.S-2H1* | 50–55 | SCRI_RS_175065 | 60.7 | 52.9 | 52.5 | 4.3 | MLOC_17443 | N/A | contig_1576718 | GT-61 | PF04577 | 1.6 | 1.0 |
| MLOC_77094 | N/A | contig_74292 | HvUAM2 | PF03214 | 68.5 | 556.9 | ||||||||
| 2H | QAX2.S-2H4** | 121–125 | SCRI_RS_221939 | 136.0 | 123.7 | 128.3 | 5.3 | MLOC_72459 | 126.7 | contig_61690 | GT-43 | PF03360 | 14.6 | 8.9 |
| MLOC_7681 | 125.3 | contig_139847 | GH-10 | PF00331 | 0.0 | 0.1 | ||||||||
| MLOC_4660 | 125.8 | contig_135374 | DUF579 | PF04669 | 3.9 | 1.9 | ||||||||
| 3H | QAX2.S-3H1* | 13–19 | SCRI_RS_192352 | 22.2 | 17.9 | 17.5 | 4.4 | MLOC_75090 | 15.3 | contig_67111 | GH-10 | PF00331 | 0.4 | 0.0 |
* > 0.05,
**> 0.01.
The allele of SCRI_RS_221939, which defines QAX2.S-2H4**, influenced average grain AX content by 0.70 μg/g (t (3.26), p = 0.0006) where accessions containing a cytosine had on average 7.27 μg/g grain AX, compared to those with a thymine at 6.57 μg/g. At QAX2.S-3H1, the average grain AX content varied by 0.38 μg/g depending on the allele of SCRI_RS_192352 present (t (3.70), p = 0.0032). At this SNP the accessions containing a cytosine had a higher average AX grain content (6.80 μg/g) compared to those containing a guanine (6.42 μg/g).
The output from the Eigenstrat analysis revealed that for SNP SCRI_RS_175065 (which represents QAX2.S-2H1) the lines containing the minor allele, at an allele frequency of 0.079, contributed to an increase in grain AX of 0.403 μg/g. For SNP SCRI_RS_192352, which represents QAX2.S-3H1, possession of the minor allele, which had a frequency of 0.378, was accompanied by a decrease in AX levels (-0.158 μg/g). Finally, for SNP SCRI_RS_221939, which represents the major QTL (QAX2.S-2H4**) on 2H, accessions that had the minor allele, with a frequency of 0.106, had a 0.358 μg/g higher grain AX level. The difference in effects for each of the SNP markers on AX levels, as indicated either by Eigenstrat analysis or the mean of differences provided with the boxplots in S1 Fig, are likely to be due to the corrections on population structure intrinsic to the Eigenstrat analysis.
The full list of QTL for grain AX content is provided in S1 Table, and will be described in the following sections. Very few of the genes identified in this study matched those identified in similar mapping experiments carried out on various wheat populations [60–62], making a comparison of candidate genes derived from this type of analyis unworkable at this stage.
Genes associated with AX in barley grain
We identified candidate genes with known map positions corresponding to the genomic regions delineated by our association analysis. These included glycosyltransferase (GTs) and glycoside hydrolase genes (GHs) previously reported to be linked to AX biosynthesis and modification or hydrolysis. Also, genes involved in the biosynthesis of nucleotide sugar donors such as arabinose mutases and two families of genes with domains of unknown function, namely DUF231 and DUF579 were coincident with the QTL. A list of selected candidate genes identified under each significant association is provided in S1 Table. For all associations identified, 2.5 cM either side of the most significant marker were searched for likely candidate genes.
Significantly associated biosynthetic genes
Interconverting enzymes
Two genes from the UDP-arabinose mutase gene family (MLOC_77094 and MLOC_63185) were identified under QAX2.S-2H1 and QAX2.S-2H3 QTL respectively. These enzymes are central to the key conversion of UDP-arabinopyranose to UDP-arabinofuranose [34]. Notably these two genes have the highest transcript levels among all genes identified here at two stages of barley grain development (caryopsis 5 and 15 days post anthesis) (S1 Table).
GT43
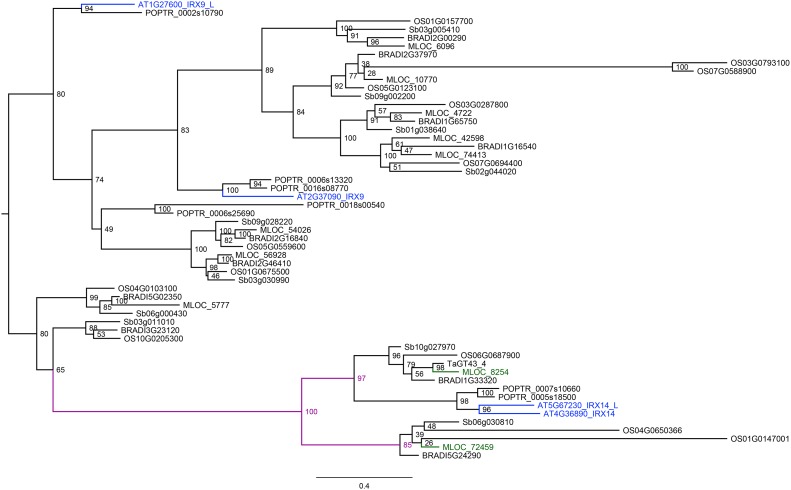
Glycosyltransferase enzymes from a number of different families have been demonstrated to be central to xylan biosynthesis. Two members of the GT43 gene family, called IRX9 and IRX14 have been shown genetically to be non-redundantly involved in the elongation of the xylan backbone [63,64] but just a single GT43 gene was found under any of the ten associations identified here (S1 Table). Using a PFAM domain search (PF03360) we identified 10 GT43 proteins in barley, 13 in rice, 11 in sorghum, ten in Brachypodium and just four in Arabidopsis. Based on data from early to mid-caryopsis development available on the morexGenes- barley RNA-seq database (https://ics.hutton.ac.uk/morexGenes/index.html) we know that five of the ten GT43 genes in barley are expressed in one or both of these stages (S2 File). To establish how closely related the HvGT43 protein (MLOC_72459) under QAX2.S-2H4 is to the Arabidopsis IRX9 and IRX14, or the related IRX9-L and IRX14-L genes, we produced a phylogenetic tree (Fig 3). This analysis was based on protein sequence translated from coding sequences of GT43 genes from various cereal species, including wheat GT43-4 that has been shown to be involved in biosynthesis of the GAX polymer [33]. It is clear that AtIRX14 and AtIRX14-L are closely related since they sit on neighbouring branches of a sub-clade which also contains wheat GT43-4, but not barley MLOC_72459 (Fig 3). Instead, a different barley protein from the GT43 family, located on chromosome 7H and not associated with any significant QTL, MLOC_8254, is most closely related to wheat GT43-4 and Arabidopsis IRX14 and IRX14-L. Barley MLOC_72459 under QAX2.S-2H4 sits within a separate sub-clade (Fig 3). At the nucleotide level MLOC_72459 and MLOC_8254 share only 50.6% sequence identity (data not shown). The tree also indicates that AtIRX9 and AtIRX9L are not closely related, fall into separate clades and neither closely match a cereal GT43. The lack of obvious orthologues of Arabidopsis IRX9 or IRX9-L may not be surprising when the structure of the xylans in cereals versus eudicots is considered, although this is likely to be more strongly linked to the nature of the substituents rather than intrinsic differences in the backbone [65,66]. Xylans are also present in more restricted tissues of eudicots and in smaller amounts than in cereals, however until the function of individual proteins is ascertained the reason for the presence or absence of particular orthologues is impossible to define. The importance of subtle differences could be key, for example expression of four rice GT-43 genes in Arabidopsis irx9 mutants showed that only two genes (Os05g03174 and Os05g48600) were capable of restoring a wild type phenotype whilst one gene (Os06g47340) was capable of complementing the mutant phenotype of irx14 [64].
Fig 3. Phylogenetic tree of GT-43 genes from 6 species.
Phylogenetic tree of GT-43 from Arabidopsis (At), barley (Hv), brachypodium (BRADI), sorghum (Sb), rice (OS) and the wheat TaGT43-4. Coding sequences of all genes were aligned using translation alignment. Phylogenetic trees were constructed using the FastTree function available in the Geneious software package. Branch labels represent FastTree support values. MLOC_ 8254 is on the same branch as the wheat TaGT43-4 and the Arabidopsis IRX14 and IRX14-L. MLOC_72459 is the only other barley gene closely related to the Arabidopsis IRX14 and IRX14-L. Arabidopsis IRX9 and IRX9-L are also shown in different colours.
GT47
GT47 genes were found under two of the ten peaks (Table 1), one on 2H and one on 5H (MLOC_61178, and MLOC_12869). Genes in this family have been identified as IRX10 or IRX10-L, and are xylan xylosyltransferases [67]. Downregulation of the Arabidopsis IRX10 orthologue in rice resulted in a 10% decrease in xylan levels in stem cell walls [68]. RNAi silencing of TaGT47-2, the orthologue of IRX10 in wheat caused a dramatic decrease in AX content in transgenic lines as well as an increase in arabinosyl substitutions [69]. None of the GT47 genes in this study were identified as orthologues of Arabidopsis IRX10 (data not shown) but nevertheless they remain candidates for AX biosynthesis in barley grain. Using a PFAM domain search for GT47 genes in barley (PF03016) we identified 30 family members, 10 of which are expressed in barley grain (S2 File). Given this large number of genes and the fact that the focus has been only on orthologous of IRX10 in grasses [68,69], we were unable to draw any conclusions regarding the involvement of the GT47 genes identified here in the biosynthesis of AX polysaccharides in barley grain. A different approach such as RNA-Seq and transcript analysis by QPCR in an AX-depositing grass tissue could provide clearer evidence supporting the involvement of certain GT47 members in AX biosynthesis in grasses.
GT61
Members of the GT61 family are being progressively identified as the proteins responsible for the addition of a range of xylan backbone substitutions in an increasing number of species [16,35–37,70]. Two genes from the GT61 gene family (MLOC_68728 and MLOC_17443) were identified in this study with MLOC_68728 located under QAX2.S-1H1 on 1H and MLOC_17443 found under QAX2.S-2H1*. Using a PFAM domain search tool we identified more than 30 members of the GT61 gene family in barley with at least 11 genes being expressed in developing barley grain (S2 File). Analysis of a rice mutant for a GT61 gene from the grass specific clade (xax1: Os02g22380) revealed that xylan from the mutant plants lacked β- Xylp-(1→2)-α-Araf-(1→3) structure substitutions, suggesting that Os02g22380 is a xylosyl transferase [36]. Interestingly, the mutant plants also lacked ferulic and p-coumaric acid, and exhibited an increase in the extractability of xylan and generally higher saccharification [36]. This was attributed to the lower degree of ferulic acid dehydrodimer cross-linking [36]. Further phylogenetic and transcript analysis of the GT61 genes identified here is required as these genes could be potential targets for modification of barley grain AX to enable increased release of xylan and other cell wall polysaccharides in a number of industrial processes.
DUF579
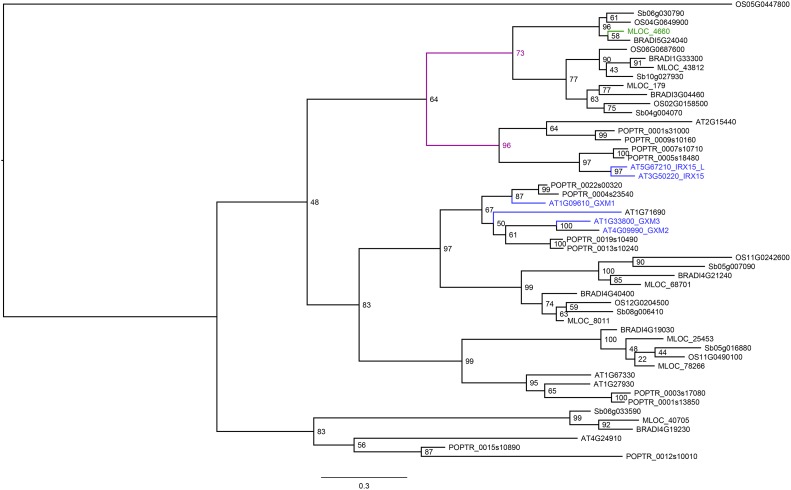
A potential candidate for QAX2.S-2H4 is a DUF579 gene (MLOC_4660). Arabidopsis has ten members in this gene family, five of which are co-expressed with genes known to be involved in secondary cell wall development [29]. Two members of the DUF579 gene family in Arabidopsis known as IRX15 (AT3G50220) and IRX15-L (AT5G67210) have been associated with xylan synthesis and deposition, as irx15 irx15-L double mutants exhibited irregular deposition of xylan in their secondary cell walls and contained xylan with a lower degree of polymerization [28,29]. However, three other members of this family, known as GXM1 (AT1G09610), GXM2 (AT4G09990) and GXM3 (AT1G33800), are believed to be involved in methylation of glucuronic acid residues attached to the xylan backbone [71]. Both a single mutation in AT1G33800 and double gxm mutants caused a significant reduction in xylan-bound methylated glucuronic acid [71]. Further characterization of AT1G33800 provided evidence that the protein encoded by this gene transfers a methyl group to α-D-glucopyranosyluronic acid residue linked to the xylan backbone [27]. Through a PFAM domain search (PF04669), eight DUF759 genes were identified in the barley genome, which were aligned with the four distinct phylogenetic clades that exist within the DUF579 gene family in Arabidopsis [27] and DUF579 proteins from poplar, rice, Brachypodium and sorghum. Our phylogenetic analysis also shows that DUF579 proteins fall into distinct clades (Fig 4). Certain members of the DUF579 proteins from all species included in the tree clustered with Arabidopsis IRX15 and IRX-15. Three barley proteins including MLOC_4660 clustered in a clade closely related to the Arabidopsis IRX15 and IRX15-L. However, it is not clear which barley gene is the orthologue of IRX15 or IRX15-L (Fig 4). Although experimental work has been carried out on DUF579 proteins from Arabidopsis and poplar [72,73], such information is still unavailable for members of this family from grasses. Whether MLOC_4660 is associated with xylan deposition, as it is the case with IRX 15 and IRX-15, is involved in methylation of glucuronic acid or plays a different role in AX biosynthesis needs to be further investigated. Nevertheless, this gene remains a potential candidate for the QAX2.S-2H4 QTL.
Fig 4. Phylogenetic tree of DUF579 genes from five species.
Phylogenetic tree of DUF579 from Arabidopsis (At), barley (Hv), Brachypodium (BRADI), sorghum (Sb) and rice (Os). Coding sequences of all genes were converted using the translation alignment. Phylogenetic trees were constructed using the FastTree function available in the Geneious software package. Branch labels represent FastTree support values. Arabidopsis IRX15 and IRX15-L, and the barley gene MLOC_4660 identified in this study are shown in different colours.
Significantly associated modifying and hydrolytic genes
Glycosyl hydrolases
It has been observed that there may be a finely tuned balance between biosynthetic and hydrolytic enzyme activity in the overall synthesis of a number of plant polysaccharides [74,75], although such hydrolases may also be key players in the modification and breakdown of these polymers. Members of the GH10 and GH11 [76,77], GH16 [78], GH51 [79,80] and GH79 [81] gene families have previously been associated with AX turnover and representatives were found under QAX2.S-3H1 (GH10; MLOC_75090), QAX2.S-5H1 (GH51; MLOC_56099 and GH79; MLOC_15027), and QAX2.S-5H2 (GH16; MLOC_80451). GH10 enzymes are endo-β-1, 4-xylanases and are involved in the hydrolysis of glycoside linkages of the xylan backbone [82]. Unlike xylanases from family 11 (GH11), GH10 xylanases may also be active on low molecular mass cellulose substrates [83]. However, both GH10 and GH11 xylanases are active on xylobiose and xylotriose substrates [84]. GH16 enzymes are mainly associated with the hydrolysis of (1,3–1,4)-β-glucan polysaccharides [85–87]. Based on the similarities in 3D structure between certain subgroups of GH16 enzymes and GH11 xylanases, it has been suggested that particular subgroups of GH16s might be active on AX [78]. However, this is yet to be functionally confirmed. GH51 enzymes are arabinofuranohydrolases, involved in the removal of arabinosyl side chains from AX [43]. GH79 enzymes exhibit a β-D-glucuronic acid activity [88] and thus could be involved in modification of GAX. Combined with other enzymes such as α-amylases, cellulases and pectinases, xylanases have important industrial applications in animal feed, food and drink and bread making industries [89], and thus offer a target for the manipulation of AX structure.
GT31 and DUF231
A GT31 gene (MLOC_70708) was identified at QAX2.S-7H1. GT31 genes have been shown to have galactosyltransferase activity, and play a role in the biosynthesis of arabinogalactan peptides [90,91]. An α-L-galactopyranosyl-(1→2)-β-D-xylopyranosyl-(1→2)-5-O-trans-feruloyl-L-arabinofuranose structure has previously been reported for AX from maize bran [92], and has more recently been associated with the AX from other cereal grains, including barley [93]. Therefore it is possible that GT31 genes are involved in the transfer of galactosyl units during the biosynthesis of AX in barley grain and this may have an influence on the overall amounts in grain tissues.
One gene from the DUF231 (MLOC_81823) family was identified at QAX2.S-5H1, and some members of this family have been linked with the acetylation of the xylan backbone [94]. A double knockout of the TBL32 and TBL33 genes in Arabidopsis resulted in a significant decrease in xylan acetyl content [95], but had no effect on overall cell wall composition. However, there are indications that these genes, with many others, are under the control of the secondary wall master transcriptional regulators SND1 and NST1, the perturbation of which may lead to broad-reaching pleiotropic effects on cell wall composition and integrity.
Conclusions
This GWAS study defined 10 QTL for the AX content of mature barley grain allowing candidate genes potentially involved in the biosynthesis of this important polysaccharide in cereals to be identified. Phylogenetic analysis of gene families suggest that a significant number of these genes may not be direct orthologues of AX-associated sequences in dicot plants such as Arabidopsis, indicating a need for further study of prime candidates, including transcript abundance and functional analysis. This could allow the use of promising candidates in conventional breeding efforts to manipulate AX levels in barley and other cereals, an industrially relevant goal for which there are currently few markers available.
Materials and methods
Plant material and growth conditions
A population of 2-row spring type barley was used in this study [96], (S1 File). This population comprised of 128 elite lines grown in a glasshouse compartment in a mix of clay-loam and cocopeat (50:50 v/v) at daytime and night-time temperatures of 22°C and 15°C respectively in The Plant Accelerator, Adelaide, Australia. This set was in particular selected to contain minimum population structure while maintaining as much diversity as possible based on population structure analysis and sequence homology. Mature grains were harvested and stored until monosaccharide analysis. For each line, five whole grains were ground to a fine powder using a ball mill (Mixer Mill MM400; Retsch Haan Germany) and the flour stored under dry conditions until the HPLC analysis.
Genotyping of SNP markers
All lines were genotyped using the 9K iSelect SNP genotyping platform described previously [96]. Prior to marker-trait association analysis, all monomorphic markers with an allele frequency of > 95% and markers with missing data > 5% were excluded from the analysis.
Monosaccharide analysis
A ~ 20 mg amount of wholegrain ground barley was used per sample. Monosaccharide analysis was carried out essentially as described by Comino et al. [97] with some modifications. Samples were treated with 1 mL 1 M sulphuric acid at 100°C for 3 hours. A 20-fold dilution of the hydrolysates was carried out prior to derivatization with 1-phenyl-3-methyl-5-pyrazolone (PMP). As an internal standard, 20 μL 0.5 mM 2-deoxy glucose was added to each sample. Excessive PMP was removed by dibutyl ether. A Phenomenex Kinetex 2.6 μm C18 100 × 3 mm 100A column installed on an Agilent 1260LC was used to separate the monosaccharides on an RP-HPLC. The flow rate was set to 0.8 mL/min. Eluents were (A) 10% acetonitrile, 40 mM ammonium acetate, and (B) 70% acetonitrile. The start condition was 85% A and 15% B and the gradient was 8 to 16% (B) over 12 mins. Detection was carried out at 250 nm. Calibration curves of standards of xylose and arabinose were used to quantify the area under the peaks.
GWAS analysis
Marker-trait association analysis was carried out in GenStat 15th Edition using the Eigenanalysis relationship model with a naïve model for comparison of each analysis (S2 Fig). For phenotype values, the mean values of the barley wholegrain total arabinose + xylose (w/w) was used. The false discovery rate (FDR) < 5% was calculated using the q value package in R [98] version 3.1.1. Boxplots to show the effect of SNPs from the three major QTL that passed the FDR test were produced using R version 3.2.2. To identify genes within intervals associated with AX content, the Barleymap website (http://floresta.eead.csic.es/barleymap/) was used. The intervals were extended by 2.5 cM either side of the SNP (s) with the highest LOD score to account for marker order uncertainty. SNPs significantly associated with the trait of interest within 5 cM of each other were considered to be linked to the same QTL and the SNP with the highest LOD score was used to represent the QTL. To obtain more consistent map positions, we compared the position of markers on three maps described in Comadran et al. [96], IBGS Consortium [51], and Mascher et al. [99]. QTL nomenclature is as described by Szűcs et al. [100] and available at (http://wheat.pw.usda.gov/ggpages/maps/OWB/).
Bioinformatics and gene identification
Different tools were employed to find annotation for unknown genes under the intervals. For genes under the associations that had Accession numbers, the nucleotide sequences were downloaded from the NCBI database https://www.ncbi.nlm.nih.gov/gquery/) and then Blasted to the barley genome MLOC loci (http://plants.ensembl.org/index.html). The annotation for these MLOCs was established with a combination of PFAM analysis and by orthology to the other well annotated cereal genomes, Brachypodium distachyon, Sorghum bicolor and rice (Oryza sativa) (http://plants.ensembl.org/biomart/martview). MLOC numbers were used to search the morexGenes- barley RNA-seq database (https://ics.hutton.ac.uk/morexGenes/index.html) to identify potential Arabidopsis and or rice orthologs and also to download the transcript profile of the candidate genes across eight developmental stages. Other tools used included PFAM domain search (http://pfam.xfam.org/). The CAZY database (http://www.cazy.org/) was used as a reference for the potential glycosyltransferases (GT) and glycoside hydrolases (GH).
Phylogenetic analysis
Amino acid sequences of barley, rice, sorghum and Arabidopsis glycosyltransferases were obtained from Ensemble Plants database (http://plants.ensembl.org/index.html) using a PFAM domain search. For GT43 the conserved PF03360 domain was used [101] and the protein sequence of TaGT43-4 described in Zeng et al. (ADK56174) [33] was included in the phylogeny analysis along with Arabidopsis IRX14 (AT5G67230) and IRX14-L (AT4G36890), IRAX9 (AT2G37090) and IRX9-L (AT1G27600). The MLOC_72459 sequence from the (https://ics.hutton.ac.uk/morexGenes/index.html) was used in a blastn search against the barley nucleotide sequences available on the NCBI database to obtain a full length gene sequence. The amino acid sequence of this gene was aligned with other sequences from rice, sorghum, Arabidopsis, barley and wheat. The MUSCLE alignment tool available in the Geneious software package version 8.1.3. [102] was used to align all sequences, and gaps were deleted from the alignment. A phylogenetic tree of the alignment was then produced using the RAxML [103] tool available in the same software package. Protein model was set to GAMMA GTR with 1000 bootstraps. For DUF579, the PFAM PF04669 was used to search for members of this family in selected species.
Supporting information
(CSV)
PFMA domains representing these families are listed in Table 1. Barley Gene id/ transcript (MLOC), Morex Contig, chromosomal location and developing grain without bracts 5 days post anthesis (CAR 5 DPA FPKM) and CAR 15 DPA FPKM (fragments per kilobase of exon per million fragments mapped) from ics.hutton.ac.uk/morexGenes.
(XLSX)
A description of QTL and potential genes identified under the peaks that passed the FDR test. Number of asterisks indicates the significance level for the adjusted P value (q value). * > 0.05, **> 0.01, ***> 0.001.
(DOCX)
A. SCRI_RS_175065 (QAX2.S-2H1), B. SCRI_RS_221939 (QAX2.S-2H4), and C. SCRI_RS_192352 (QAX2.S-3H1*). p <0.01 = **, p<0.001 = ***.
(TIF)
The–Log 10 (P-value) is shown on the Y axis. The X axis shows the seven barley chromosomes. Total AX content of wholegrain expressed as w/w was used for marker-trait association analysis.
(TIF)
Acknowledgments
This work was supported by grants from the Australian Research Council. We would like to acknowledge The Plant Accelerator, a National Collaborative Research Infrastructure Strategy facility, for the importation of plant lines through quarantine and the expert plant growth and care provided by its staff, particularly Dr. Bettina Berger, Lidia Mischis, Nicole Bond and Fiona Groskreutz. KH and RW would like to acknowledge funding from the Scottish Rural and Environment Science and Analytical Services Division (RESAS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the University of Adelaide www.adelaide.edu.au – ASH; Australian Research Council Centre of Excellence in Plant Cells Walls CE110001007 www.plantcellwalls.org.au - ASH, RAB, JL, NS, JGS, MJG, AL; Scottish Rural and Environment Science and Analytical Services Division http://www.gov.scot/Topics/Research/About/EBAR/StrategicResearch/future-research-strategy KH, RW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rennie EA, Scheller HV (2014) Xylan biosynthesis. Current Opinion in Biotechnology 26: 100–107. doi: 10.1016/j.copbio.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 2.Wilkie KCB (1979) The Hemicelluloses of Grasses and Cereals In: Tipson RS, Derek H, editors. Advances in Carbohydrate Chemistry and Biochemistry: Academic Press; pp. 215–264. [Google Scholar]
- 3.Carpita NC (1996) Structure and biogenesis of the cell walls of grasses. Annual Review of Plant Biology 47: 445–476. [DOI] [PubMed] [Google Scholar]
- 4.Bacic A, Stone B (1981) Chemistry and Organization of Aleurone Cell Wall Components From Wheat and Barley. Functional Plant Biology 8: 475–495. [Google Scholar]
- 5.Aspinall GO (1959) Structural Chemistry of the Hemicelluloses In: Melville LW, editor. Advances in Carbohydrate Chemistry: Academic Press; pp. 429–468. [DOI] [PubMed] [Google Scholar]
- 6.Höije A, Sandström C, Roubroeks JP, Andersson R, Gohil S, Gatenholm P. (2006) Evidence of the presence of 2-O-β-d-xylopyranosyl-α-l-arabinofuranose side chains in barley husk arabinoxylan. Carbohydrate Research 341: 2959–2966. doi: 10.1016/j.carres.2006.10.008 [DOI] [PubMed] [Google Scholar]
- 7.Sun X-F, Sun R, Fowler P, Baird MS (2005) Extraction and characterization of original lignin and hemicelluloses from wheat straw. Journal of Agricultural and Food Chemistry 53: 860–870. doi: 10.1021/jf040456q [DOI] [PubMed] [Google Scholar]
- 8.Coelho E, Rocha MAM, Moreira ASP, Domingues MRM, Coimbra MA (2016) Revisiting the structural features of arabinoxylans from brewers’ spent grain. Carbohydrate Polymers 139: 167–176. doi: 10.1016/j.carbpol.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 9.Yoshida-Shimokawa T, Yoshida S, Kakegawa K, Ishii T (2001) Enzymic feruloylation of arabinoxylan-trisaccharide by feruloyl-CoA: arabinoxylan-trisaccharide O-hydroxycinnamoyl transferase from Oryza sativa. Planta 212: 470–474. doi: 10.1007/s004250000490 [DOI] [PubMed] [Google Scholar]
- 10.de O. Buanafina MM (2009) Feruloylation in Grasses: Current and Future Perspectives. Molecular Plant 2: 861–872. doi: 10.1093/mp/ssp067 [DOI] [PubMed] [Google Scholar]
- 11.Jääskeläinen A-S, Holopainen-Mantila U, Tamminen T, Vuorinen T (2013) Endosperm and aleurone cell structure in barley and wheat as studied by optical and Raman microscopy. Journal of Cereal Science 57: 543–550. [Google Scholar]
- 12.Adom KK, Liu RH (2002) Antioxidant Activity of Grains. Journal of Agricultural and Food Chemistry 50: 6182–6187. [DOI] [PubMed] [Google Scholar]
- 13.Slavin J (2004) Whole grains and human health. Nutrition Research Reviews 17: 99–110. doi: 10.1079/NRR200374 [DOI] [PubMed] [Google Scholar]
- 14.Tosh SM, Shea Miller S (2016) Health Effects of β-Glucans Found in Cereals A2—Seetharaman, Colin WrigleyHarold CorkeKoushik In: Faubion J, editor. Encyclopedia of Food Grains (Second Edition). Oxford: Academic Press; pp. 236–240. [Google Scholar]
- 15.Westendorf ML, Wohlt JE (2002) Brewing by-products: their use as animal feeds. Veterinary Clinics of North America: Food Animal Practice 18: 233–252. [DOI] [PubMed] [Google Scholar]
- 16.Pellny TK, Lovegrove A, Freeman J, Tosi P, Love CG, Knox JP, et al. (2012) Cell Walls of Developing Wheat Starchy Endosperm: Comparison of Composition and RNA-Seq Transcriptome. Plant Physiology 158: 612–627. doi: 10.1104/pp.111.189191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamforth CW, Kanauchi M (2001) A Simple Model for the Cell Wall of the Starchy Endosperm in Barley*. Journal of the Institute of Brewing 107: 235–240. [Google Scholar]
- 18.Shang Y-L, Li X-M, Cai G-L, Lu J (2015) The role of ferulic acid and arabinoxylan in inducing premature yeast flocculation. Journal of the Institute of Brewing 121: 49–54. [Google Scholar]
- 19.Izydorczyk M, MacGregor A (2000) Evidence of intermolecular interactions of β-glucans and arabinoxylans. Carbohydrate Polymers 41: 417–420. [Google Scholar]
- 20.Mortimer JC, Miles GP, Brown DM, Zhang Z, Segura MP, Weimar T, et al. (2010) Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proceedings of the National Academy of Sciences 107: 17409–17414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach Knudsen KE (2015) Microbial Degradation of Whole-Grain Complex Carbohydrates and Impact on Short-Chain Fatty Acids and Health. Advances in Nutrition: An International Review Journal 6: 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee C, O’Neill MA, Tsumuraya Y, Darvill AG, Ye Z-H (2007) The irregular xylem9 Mutant is Deficient in Xylan Xylosyltransferase Activity. Plant and Cell Physiology 48: 1624–1634. doi: 10.1093/pcp/pcm135 [DOI] [PubMed] [Google Scholar]
- 23.Keppler BD, Showalter AM (2010) IRX14 and IRX14-LIKE, Two Glycosyl Transferases Involved in Glucuronoxylan Biosynthesis and Drought Tolerance in Arabidopsis. Molecular Plant 3: 834–841. doi: 10.1093/mp/ssq028 [DOI] [PubMed] [Google Scholar]
- 24.Lee C, Zhong R, Ye Z-H (2012) Arabidopsis Family GT43 Members are Xylan Xylosyltransferases Required for the Elongation of the Xylan Backbone. Plant and Cell Physiology 53: 135–143. doi: 10.1093/pcp/pcr158 [DOI] [PubMed] [Google Scholar]
- 25.Brown DM, Zhang Z, Stephens E, Dupree P, Turner SR (2009) Characterization of IRX10 and IRX10-like reveals an essential role in glucuronoxylan biosynthesis in Arabidopsis. The Plant Journal 57: 732–746. doi: 10.1111/j.1365-313X.2008.03729.x [DOI] [PubMed] [Google Scholar]
- 26.Rennie EA, Hansen SF, Baidoo EEK, Hadi MZ, Keasling JD, Scheller HV. (2012) Three Members of the Arabidopsis Glycosyltransferase Family 8 Are Xylan Glucuronosyltransferases. Plant Physiology 159: 1408–1417. doi: 10.1104/pp.112.200964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbanowicz BR, Peña MJ, Ratnaparkhe S, Avci U, Backe J, Steet HF, et al. (2012) 4-O-methylation of glucuronic acid in Arabidopsis glucuronoxylan is catalyzed by a domain of unknown function family 579 protein. Proceedings of the National Academy of Sciences 109: 14253–14258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen JK, Kim H, Cocuron JC, Orler R, Ralph J, Wilkerson CG. (2011) The DUF579 domain containing proteins IRX15 and IRX15-L affect xylan synthesis in Arabidopsis. The Plant Journal 66: 387–400. doi: 10.1111/j.1365-313X.2010.04475.x [DOI] [PubMed] [Google Scholar]
- 29.Brown D, Wightman R, Zhang Z, Gomez LD, Atanassov I, Bukowski JP, et al. (2011) Arabidopsis genes IRREGULAR XYLEM (IRX15) and IRX15L encode DUF579-containing proteins that are essential for normal xylan deposition in the secondary cell wall. The Plant Journal 66: 401–413. doi: 10.1111/j.1365-313X.2011.04501.x [DOI] [PubMed] [Google Scholar]
- 30.Burget EG, Reiter W-D (1999) The mur4 Mutant of Arabidopsis Is Partially Defective in the de Novo Synthesis of Uridine Diphosphol-Arabinose. Plant Physiology 121: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotake T, Takata R, Verma R, Takaba M, Yamaguchi D, Orita T, et al. (2009) Bifunctional cytosolic UDP-glucose 4-epimerases catalyse the interconversion between UDP-D-xylose and UDP-L-arabinose in plants. Biochemical Journal 424: 169–177. doi: 10.1042/BJ20091025 [DOI] [PubMed] [Google Scholar]
- 32.Rautengarten C, Ebert B, Herter T, Petzold CJ, Ishii T, Mukhopadhyay A, et al. (2011) The Interconversion of UDP-Arabinopyranose and UDP-Arabinofuranose Is Indispensable for Plant Development in Arabidopsis. The Plant Cell Online 23: 1373–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng W, Jiang N, Nadella R, Killen TL, Nadella V, Faik A. (2010) A Glucurono(arabino)xylan Synthase Complex from Wheat Contains Members of the GT43, GT47, and GT75 Families and Functions Cooperatively. Plant Physiology 154: 78–97. doi: 10.1104/pp.110.159749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh YS, Zhang Q, Yap K, Shirley NJ, Lahnstein J, Nelson CJ, et al. (2015) The Genetics, Transcriptional Profiles and Catalytic Properties of the UDP-Arabinose Mutase Family from Barley. Biochemistry. [DOI] [PubMed] [Google Scholar]
- 35.Anders N, Wilkinson MD, Lovegrove A, Freeman J, Tryfona T, Pellny TK, et al. (2012) Glycosyl transferases in family 61 mediate arabinofuranosyl transfer onto xylan in grasses. Proceedings of the National Academy of Sciences 109: 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiniquy D, Sharma V, Schultink A, Baidoo EE, Rautengarten C, Cheng K, et al. (2012) XAX1 from glycosyltransferase family 61 mediates xylosyltransfer to rice xylan. Proceedings of the National Academy of Sciences 109: 17117–17122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suliman M, Chateigner-Boutin AL, Francin-Allami M, Partier A, Bouchet B, Salse J, et al. (2013) Identification of glycosyltransferases involved in cell wall synthesis of wheat endosperm. Journal of Proteomics 78: 508–521. doi: 10.1016/j.jprot.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 38.Bartley LE, Peck ML, Kim S-R, Ebert B, Manisseri C, Chiniquy DM, et al. (2013) Overexpression of a BAHD Acyltransferase, OsAt10, Alters Rice Cell Wall Hydroxycinnamic Acid Content and Saccharification. Plant Physiology 161: 1615–1633. doi: 10.1104/pp.112.208694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piston F, Uauy C, Fu L, Langston J, Labavitch J, Dubcovsky J. (2009) Down-regulation of four putative arabinoxylan feruloyl transferase genes from family PF02458 reduces ester-linked ferulate content in rice cell walls. Planta 231: 677–691. doi: 10.1007/s00425-009-1077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Y, Teng Q, Zhong R, Ye Z-H (2013) The Arabidopsis DUF231 Domain-Containing Protein ESK1 Mediates 2-O- and 3-O-Acetylation of Xylosyl Residues in Xylan. Plant and Cell Physiology 54: 1186–1199. doi: 10.1093/pcp/pct070 [DOI] [PubMed] [Google Scholar]
- 41.Xiong G, Cheng K, Pauly M (2013) Xylan O-Acetylation Impacts Xylem Development and Enzymatic Recalcitrance as Indicated by the Arabidopsis Mutant tbl29. Molecular Plant 6: 1373–1375. doi: 10.1093/mp/sst014 [DOI] [PubMed] [Google Scholar]
- 42.Franková L, Fry SC (2011) Phylogenetic variation in glycosidases and glycanases acting on plant cell wall polysaccharides, and the detection of transglycosidase and trans-β-xylanase activities. The Plant Journal 67: 662–681. doi: 10.1111/j.1365-313X.2011.04625.x [DOI] [PubMed] [Google Scholar]
- 43.Burton R, Hrmova M, Fincher GB (2001) Barley arabinoxylan arabinofuranohydrolases: purification, characterization and determination of primary structures from cDNA clones. Biochemical Journal 356: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratke C, Pawar PMA, Balasubramanian VK, Naumann M, Duncranz ML, Derba-Maceluch M, et al. (2015) Populus GT43 family members group into distinct sets required for primary and secondary wall xylan biosynthesis and include useful promoters for wood modification. Plant Biotechnology Journal 13: 26–37. doi: 10.1111/pbi.12232 [DOI] [PubMed] [Google Scholar]
- 45.Zeng W, Lampugnani ER, Picard KL, Song L, Wu A-M, Farion IM, et al. (2016) Asparagus IRX9, IRX10, and IRX14A Are Components of an Active Xylan Backbone Synthase Complex that Forms in the Golgi Apparatus. Plant Physiology 171: 93–109. doi: 10.1104/pp.15.01919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S, Lei L, Yingling YG, Gu Y (2015) Microtubules and cellulose biosynthesis: the emergence of new players. Current Opinion in Plant Biology 28: 76–82. doi: 10.1016/j.pbi.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 47.Shi Y-n, Liu X-f, Li X, Dong W-c, Grierson D, Yin X-r, et al. (2017) SIMYB1 and SIMYB2, two new MYB genes from tomato, transcriptionally regulate cellulose biosynthesis in tobacco. Journal of Integrative Agriculture 16: 65–75. [Google Scholar]
- 48.Wang H-Z, Dixon RA (2012) On–off switches for secondary cell wall biosynthesis. Molecular Plant 5: 297–303. doi: 10.1093/mp/ssr098 [DOI] [PubMed] [Google Scholar]
- 49.Zhong R, Demura T, Ye Z-H (2006) SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. The Plant Cell 18: 3158–3170. doi: 10.1105/tpc.106.047399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi M, Demura T (2010) Transcriptional regulation of secondary wall formation controlled by NAC domain proteins. Plant Biotechnology 27: 237–242. [Google Scholar]
- 51.(2012) A physical, genetic and functional sequence assembly of the barley genome. Nature 491: 711–716. doi: 10.1038/nature11543 [DOI] [PubMed] [Google Scholar]
- 52.Izydorczyk MS, Dexter JE (2008) Barley β-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products–a Review. Food Research International 41: 850–868. [Google Scholar]
- 53.Rakha A (2011) Characterisation of dietary fibre in cereal grains and products.
- 54.Boskov Hansen H, Andreasen M, Nielsen M, Larsen L, Knudsen BK, Meyer A, et al. (2002) Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. European Food Research and Technology 214: 33–42. [Google Scholar]
- 55.Saulnier L, Peneau N, Thibault JF (1995) Variability in grain extract viscosity and water-soluble arabinoxylan content in wheat. Journal of Cereal Science 22: 259–264. [Google Scholar]
- 56.Gebruers K, Dornez E, Bedo Z, Rakszegi M, Frás A, Boros D, et al. (2010) Environment and Genotype Effects on the Content of Dietary Fiber and Its Components in Wheat in the HEALTHGRAIN Diversity Screen†. Journal of Agricultural and Food Chemistry 58: 9353–9361. doi: 10.1021/jf100447g [DOI] [PubMed] [Google Scholar]
- 57.Höije A, Gröndahl M, Tømmeraas K, Gatenholm P (2005) Isolation and characterization of physicochemical and material properties of arabinoxylans from barley husks. Carbohydrate Polymers 61: 266–275. [Google Scholar]
- 58.Krawczyk H, Persson T, Andersson A, Jönsson AS (2008) Isolation of hemicelluloses from barley husks. Food and Bioproducts Processing 86: 31–36. [Google Scholar]
- 59.Houston K, Russell J, Schreiber M, Halpin C, Oakey H, Washington J, et al. (2014) A genome wide association scan for (1,3;1,4)-beta-glucan content in the grain of contemporary 2-row Spring and Winter barleys. BMC Genomics 15: 907 doi: 10.1186/1471-2164-15-907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marcotuli I, Houston K, Waugh R, Fincher GB, Burton RA, Blanco A, et al. (2015) Genome Wide Association Mapping for Arabinoxylan Content in a Collection of Tetraploid Wheats. PLoS ONE 10: e0132787 doi: 10.1371/journal.pone.0132787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang L, Zhao D, Yan J, Zhang Y, Xia X, Tian Y, et al. (2016) QTL mapping of grain arabinoxylan contents in common wheat using a recombinant inbred line population. Euphytica 208: 205–214. [Google Scholar]
- 62.Nguyen V-L, Huynh B-L, Wallwork H, Stangoulis J (2011) Identification of Quantitative Trait Loci for Grain Arabinoxylan Concentration in Bread Wheat All rights reserved. Crop Science 51: 1143–1150. [Google Scholar]
- 63.Lee C, Teng Q, Huang W, Zhong R, Ye Z-H (2010) The Arabidopsis Family GT43 Glycosyltransferases Form Two Functionally Nonredundant Groups Essential for the Elongation of Glucuronoxylan Backbone. Plant Physiology 153: 526–541. doi: 10.1104/pp.110.155309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee C, Teng Q, Zhong R, Yuan Y, Ye Z-H (2014) Functional roles of rice glycosyltransferase family GT43 in xylan biosynthesis. Plant Signaling & Behavior 9: e27809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burton RA, Fincher GB (2014) Evolution and development of cell walls in cereal grains. Frontiers in Plant Science 5: 456 doi: 10.3389/fpls.2014.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faik A (2010) Xylan Biosynthesis: News from the Grass. Plant Physiology 153: 396–402. doi: 10.1104/pp.110.154237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu A-M, Rihouey C, Seveno M, Hörnblad E, Singh SK, Matsunaga T, et al. (2009) The Arabidopsis IRX10 and IRX10-LIKE glycosyltransferases are critical for glucuronoxylan biosynthesis during secondary cell wall formation. The Plant Journal 57: 718–731. doi: 10.1111/j.1365-313X.2008.03724.x [DOI] [PubMed] [Google Scholar]
- 68.Chen X, Vega-Sánchez ME, Verhertbruggen Y, Chiniquy D, Canlas PE, Fagerström A, et al. (2013) Inactivation of OsIRX10 Leads to Decreased Xylan Content in Rice Culm Cell Walls and Improved Biomass Saccharification. Molecular Plant 6: 570–573. doi: 10.1093/mp/sss135 [DOI] [PubMed] [Google Scholar]
- 69.Lovegrove A, Wilkinson MD, Freeman J, Pellny TK, Tosi P, Saulnier L, et al. (2013) RNA Interference Suppression of Genes in Glycosyl Transferase Families 43 and 47 in Wheat Starchy Endosperm Causes Large Decreases in Arabinoxylan Content. Plant Physiology 163: 95–107. doi: 10.1104/pp.113.222653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh A, Singh U, Mittal D, Grover A (2010) Transcript expression and regulatory characteristics of a rice glycosyltransferase OsGT61-1 gene. Plant Science 179: 114–122. [Google Scholar]
- 71.Lee C, Teng Q, Zhong R, Yuan Y, Haghighat M, Ye Z-H. (2012) Three Arabidopsis DUF579 Domain-Containing GXM Proteins are Methyltransferases Catalyzing 4-O-Methylation of Glucuronic Acid on Xylan. Plant and Cell Physiology 53: 1934–1949. doi: 10.1093/pcp/pcs138 [DOI] [PubMed] [Google Scholar]
- 72.Yuan Y, Teng Q, Zhong R, Ye Z-H (2014) Identification and Biochemical Characterization of Four Wood-Associated Glucuronoxylan Methyltransferases in Populus. PLoS ONE 9: e87370 doi: 10.1371/journal.pone.0087370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song D, Gui J, Liu C, Sun J, Li L (2016) Suppression of PtrDUF579-3 Expression Causes Structural Changes of the Glucuronoxylan in Populus. Frontiers in Plant Science 7: 493 doi: 10.3389/fpls.2016.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Minic Z, Jouanin L (2006) Plant glycoside hydrolases involved in cell wall polysaccharide degradation. Plant Physiology and Biochemistry 44: 435–449. doi: 10.1016/j.plaphy.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 75.Kuntothom T, Luang S, Harvey AJ, Fincher GB, Opassiri R, Hrmova M, et al. (2009) Rice family GH1 glycoside hydrolases with β-d-glucosidase and β-d-mannosidase activities. Archives of Biochemistry and Biophysics 491: 85–95. doi: 10.1016/j.abb.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 76.Kolenová K, Vršanská M, Biely P (2006) Mode of action of endo-β-1,4-xylanases of families 10 and 11 on acidic xylooligosaccharides. Journal of Biotechnology 121: 338–345. doi: 10.1016/j.jbiotec.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 77.Zheng H, Guo B, Chen X-L, Fan S-J, Zhang Y-Z (2011) Improvement of the quality of wheat bread by addition of glycoside hydrolase family 10 xylanases. Applied Microbiology and Biotechnology 90: 509–515. doi: 10.1007/s00253-011-3088-7 [DOI] [PubMed] [Google Scholar]
- 78.Strohmeier M, Hrmova M, Fischer M, Harvey AJ, Fincher GB, Pleiss J. (2004) Molecular modeling of family GH16 glycoside hydrolases: Potential roles for xyloglucan transglucosylases/hydrolases in cell wall modification in the poaceae. Protein Science 13: 3200–3213. doi: 10.1110/ps.04828404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rémond C, Boukari I, Chambat G, O’Donohue M (2008) Action of a GH 51 α-l-arabinofuranosidase on wheat-derived arabinoxylans and arabino-xylooligosaccharides. Carbohydrate Polymers 72: 424–430. [Google Scholar]
- 80.Laidlaw HKC, Lahnstein J, Burton RA, Fincher GB, Jobling SA (2012) Analysis of the arabinoxylan arabinofuranohydrolase gene family in barley does not support their involvement in the remodelling of endosperm cell walls during development. Journal of Experimental Botany 63: 3031–3045. doi: 10.1093/jxb/ers019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Michikawa M, Ichinose H, Momma M, Biely P, Jongkees S, Yoshida M, et al. (2012) Structural and biochemical characterization of glycoside hydrolase family 79 β-glucuronidase from Acidobacterium capsulatum. Journal of Biological Chemistry 287: 14069–14077. doi: 10.1074/jbc.M112.346288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiology Reviews 29: 3–23. doi: 10.1016/j.femsre.2004.06.005 [DOI] [PubMed] [Google Scholar]
- 83.Gilkes NR, Claeyssens M, Aebersold R, Henrissat B, Meinke A, Morrison HD, et al. (1991) Structural and functional relationships in two families of β-1, 4-glycanases. The FEBS Journal 202: 367–377. [DOI] [PubMed] [Google Scholar]
- 84.Biely P, Kluepfel D, Morosoli R, Shareck F (1993) Mode of action of three endo-β-1, 4-xylanases of Streptomyces lividans. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology 1162: 246–254. [DOI] [PubMed] [Google Scholar]
- 85.Furtado GP, Ribeiro LF, Santos CR, Tonoli CC, de Souza AR, Oliveira RR, et al. (2011) Biochemical and structural characterization of a β-1,3–1,4-glucanase from Bacillus subtilis 168. Process Biochemistry 46: 1202–1206. [Google Scholar]
- 86.Chen C-C, Huang J-W, Zhao P, Ko T-P, Huang C-H, Chan H-C, et al. (2015) Structural analyses and yeast production of the β-1,3–1,4-glucanase catalytic module encoded by the licB gene of Clostridium thermocellum. Enzyme and Microbial Technology 71: 1–7. doi: 10.1016/j.enzmictec.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 87.Fibriansah G, Masuda S, Koizumi N, Nakamura S, Kumasaka T (2007) The 1.3 Å crystal structure of a novel endo-β-1,3-glucanase of glycoside hydrolase family 16 from alkaliphilic Nocardiopsis sp. strain F96. Proteins: Structure, Function, and Bioinformatics 69: 683–690. [DOI] [PubMed] [Google Scholar]
- 88.Michikawa M, Ichinose H, Momma M, Biely P, Jongkees S, Yoshida M, et al. (2012) Structural and Biochemical Characterization of Glycoside Hydrolase Family 79 β-Glucuronidase from Acidobacterium capsulatum. Journal of Biological Chemistry 287: 14069–14077. doi: 10.1074/jbc.M112.346288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS. (2005) Xylanases from fungi: properties and industrial applications. Applied Microbiology and Biotechnology 67: 577–591. doi: 10.1007/s00253-005-1904-7 [DOI] [PubMed] [Google Scholar]
- 90.Penning BW, Hunter CT, Tayengwa R, Eveland AL, Dugard CK, Olek AT, et al. (2009) Genetic Resources for Maize Cell Wall Biology. Plant Physiology 151: 1703–1728. doi: 10.1104/pp.109.136804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Basu D, Liang Y, Liu X, Himmeldirk K, Faik A, Kieliszewski M, et al. (2013) Functional Identification of a Hydroxyproline-O-galactosyltransferase Specific for Arabinogalactan Protein Biosynthesis in Arabidopsis. Journal of Biological Chemistry 288: 10132–10143. doi: 10.1074/jbc.M112.432609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saulnier L, Vigouroux J, Thibault J-F (1995) Isolation and partial characterization of feruloylated oligosaccharides from maize bran. Carbohydrate Research 272: 241–253. [DOI] [PubMed] [Google Scholar]
- 93.Schendel RR, Meyer MR, Bunzel M (2016) Quantitative profiling of feruloylated arabinoxylan side chains from graminaceous cell walls. Frontiers in Plant Science 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan Y, Teng Q, Zhong R, Ye Z-H (2013) The Arabidopsis DUF231 domain-containing protein ESK1 mediates 2-O-and 3-O-acetylation of xylosyl residues in xylan. Plant and Cell Physiology: pct070. [DOI] [PubMed] [Google Scholar]
- 95.Yuan Y, Teng Q, Zhong R, Haghighat M, Richardson EA, Ye Z-H. (2016) Mutations of Arabidopsis TBL32 and TBL33 Affect Xylan Acetylation and Secondary Wall Deposition. PLoS ONE 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Comadran J, Kilian B, Russell J, Ramsay L, Stein N, Ganal M, et al. (2012) Natural variation in a homolog of Antirrhinum CENTRORADIALIS contributed to spring growth habit and environmental adaptation in cultivated barley. Nature genetics 44: 1388–1392. doi: 10.1038/ng.2447 [DOI] [PubMed] [Google Scholar]
- 97.Comino P, Shelat K, Collins H, Lahnstein J, Gidley MJ (2013) Separation and purification of soluble polymers and cell wall fractions from wheat, rye and hull less barley endosperm flours for structure-nutrition studies. Journal of Agricultural and Food Chemistry 61: 12111–12122. doi: 10.1021/jf403558u [DOI] [PubMed] [Google Scholar]
- 98.Dabney A, Storey J, Warnes G (2011) qvalue: Q-value estimation for false discovery rate control. R package version 1.26. 0.
- 99.Mascher M, Muehlbauer GJ, Rokhsar DS, Chapman J, Schmutz J, Barry K, et al. (2013) Anchoring and ordering NGS contig assemblies by population sequencing (POPSEQ). The Plant Journal 76: 718–727. doi: 10.1111/tpj.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Szűcs P, Blake VC, Bhat PR, Chao S, Close TJ, Cuesta-Marcos A, et al. (2009) An Integrated Resource for Barley Linkage Map and Malting Quality QTL Alignment. Plant Gen 2: 134–140. [Google Scholar]
- 101.Kulkarni AR, Peña MJ, Avci U, Mazumder K, Urbanowicz BR, Pattathil S, et al. (2012) The ability of land plants to synthesize glucuronoxylans predates the evolution of tracheophytes. Glycobiology 22: 439–451. doi: 10.1093/glycob/cwr117 [DOI] [PubMed] [Google Scholar]
- 102.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. (2012) Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. doi: 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. doi: 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
PFMA domains representing these families are listed in Table 1. Barley Gene id/ transcript (MLOC), Morex Contig, chromosomal location and developing grain without bracts 5 days post anthesis (CAR 5 DPA FPKM) and CAR 15 DPA FPKM (fragments per kilobase of exon per million fragments mapped) from ics.hutton.ac.uk/morexGenes.
(XLSX)
A description of QTL and potential genes identified under the peaks that passed the FDR test. Number of asterisks indicates the significance level for the adjusted P value (q value). * > 0.05, **> 0.01, ***> 0.001.
(DOCX)
A. SCRI_RS_175065 (QAX2.S-2H1), B. SCRI_RS_221939 (QAX2.S-2H4), and C. SCRI_RS_192352 (QAX2.S-3H1*). p <0.01 = **, p<0.001 = ***.
(TIF)
The–Log 10 (P-value) is shown on the Y axis. The X axis shows the seven barley chromosomes. Total AX content of wholegrain expressed as w/w was used for marker-trait association analysis.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.