Abstract
Background and Purpose
To determine associations between stenosis, measures of plaque burden, and compositional features of carotid atherosclerosis, including high-risk features of intraplaque hemorrhage (IPH) and surface disruption.
Methods
Institutional Review Board approval and informed consent for all participants were obtained before study initiation. Patients with either carotid stenosis >50% by duplex ultrasound or suspected coronary artery disease underwent multi-contrast carotid MRI at 3.0 T. For each artery, stenosis, percent wall volume (PWV=100%×wall volume/total vessel volume), and mean wall thickness (MWT) were measured. Presence or absence of a lipid-rich necrotic core, calcification, IPH, and surface disruption were recorded.
Results
One hundred eighty-one patients were included in the final analysis. The area under the curve (AUC) calculated from receiver-operating-characteristics analysis found the presence of IPH was similarly classified by stenosis (AUC=0.82), PWV (AUC=0.88), and MWT (AUC=0.88). Notably, IPH was present in the lowest category of each parameter. Prevalence of IPH in arteries with 0% stenosis was 4.4%. In arteries with PWV <40%, prevalence was 3.2%; in arteries with MWT <1.0 mm, prevalence was 2.3%. Strength of classification for surface disruption was similarly classified by stenosis (AUC=0.87), PWV (AUC=0.93), and MWT (AUC=0.94).
Conclusions
Measures of plaque burden do not substantially improve disease assessment compared to stenosis. The finding of IPH in all categories of stenosis and plaque burden suggests that direct characterization of plaque composition and surface status is necessary to fully discriminate disease severity.
Keywords: atherosclerosis, carotid artery, magnetic resonance imaging, plaque burden, stenosis
Carotid artery atherosclerosis is a major etiology of cerebrovascular events such as transient ischemia attack and stroke.1 Because of the well-defined association between severity of luminal stenosis and clinical events, carotid stenosis currently represents the principal criterion for ischemic risk stratification and surgical intervention.2 However, the occurrence of stroke in patients with mild to moderate (<70%) carotid stenosis suggests that stenosis may not be the strongest classifier of atherosclerotic disease severity.3 In addition, high-risk features of atherosclerosis, such as intraplaque hemorrhage (IPH) and surface disruption, have been reported across all stenotic categories (0%–99%).4,5 As such, alternate or complementary strategies for evaluating carotid atherosclerotic disease have become increasingly desirable.
The arterial remodeling hypothesis,6 defined as the preservation of the lumen via outward expansion of the arterial wall in response to atherosclerosis, has led to the development of strategies for evaluating the arterial wall. In the coronary artery, measures of plaque burden have been used to characterize disease severity and occur after response to therapy. In particular, Nissen et al7 have proposed percent atheroma volume (100%×atheroma volume/total vessel volume) during invasive intravascular ultrasound investigations as a parameter that captures plaque burden while accounting for inherent differences in arterial size. An analogous measurement in the carotid artery obtained noninvasively via high-resolution black-blood MRI is percent wall volume (PWV=100%×wall volume/total vessel volume). As an alternative to PWV, arterial wall thickness might also represent a viable measure of local disease severity. B-mode ultrasound has been shown to effectively measure the intima-media thickness of the arterial wall.8 Multiple long-term, prospective investigations have shown that carotid intima-media thickness is associated with stroke.9 Recently, a strong association has been shown between intima-media thickness measured by B-mode ultrasound and mean wall thickness (MWT) measured by carotid MRI.10
We hypothesized that measures of plaque burden (ie, PWV and MWT) would be stronger classifiers of high-risk features, specifically IPH and surface disruption, than stenosis. This study sought to determine: (1) associations between stenosis, PWV, MWT, and the compositional features of carotid atherosclerotic disease; and (2) strength of plaque burden in classifying carotid high-risk features. Identification of a parameter that accurately classifies the presence or absence of these features may prove valuable in advancing the accurate assessment of carotid disease severity.
Materials and Methods
Study Sample
Carotid MRI data were pooled from participants recruited at a single academic medical center either for >50% stenosis by duplex ultrasound in at least 1 carotid artery or for coronary CTA for suspected coronary artery disease attributable to chest pain. Carotid duplex ultrasound examinations were performed by a singular operator with 5 years of experience. Utilization of images from the cohort with suspected coronary artery disease has been previously shown to yield arteries with normal to moderate carotid atherosclerotic disease,11 thus ensuring a broad distribution of carotid atherosclerosis for the sample population as a whole. For each cohort, the artery selected for imaging with carotid MRI, termed the index artery, was determined by recruitment strategy. In subjects with >50% stenosis in at least 1 carotid artery, the artery with the greatest amount of stenosis on duplex ultrasound was assigned as the index artery. For subjects recruited from coronary CTA, the index artery was randomly assigned. Clinical information was obtained through chart review. The Institutional Review Board approved the research protocol before study initiation and informed consent was obtained from all participants.
Carotid MRI Protocol
All images were acquired on a 3.0-T whole-body scanner (Signa Excite; General Electric Medical Systems) utilizing a 4-channel, phased-array carotid surface coil. A standardized protocol adapted for imaging at 3.0 T was used to obtain 4 contrast-weighted images of the index carotid artery in the transverse plane: T1-weighted, proton-density weighted, T2-weighted, and 3-dimensional time-of-flight MRA. The scan was longitudinally centered on the bifurcation of the index artery. MRI parameters are listed in the Supplemental Table (available online at http://stroke.ahajournals.org). Postcontrast T1-weighted images were acquired for subjects with >50% carotid stenosis on duplex ultrasound. Gadodiamide (Omniscan; GE Health-care) was administrated intravenously with a dose of 0.1 mmol/kg at a rate of 1 mL/sec 5 minutes before contrast-enhanced T1-weighted image acquisition. For patients with carotid >50% stenosis, the carotid MRI was performed within 2 weeks after carotid ultrasound examinations. For patients with suspected coronary artery disease, the carotid arteries were imaged by MR within 1 week after coronary CTA.
Image Analysis
Two trained reviewers with 2 years of experience in reading carotid MR images, blinded to clinical information and stenosis measurements, evaluated image quality and interpreted the multi-contrast images of the index carotid artery via consensus opinion. For each index artery, an image quality rating was assigned using a 4-point scale (1=poor, 4=excellent). Arteries with image quality <2 were excluded from analysis. For the remaining interpretable arteries, a software package (CASCADE, Seattle, WA) was used to measure the lumen area, wall area, total vessel area, and mean wall thickness at each axial location. The PWV was subsequently determined from the wall volume and total vessel volume for each index artery. The average MWT across all axial locations defined the MWT for each index artery. The presence or absence of carotid compositional features, including calcification, lipid-rich necrotic core (LRNC), IPH, and surface disruption (ulceration or fibrous cap rupture), was determined using previously published multi-contrast criteria validated with histology.12,13 Using CASCADE, the intrareader and inter-reader coefficients of variation were from 3.0% to 7.2% in quantifying the carotid lumen and wall area,14 and the intraclass correlation coefficient was from 0.73 to 0.95 in quantitative evaluation of carotid plaque compositions.15
Maximum intensity projection images were reconstructed from the 3-dimensional time-of-flight MRA images using GE software (GE Medical System Advantage Workstation 4.3). Using the same GE software package, a single reviewer with 3 years of experience in cardiovascular radiology, blinded to images and results from multi-contrast carotid MRI, measured luminal stenosis using NASCET criteria (percent stenosis=100%×[1 − luminal diameter at the point of maximal narrowing/the diameter of the normal distal internal carotid artery]).
Data Analysis
The stenosis, PWV, and MWT between index arteries with and without each carotid compositional feature, such as calcification, LRNC, IPH, and surface disruption, were compared using the independent t test. Logistic regression analysis was used to calculate the OR and corresponding 95% CI for stenosis, PWV, and MWT. Receiver-operating characteristics analysis was used to determine the strength of classification for stenosis, PWV, and MWT for determining the presence vs absence of each compositional feature. The area under the curve and corresponding 95% CI are reported. Prevalence of carotid compositional features for different categories of stenosis, PWV, and MWT are reported. Stenosis and PWV were categorized by 10% intervals. MWT was partitioned into the following 5 categories: <1.0 mm, 1.0 to 1.5 mm, 1.5 to 2.0 mm, 2.0 to 2.5 mm, and >2.5 mm. Pearson correlation coefficient, r, was used to determine associations between stenosis and PWV. All analyses were performed with SPSS for Windows (version 12.0; SPSS). Statistical significance was defined as a value of P<0.05.
Results
From October 2005 to February 2009, 190 participants (N=67, duplex stenosis >50%; N=123, coronary CTA) underwent carotid MRI. Of the 190 participants, 9 (4.7%) were excluded because of total occlusion in the index carotid artery. All of the remaining 181 index arteries had sufficient image quality for comprehensive plaque interpretation. The demographics and arterial characteristics for this cohort are reported in Table 1. The mean±SD of stenosis, PWV, and MWT for this population were 21.3%±27.5%, 43.7%±14.5%, and 1.3±0.6 mm, respectively.
Table 1.
Demographics of the Study Population (N = 181)
| Mean±SD or %
|
P* | ||
|---|---|---|---|
| Patients With Carotid Stenosis >50% (N=64) | Patients With Suspected Coronary Artery Disease (N=117) | ||
| Age, y | 66.9±11.5 | 58.1±9.6 | <0.001 |
| Male, % | 85.9 | 78.6 | 0.230 |
| Height, cm | 169.3±6.1 | 169.9±7.0 | 0.527 |
| Weight, kg | 69.1±9.7 | 75.3±10.9 | <0.001 |
| Body mass index, kg/m2 | 24.1±3.0 | 26.0±3.0 | <0.001 |
| Hypertension, % | 67.2 | 58.1 | 0.232 |
| Smoker, % | 40.6 | 54.7 | 0.071 |
| Diabetes mellitus, % | 15.6 | 23.1 | 0.236 |
| History of coronary artery disease, % | 32.8 | 26.5 | 0.371 |
| Neurological symptoms, % | 40.6 | 17.9 | 0.001 |
| Carotid stenosis, % | 44.4±27.9 | 8.7±17.1 | <0.001 |
| Mean wall thickness, mm | 1.9±0.6 | 1.0±0.3 | <0.001 |
| Percent wall volume, % | 58.0±11.3 | 35.9±9.0 | <0.001 |
| Presence of calcification, % | 78.1 | 38.5 | <0.001 |
| Presence of lipid-rich necrotic core, % | 92.2 | 62.4 | <0.001 |
| Presence of intraplaque hemorrhage, % | 45.3 | 8.5 | <0.001 |
| Presence of surface disruption, % | 35.9 | 3.4 | <0.001 |
Independent t test was used to compare continuous variables. The χ2 test was conducted for comparison of binary variables.
Compared to carotid arteries without each compositional feature, those arteries with the corresponding compositional feature exhibited significantly greater stenosis, PWV, and MWT (all P<0.001; Table 2). In addition, logistic regression analysis found that stenosis, PWV, and MWT were significant predictors of all carotid compositional features (OR, 1.42–4.51; all P<0.001; Table 2). Receiver-operating characteristic analysis found that measurements of stenosis, PWV, and MWT were strong classifiers of carotid compositional features (areas under the curve=0.78–0.94; all P<0.001; Table 2 and Figure 1). Although measures of plaque burden (PWV and MWT) had the highest area under the curve for classifying the presence of calcification, LRNC, IPH, and surface disruption, performance was not substantially higher than stenosis (Table 2, Figure 1).
Table 2.
Association of Carotid Plaque Features With Stenosis and Plaque Burden
| Presence (Mean±SD) | Absence (Mean±SD) | P* | OR (95% CI) | P† | Area Under the Curve (95% CI) | P‡ | |
|---|---|---|---|---|---|---|---|
| Calcification | |||||||
| Stenosis (%) | 33.1±28.7 | 8.2±19.0 | <0.001 | 1.57 (1.33–1.86) | <0.001 | 0.78 (0.71–0.83) | <0.001 |
| PWV (%) | 50.6±13.7 | 36.1±11.0 | <0.001 | 2.49 (1.85–3.35) | <0.001 | 0.81 (0.75–0.87) | <0.001 |
| MWT (mm) | 1.6±0.6 | 1.1±0.6 | <0.001 | 1.42 (1.24–1.64) | <0.001 | 0.84 (0.78–0.89) | <0.001 |
| Lipid-rich necrotic core | |||||||
| Stenosis (%) | 27.6±28.3 | 4.4±15.5 | <0.001 | 1.83 (1.38–2.43) | <0.001 | 0.78 (0.71–0.83) | <0.001 |
| PWV (%) | 47.9±14.3 | 32.5±6.7 | <0.001 | 4.03 (2.37–6.86) | <0.001 | 0.84 (0.78–0.89) | <0.001 |
| MWT (mm) | 1.5±0.7 | 0.9±0.2 | <0.001 | 3.58 (2.07–6.19) | <0.001 | 0.89 (0.83–0.93) | <0.001 |
| Intraplaque hemorrhage | |||||||
| Stenosis (%) | 46.4±27.0 | 14.4±23.3 | <0.001 | 1.51 (1.31–1.74) | <0.001 | 0.82 (0.76–0.87) | <0.001 |
| PWV (%) | 59.5±11.5 | 39.4±12.0 | <0.001 | 3.09 (2.17–4.37) | <0.001 | 0.88 (0.82–0.92) | <0.001 |
| MWT (mm) | 2.0±0.7 | 1.1±0.5 | <0.001 | 1.59 (1.37–1.84) | <0.001 | 0.88 (0.82–0.92) | <0.001 |
| Surface disruption | |||||||
| Stenosis (%) | 53.5±23.7 | 15.6±24.0 | <0.001 | 1.58 (1.34–1.86) | <0.001 | 0.87 (0.81–0.92) | <0.001 |
| PWV (%) | 64.1±8.7 | 40.1±12.1 | <0.001 | 4.51 (2.68–7.60) | <0.001 | 0.93 (0.88–0.96) | <0.001 |
| MWT (mm) | 2.3±0.6 | 1.2±0.5 | <0.001 | 1.96 (1.56–2.48) | <0.001 | 0.94 (0.90–0.97) | <0.001 |
MWT indicates mean wall thickness; PWV, percent wall volume.
P is calculated by independent t test.
P is calculated by logistic regression with 10% increment for both stenosis and PWV and 0.2-mm increment for MWT, respectively.
P is calculated by receiver-operating characteristic analysis.
Figure 1.
The area under the curve graphs from receiver-operating characteristic analysis represent the strength of stenosis, percent wall volume (PWV), and mean wall thickness (MWT) in classifying presence or absence of carotid calcification (A), lipid-rich necrotic core (B), intraplaque hemorrhage (C), and surface disruption (D). These graphs indicate that stenosis, PWV, and MWT are strong classifiers for each carotid atherosclerotic feature.
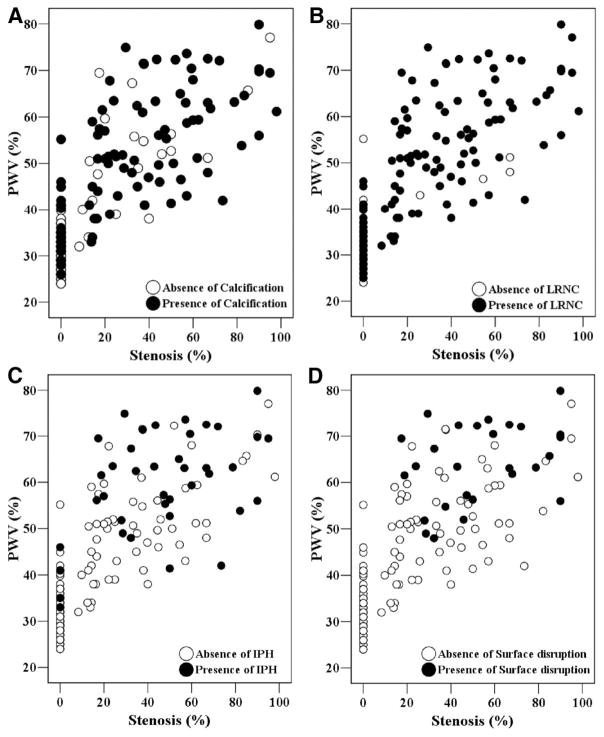
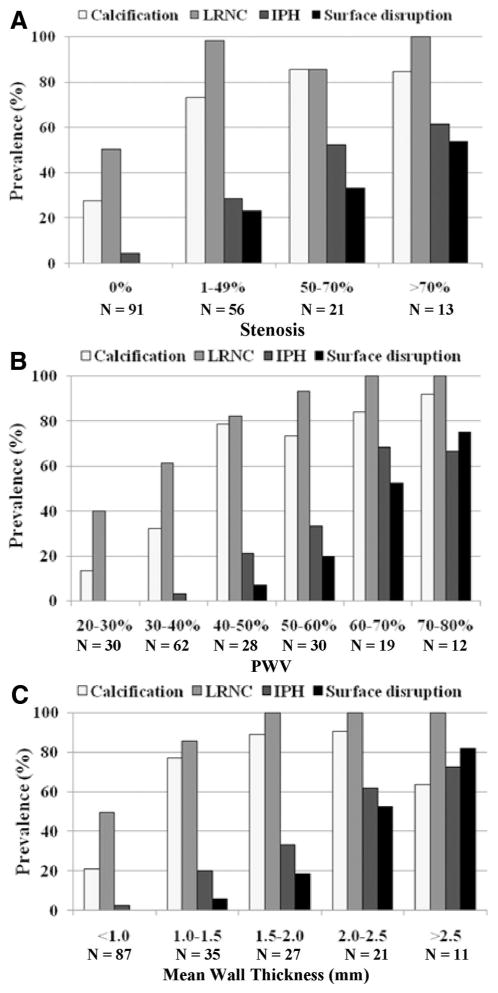
There was a significant correlation between PWV and stenosis (r=0.80; P<0.001; Figure 2). Scatter plots (Figure 2) demonstrate a general linear trend, but a wide distribution of PWV for all levels of stenosis was evident. Points labeled according to the presence or absence of calcification (Figure 2A), LRNC (Figure 2B), IPH (Figure 2C), or surface disruption (Figure 2D) indicate that each plaque feature occurred across a range of both PWV and stenosis. Calcification, LRNC, and IPH were present in nearly the full range of stenosis and PWV, whereas surface disruption started to occur in arteries with greater stenosis and PWV than other compositional features. Figure 3 shows a general increasing prevalence of carotid compositional features with increasing categories of stenosis (Figure 3A), PWV (Figure 3B), and MWT (Figure 3C). Of note, IPH was identified in lesions with 0% stenosis, with PWV <40%, and in the lowest MWT category (<1.0 mm; Figure 4). In contrast, surface disruption occurred in arteries with stenosis ≥17.4%, PWV ≥48.0%, or MWT ≥1.29 mm in this cohort.
Figure 2.
Scatter plots represent a general linear correlation between percent wall volume (PWV) and stenosis. However, there is a wide spectrum of PWV at each level of stenosis. Points labeled according to the presence or absence of calcification (A), LRNC (B), IPH (C), or surface disruption (D) indicate that each plaque feature occurred across a range of both PWV and stenosis. Notice that each compositional feature occurs across the full range of stenosis and PWV, with the exception of surface disruption.
Figure 3.
Prevalence of carotid compositional features in different categories of stenosis (A), percent wall volume (PWV) (B), and mean wall thickness (C). An increasing trend for prevalence of carotid features with increasing severity of stenosis, PWV, and MWT is present.
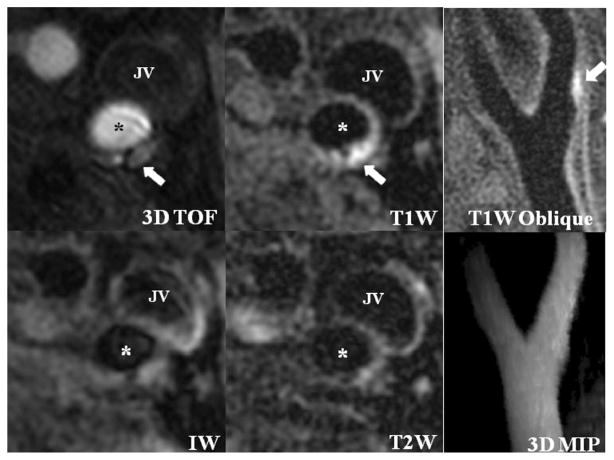
Figure 4.
An atherosclerotic plaque with intraplaque hemorrhage (white arrows) can be found in the left internal carotid artery (*). This lesion has low plaque burden (percent wall volume=36%) and normal MRA (3-dimensional maximum intensity projection). JV, jugular vein; ECA, external carotid artery.
Discussion
This study is one of the first to our knowledge to directly compare carotid luminal stenosis and plaque burden measurements in classifying presence or absence of carotid compositional features. In this study, we found that greater plaque burden, as measured by PWV and MWT, and increased luminal stenosis were significantly associated with the presence of calcification, LRNC, IPH, and surface disruption. In addition, plaque burden and stenosis were strong classifiers for the presence of each of these compositional features. However, high-risk features (ie, IPH and surface disruption) were identified across a wide range of plaque burden parameters and stenosis. Moreover, IPH was present not only in lesions with 0% stenosis but also within the plaque burden categories indicative of the earliest disease. Although unexpected, these findings offer compelling evidence for direct evaluation of plaque composition and surface status to adequately differentiate severity of disease rather than the continued reliance on conventional utilization of indirect markers (eg, stenosis and plaque burden measurements).
The identification of IPH and surface disruption across the full range of stenosis has been previously reported. Saam et al4 found that complicated plaques, which included lesions with either IPH or surface disruption, occurred in 21.7% of arteries with 16% to 49% stenosis and in 8.1% of arteries with 1% to 15% stenosis by duplex ultrasound. Recently, Dong et al5 reported that in individuals with 0% stenosis on contrast-enhanced MRA, the prevalence of IPH and surface disruption were 8.7% and 4.3%, respectively. For IPH, our findings are in strong agreement with these previous studies.4,5 For surface disruption, we found a lower prevalence in lesions with 0% stenosis compared to that in the study by Dong et al.5 Differences may be consequent of the disparity in the sample populations. Dong et al5 recruited patients with >50% stenosis in 1 carotid artery and used the contralateral artery for their analysis. The presence of advanced carotid disease in the contralateral artery may have predisposed the sample population to have a higher prevalence of surface disruption in the index artery, despite no measurable luminal encroachment. In the study reported herein, the artery with greater stenosis was utilized in patients with known preexisting carotid atherosclerosis. We observed the development of stenosis and increased plaque burden before surface disruption in our cohort. These findings are consistent with the development of a sufficiently large LRNC before disruption, which recently has been reported as the strongest predictor of future surface disruptions.16 However, in consideration of the findings by Dong et al,5 advanced disease in 1 carotid artery may predispose plaque instability on the contralateral side, regardless of the measure of disease severity. Longitudinal studies that follow bilateral carotid disease are necessary to better-appreciate the relationships or, perhaps, independence of carotid disease within the same individual.
The role of IPH in carotid atherosclerotic disease has become increasingly prominent. Prospective studies17,18 have demonstrated a significant association between IPH and ischemic events. In addition, IPH has been reported to accelerate plaque progression in carotid arteries19 and predict recurrent cerebrovascular events.20,21 Notably, Underhill et al22 recently reported that the presence of IPH in lesions with 16% to 49% stenosis at baseline not only accelerated plaque growth but also was associated with inward remodeling. Moreover, they observed that IPH may mitigate the positive effects of statin therapy.22 Collectively, these studies indicate that identification of IPH may be a necessary and critical aspect in differentiating disease severity. Although there was a higher prevalence of IPH in lesions with more stenosis and increased plaque burden, the presence of IPH spanned the entire range of these parameters. Accordingly, patients with negative traditional imaging findings but with a history of ischemic events or an increased clinical risk of ischemic events may warrant imaging for the detection of IPH. Sequences developed specifically for IPH detection in the carotid artery have been developed and validated at 3.0 T.23 The utilization of a single contrast weighting for IPH detection may prove cost-effective in the management of susceptible individuals for the prevention of stroke.
In this study, we used PWV as a plaque burden measurement in the carotid artery. Beyond the correspondence to a well-established parameter in the coronary artery, there are additional factors that motivate the selection of PWV for assessment of disease severity in the carotid artery. First, PWV has been shown to have the highest measure of reproducibility across all measures of carotid plaque morphology with MRI.24 Second, PWV fully exploits the 3-dimensional data acquisition inherent to carotid MRI. Finally, high levels of in vivo plaque burden, as measured by PWV, have been identified across the full spectrum of stenosis in the carotid artery.25 Importantly, the latter factor suggests that PWV may provide unique information compared to stenosis measurements.
Similar to Babiarz et al,25 we found a significant association between PWV and stenosis. However, at each level of stenosis there was a wide spread of PWV. Glagov et al6 originally observed from observations of postmortem coronary arteries that luminal narrowing occurred after the atherosclerotic lesion occupied >40% of the internal elastic lamina area. Our data suggest that arterial remodeling may occur differently in the carotid artery and/or may be dependent on additional factors beyond plaque burden. Underhill et al16 hypothesized that the presence of IPH or alternative change in composition may alter the biological behavior of the lesion. Such a phenomenon previously has been described in balloon-injured rabbits.26 Other factors beyond plaque composition may also govern arterial remodeling, such as shear stress.6 Accordingly, large prospective studies that investigate a variety of demographic, serological, and imaging parameters (eg, inflammation,27 neovasculature28) are necessary to identify factors that contribute to differential remodeling patterns.
In this study, the carotid black-blood MRI technique was utilized to determine the compositional features of atherosclerotic plaque. This histologically validated technique provided unique plaque compositional information related to plaque vulnerability beyond luminal stenosis. However, there were several study limitations. First, 2 groups of patients with heterogeneous demographics were recruited. Second, slightly different imaging protocols were used in these 2 groups of subjects. A better-designed future study may include a larger sample size, homogenous inclusion criteria and imaging protocol, multiple races, and fair gender distribution. Additionally, the new developed imaging techniques for detecting IPH29 and thrombus30 might be helpful in characterizing vulnerable plaques in future studies.
Conclusion
In conclusion, luminal stenosis and measures of plaque burden are associated with the composition of carotid plaques. However, high-risk features such as IPH and surface disruption occur across a wide range of both parameters. In addition, measures of plaque burden do not substantially improve assessment of carotid disease severity compared to the traditional criterion of luminal stenosis. As such, direct imaging assessment for the detection of IPH and surface disruption appears necessary to adequately assess disease severity. Additionally, patients with negative imaging findings by traditional criteria but with a history of ischemic events or strong clinical risk factors for ischemic events may warrant vessel wall imaging.
Supplementary Material
Supplemental Table. Carotid MR imaging parameters.
Acknowledgments
Sources of Funding
This study is supported in part by a grant from GE Healthcare and the National Institutes of Health (RO1 HL 56874).
Footnotes
The online-only Data Supplement is available at http://stroke.ahajournals.org/cgi/content/full/STROKEAHA.110.597328/DC1.
Disclosure
None.
References
- 1.Hollander M, Bots ML, Del Sol AI, Koudstaal PJ, Witteman JC, Grobbee DE, Hofman A, Breteler MM. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: the Rotterdam study. Circulation. 2002;105:2872–2877. doi: 10.1161/01.cir.0000018650.58984.75. [DOI] [PubMed] [Google Scholar]
- 2.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi M, Ogasawara K, Inoue T, Saito H, Suga Y, Ogawa A. Endarterectomy for mild cervical carotid artery stenosis in patients with ischemic stroke events refractory to medical treatment. Neurol Med Chir (Tokyo) 2008;48:211–215. doi: 10.2176/nmc.48.211. [DOI] [PubMed] [Google Scholar]
- 4.Saam T, Underhill HR, Chu B, Takaya N, Cai J, Polissar NL, Yuan C, Hatsukami TS. Prevalence of American Heart Association type VI carotid atherosclerotic lesions identified by magnetic resonance imaging for different levels of stenosis as measured by duplex ultrasound. J Am Coll Cardiol. 2008;51:1014–1021. doi: 10.1016/j.jacc.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 5.Dong L, Underhill HR, Yu W, Ota H, Hatsukami TS, Gao TL, Zhang Z, Oikawa M, Zhao X, Yuan C. Geometric and compositional appearance of atheroma in an angiographically normal carotid artery in patients with atherosclerosis. AJNR Am J Neuroradiol. 2010;31:311–316. doi: 10.3174/ajnr.A1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 7.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 8.Tang R, Hennig M, Thomasson B, Scherz R, Ravinetto R, Catalini R, Rubba P, Zanchetti A, Bond MG. Baseline reproducibility of B-mode ultrasonic measurement of carotid artery intima-media thickness: The European Lacidipine Study on Atherosclerosis (ELSA) J Hypertens. 2000;18:197–201. doi: 10.1097/00004872-200018020-00010. [DOI] [PubMed] [Google Scholar]
- 9.Cao JJ, Thach C, Manolio TA, Psaty BM, Kuller LH, Chaves PH, Polak JF, Sutton-Tyrrell K, Herrington DM, Price TR, Cushman M. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–170. doi: 10.1161/01.CIR.0000079160.07364.6A. [DOI] [PubMed] [Google Scholar]
- 10.Underhill HR, Kerwin WS, Hatsukami TS, Yuan C. Automated measurement of mean wall thickness in the common carotid artery by MRI: a comparison to intima-media thickness by B-mode ultrasound. J Magn Reson Imaging. 2006;24:379–387. doi: 10.1002/jmri.20636. [DOI] [PubMed] [Google Scholar]
- 11.Underhill HR, Yuan C, Terry JG, Chen H, Espeland MA, Hatsukami TS, Saam T, Chu B, Yu W, Oikawa M, Takaya N, Yarnykh VL, Kraft R, Carr JJ, Maldjian J, Tang R, Crouse JR., III Differences in carotid arterial morphology and composition between individuals with and without obstructive coronary artery disease: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2008;10:31. doi: 10.1186/1532-429X-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–1373. doi: 10.1161/01.cir.0000028591.44554.f9. [DOI] [PubMed] [Google Scholar]
- 13.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, Takaya N, Polissar NL, Yuan C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 14.Saam T, Hatsukami TS, Yarnykh VL, Hayes CE, Underhill H, Chu B, Takaya N, Cai J, Kerwin WS, Xu D, Polissar NL, Neradilek B, Hamar WK, Maki J, Shaw DW, Buck RJ, Wyman B, Yuan C. Reader and platform reproducibility for quantitative assessment of carotid atherosclerotic plaque using 1.5T Siemens, Philips, and General Electric scanners. J Magn Reson Imaging. 2007;26:344–352. doi: 10.1002/jmri.21004. [DOI] [PubMed] [Google Scholar]
- 15.Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–239. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 16.Underhill HR, Yuan C, Yarnykh VL, Chu B, Oikawa M, Dong L, Polissar NL, Garden GA, Cramer SC, Hatsukami TS. Predictors of surface disruption with mr imaging in asymptomatic carotid artery stenosis. AJNR Am J Neuroradiol. 2010;31:487–493. doi: 10.3174/ajnr.A1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, Garden GA, Cramer SC, Maravilla KR, Hashimoto B, Hatsukami TS. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI–initial results. Stroke. 2006;37:818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 18.Singh N, Moody AR, Gladstone DJ, Leung G, Ravikumar R, Zhan J, Maggisano R. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology. 2009;252:502–508. doi: 10.1148/radiol.2522080792. [DOI] [PubMed] [Google Scholar]
- 19.Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, Ferguson MS, Hatsukami TS. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111:2768–2775. doi: 10.1161/CIRCULATIONAHA.104.504167. [DOI] [PubMed] [Google Scholar]
- 20.Altaf N, Daniels L, Morgan PS, Auer D, MacSweeney ST, Moody AR, Gladman JR. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg. 2008;47:337–342. doi: 10.1016/j.jvs.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 21.Altaf N, MacSweeney ST, Gladman J, Auer DP. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke. 2007;38:1633–1635. doi: 10.1161/STROKEAHA.106.473066. [DOI] [PubMed] [Google Scholar]
- 22.Underhill HR, Yuan C, Yarnykh VL, Chu B, Oikawa M, Polissar NL, Schwartz SM, Jarvik GP, Hatsukami TS. Arterial remodeling in the subclinical carotid artery disease. J Am Coll Cardiol Cardiovasc Imaging. 2009;2:1381–1389. doi: 10.1016/j.jcmg.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu DC, Ferguson MS, DeMarco JK. An optimized 3D inversion recovery prepared fast spoiled gradient recalled sequence for carotid plaque hemorrhage imaging at 3.0 T. Magn Reson Imaging. 2008;26:1360–1366. doi: 10.1016/j.mri.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Saam T, Kerwin WS, Chu B, Cai J, Kampschulte A, Hatsukami TS, Zhao XQ, Polissar NL, Neradilek B, Yarnykh VL, Flemming K, Huston J, III, Insull W, Jr, Morrisett JD, Rand SD, DeMarco KJ, Yuan C. Sample size calculation for clinical trials using magnetic resonance imaging for the quantitative assessment of carotid atherosclerosis. J Cardiovasc Magn Reson. 2005;7:799–808. doi: 10.1080/10976640500287703. [DOI] [PubMed] [Google Scholar]
- 25.Babiarz LS, Astor B, Mohamed MA, Wasserman BA. Comparison of gadolinium-enhanced cardiovascular magnetic resonance angiography with high-resolution black blood cardiovascular magnetic resonance for assessing carotid artery stenosis. J Cardiovasc Magn Reson. 2007;9:63–70. doi: 10.1080/10976640600843462. [DOI] [PubMed] [Google Scholar]
- 26.Courtman DW, Schwartz SM, Hart CE. Sequential injury of the rabbit abdominal aorta induces intramural coagulation and luminal narrowing independent of intimal mass: extrinsic pathway inhibition eliminates luminal narrowing. Circ Res. 1998;82:996–1006. doi: 10.1161/01.res.82.9.996. [DOI] [PubMed] [Google Scholar]
- 27.Trivedi RA, Mallawarachi C, U-King-Im JM, Graves MJ, Horsley J, Goddard MJ, Brown A, Wang L, Kirkpatrick PJ, Brown J, Gillard JH. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–1606. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 28.Kerwin WS, Oikawa M, Yuan C, Jarvik GP, Hatsukami TS. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med. 2008;59:507–514. doi: 10.1002/mrm.21532. [DOI] [PubMed] [Google Scholar]
- 29.Yamada N, Higashi M, Otsubo R, Sakuma T, Oyama N, Tanaka R, Iihara K, Naritomi H, Minematsu K, Naito H. Association between signal hyperintensity on T1-weighted MR imaging of carotid plaques and ipsilateral ischemic events. AJNR Am J Neuroradiol. 2007;28:287–292. [PMC free article] [PubMed] [Google Scholar]
- 30.Daniels LR, Gladman JR, Altaf N, Moody AR. Magnetic resonance direct thrombus imaging in moderate carotid artery stenosis. Stroke. 2006;37:767–768. doi: 10.1161/01.str.0000204239.49586.55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table. Carotid MR imaging parameters.