Summary
Sodalis praecaptivus is a close relative and putative environmental progenitor of the widely distributed, insect-associated, Sodalis-allied symbionts. Here we show that mutant strains of S. praecaptivus that lack genetic components of a quorum sensing (QS) apparatus acquire a rapid and potent killing phenotype following microinjection into an insect host. Transcriptomic and genetic analyses indicate that insect killing occurs as a consequence of virulence factors, including insecticidal toxins and enzymes that degrade the insect integument, which are normally repressed by QS at high infection densities. This method of regulation suggests that virulence factors are only utilized in early infection to initiate the insect-bacterial association. Once bacteria reach sufficient density in host tissues, the QS circuit represses expression of these harmful genes, facilitating a long-lasting and benign association. We discuss the implications of the functionality of this QS system in the context of establishment and evolution of mutualistic relationships involving these bacteria.
eTOC
Enomoto et al. show that a progenitor of the widely distributed Sodalis-allied insect endosymbionts utilizes quorum sensing to suppress virulence factors following the establishment of infection. This allows bacteria to maintain a benign and persistent infection in their insect host, possibly facilitating the evolution of mutualistic relationships.
Introduction
Many eukaryotes harbor mutualistic bacteria that increase host fitness and facilitate environmental niche diversification (Douglas, 2014). Insects serve as useful systems for the study of symbiosis because they are readily amenable to genomic and experimental study. While much is known about the evolution and functions of symbiosis in insects (Moran et al., 2008), little is known about how these mutualistic associations originate. One conspicuous finding is that certain bacterial phylotypes are predisposed to the development of these mutualisms. Examples include representatives of the Sodalis genera, which are found in a wide range of distantly related insect hosts (Snyder et al., 2011; Clayton et al., 2012).
Although certain symbionts provide specialized functions for their insect hosts (e.g. defense: Oliver et al., 2010), many just provide simple nutritional supplements, such as amino acids and vitamins (Douglas, 2009). Since these metabolites can be made by a wide-range of bacteria, the notion of specialized function cannot explain the propensity of certain phylotypes to develop these associations. Rather, selected lineages seem predisposed to developing these associations as a result of genetic adaptations (Sachs et al., 2013). One of the most significant challenges in host association involves circumventing the sophisticated physical and molecular defenses that prevent bacterial entry and colonization. To this end, pathogens have evolved numerous molecular adaptations (virulence properties) to combat these processes and enhance bacterial infection. These range from relatively innocuous physical adaptations that facilitate resistance to factors of immunity, through to the secretion of enzymes and toxins that degrade host integument and inflict damage on tissues and cells (Vallet-Gely et al., 2008). It stands to reason that mutualists would experience stronger selection to limit the application of virulence in order to maximize host fitness and the likelihood of success of the resulting association.
In this study, we explore interactions between a close free-living relative of the Sodalis-allied insect endosymbionts, Sodalis praecaptivus (Clayton et al., 2012), and a grain weevil host, Sitophilus zeamais, that harbors a very recently derived Sodalis-allied endosymbiont (Lefevre et al., 2004). Close relatives of S. praecaptivus have been identified in a wide range of insect hosts (Snyder et al., 2011; Clayton et al., 2012), and genomic analyses indicate that these mutualistic symbionts have evolved repeatedly and independently from an S. praecaptivus-like antecedent, undergoing substantial genome degeneration and size reduction as a consequence of the loss of many genes that lack adaptive value following the transition to the static symbiotic lifestyle (Clayton et al., 2012; Husnik and McCutcheon, 2016). Regarding their origin, the closest relatives of S. praecaptivus have been found in certain stinkbugs and chestnut weevils, in which these bacteria have a sporadic frequency of infection among insect populations (Kaiwa et al., 2010; Toju and Fukatsu, 2011), suggesting that they are not maternally transmitted by these insects but are instead acquired horizontally from the environment by each new insect generation. Since such a transient association is not compatible with the notion of obligate mutualism, we proposed that these insects may serve as vectors for the transmission of these bacteria to alternative hosts; namely the trees that the stinkbugs are known to feed upon (Clayton et al., 2012). In support of this, S. praecaptivus shares many genetic features (including genes encoding plant virulence factors) with the closely related phytopathogenic lineage comprising Erwinia and relatives, which are also often vectored by insects (Nadarasah and Stavrinides, 2004).
The regulation of gene expression is an important adaptive trait that facilitates the modification of physiology in accordance with the environment. One such mode of regulation is quorum sensing (QS), in which bacteria synthesize an autoinducer pheromone that increases in accordance with bacterial density and interacts at a threshold concentration with response regulators to facilitate changes in gene expression (Fuqua et al., 2001; Waters and Bassler, 2005). In the current study, we investigated the role of a QS system in S. praecaptivus and found that it negatively regulates a potent insect killing phenotype. We propose that the negative regulation of virulence by QS is an important adaptation that contributes to the ability of Sodalis-allied symbionts to develop and sustain associations with insects.
Results
S. praecaptivus and S. glossinidius maintain homologous quorum sensing systems
The tsetse fly symbiont, S. glossinidius, was previously shown to produce N-(3-oxohexanoyl) homoserine lactone (OHHL) as a QS signaling molecule via the protein product of a gene designated sogI (Pontes et al., 2008). To characterize the S. praecaptivus signaling molecule, ethyl acetate extracts of S. glossinidius and S. praecaptivus culture media were separated by TLC and overlaid with an Agrobacterium tumefaciens reporter strain that yields a blue spot in the presence of an N-acyl homoserine lactone (Zhu et al., 2003). Since the resulting spots co-localized on the TLC plate, we conclude that S. praecaptivus also produces OHHL (Figure 1A). In addition, S. praecaptivus maintains two LuxR-like response regulators (ypeR and yenR; locus tags Sant_3587 and Sant_1175, respectively) sharing >90% amino acid sequence identity with orthologs from S. glossinidius.
Figure 1.
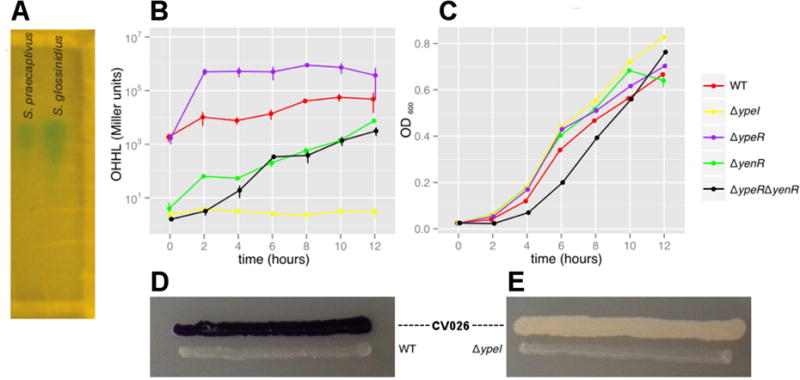
Characterization of the S. praecaptivus QS system. (A) TLC plate with ethyl acetate extracts derived from culture supernatants of S. praecaptivus and S. glossinidius. The plate was overlaid with an A. tumefaciens N-acyl homoserine lactone (AHL) reporter strain that produces blue spots in the presence of AHL molecules. This assay shows that S. praecaptivus and S. glossinidius each produce a single AHL molecule, which was previously characterized by mass spectrometry as OHHL in S. glossinidius. (B) OHHL production (assayed using the A. tumefaciens reporter strain) and (C) growth (OD600) of WT and mutant strains of S. praecaptivus. Data were obtained from three biological replicates and error bars show standard errors. (D & E) Plate-based assays of AHL production from the S. praecaptivus WT and ΔypeI strains (respectively) using the Chromobacterium violaceum CV026 reporter strain which produces a violet pigment in the presence of AHLs.
Transcriptomic analysis of the S. praecaptivus QS system
To identify genes regulated by quorum sensing, we used RNA-Seq to compare gene expression levels in a ΔypeI mutant strain of S. praecaptivus in vitro in the presence or absence of 10 μg/ml OHHL, which mimics a high cell density state. Thirty genes were found to have >4 fold changes in expression (Figure 2A) and most (26/30) showed a decrease in expression in response to OHHL. Many of the OHHL-repressed genes have putative functions associated with insect pathogenesis. These include homologs of the PirAB insecticidal toxin (Sant_2443, Sant_2444), genes encoding chitinases (Sant_1984, Sant_1963), chitin binding domain-containing proteins (Sant_1090, Sant_P0292) and collagenase-like proteases (Sant_3444, Sant_3445).
Figure 2.
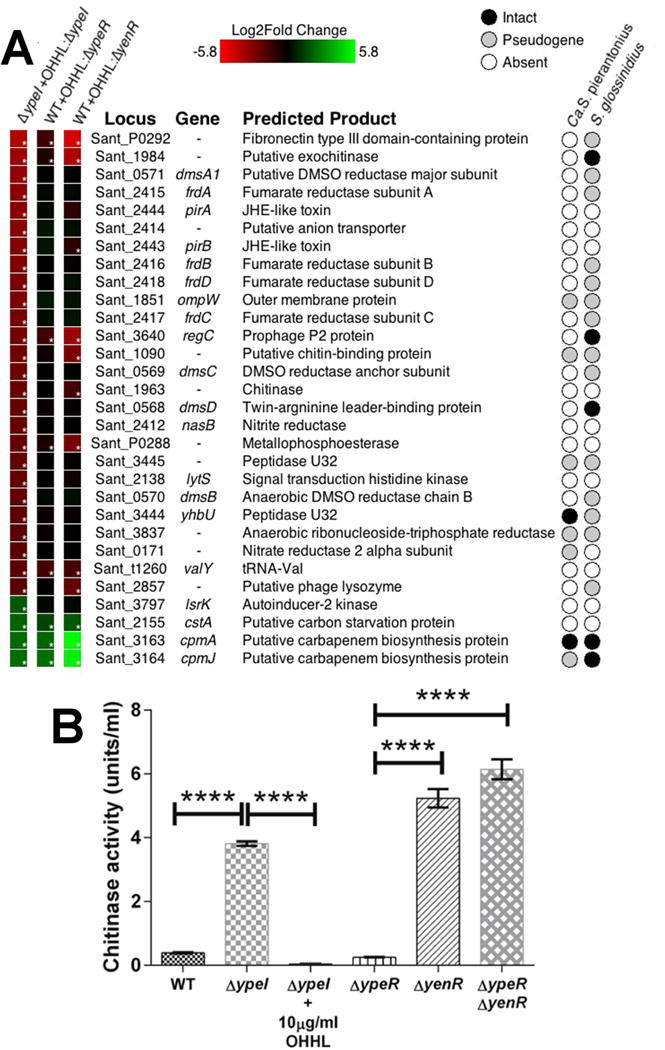
Transcriptomic (RNA-Seq) Analysis of QS-regulated genes in S. praecaptivus. (A) Heat maps showing changes in gene expression. The first column shows changes in the 30 most differentially expressed genes in the S. praecaptivus ΔypeI strain in response to addition of exogenous OHHL. The second and third columns show changes in the expression of the same 30 genes in a WT strain (supplemented with OHHL) relative to ΔypeR and ΔyenR strains (respectively), lacking exogenous OHHL. The asterisks in the boxes on the left indicate statistically significant levels of differential expression. The matrix on the right hand side of the panel reveals the status of the 30 genes in Sodalis-allied insect symbionts that are closely related to S. praecaptivus. Complete transcriptomic datasets are provided in Table S1. (B) Chitinase activities of WT and mutant S. praecaptivus strains, in the presence or absence of OHHL. All samples were assayed in triplicate and error bars show standard deviations. One way ANOVA comparisons between genotypes: ΔypeI vs. ΔypeI supplemented with 10 μg/ml OHHL, WT vs. ΔypeI, ΔypeR vs. ΔyenR and ΔypeR vs. ΔypeRyenR, all yield p <0.0001; ΔyenR vs. ΔypeR ΔyenR double mutant yields p = 0.0006.
Only four genes demonstrated a significant increase in expression in response to OHHL. Curiously, the top two induced genes, cpmA and cpmJ (Sant_3163, Sant_3164), were also induced in S. glossinidius and are homologous to components of the carbapenem biosynthesis gene cluster that is present in some enteric bacteria (Pontes et al., 2008). The other two genes induced by OHHL were a putative carbon starvation protein (cstA; Sant_2155) and a putative autoinducer 2 kinase (lsrK; Sant_3797) that may be involved in the regulation of another quorum sensing pathway.
To determine the roles of YpeR and YenR in the regulation of expression via QS, we compared the RNA-Seq profiles of ΔypeR and ΔyenR mutant strains with the WT strain in the presence of OHHL (Figure 2A). Although both regulators clearly contribute to the differential regulation of the genes identified in the experiment involving the ΔypeI mutant strain, the solo- response regulator (YenR) has a more potent effect on differential regulation, given that the transcriptomic profile of the ΔyenR mutant strain more closely mimics that of a ΔypeI mutant strain lacking OHHL. However, this seems to be due to the loss of OHHL synthase (YpeI) expression in the ΔyenR strain, which has ~50-fold less ypeI transcripts relative to the WT strain (Table S1; see entries highlighted in green on pages 2 & 3). To explore this further, we assayed the amount of OHHL produced by the WT, ΔypeI, ΔypeR and ΔyenR strains. In liquid culture, the ΔypeI strain failed to produce any OHHL (Figures 1B & 1C), consistent with the notion that ypeI is the only gene encoding an N-acyl HSL synthase in S. praecaptivus. This was further corroborated using an alternative reporter (Chromobacterium violaceum strain CV026; McClean et al., 1997), which also only detected a molecule produced by the WT strain (Figures 1D & 1E). In agreement with the transcriptomic data, the amount of OHHL produced by the ΔyenR mutant was greatly reduced relative to WT (~10-fold at 12h; Figure 1B), consistent with the notion that YenR enhances ypeI transcription and/or YpeR represses ypeI transcription. This is further supported by the fact that a ΔyenR ΔyenR double mutant strain also produces a greatly reduced amount of OHHL and a ΔypeR mutant strain shows an elevated level of OHHL production relative to WT (~10-fold at 12h; Figure 1B). However, the transcriptomic data shows that the counts of ypeI transcripts are actually (~2-fold) lower in the ΔypeR mutant relative to the WT strain, suggesting that the OHHL level in the culture media is also controlled by mechanisms other than the modulation of ypeI transcription. Interestingly, some LuxR homologs regulate transcription in the absence of their signaling molecules and their regulatory functions are antagonized by AHLs (Tsai and Winans, 2010). Genes encoding these particular luxI and luxR homolog (including those in S. praecaptivus) often overlap at their 3′-ends to mediating transcriptional antagonism. Taken together, these results indicate that there is a complex regulatory interplay between YpeI, YpeR and YenR that facilitates QS-based signaling.
Validation of transcriptomic data
Since two S. praecaptivus genes encoding chitinases were significantly downregulated in response to OHHL, we performed chitinase assays in WT, ΔypeI, ΔypeR and ΔyenR strains of S. praecaptivus, The highest activity was found in the ΔypeI strain lacking OHHL (Figure 2B), consistent with the notion of QS-based repression. In addition, the ΔypeR strain had a chitinase activity similar to the WT strain, indicating that YenR alone can repress chitinase in the presence of OHHL. Also, the ΔyenR mutant strain had significantly higher chitinase activity, consistent with the repression of OHHL synthesis. Together with the observation that the ΔyenR ΔypeR double mutant has high chitinase activity, these results corroborate those obtained from RNA-Seq analysis and the OHHL assays.
Quorum sensing suppresses growth of S. praecaptivus in vitro.
During the course of our work, we noticed that the S. praecaptivus WT strain had a growth rate that was significantly reduced relative to the ΔypeI mutant (Figure 1C). This was corroborated by a series of experiments performed on solid media, which show that OHHL inhibits growth of both the S. praecaptivus WT and ΔypeR mutant strains, but not the ΔyenR strain (Figure 3A). In addition, a plasmid overexpressing the OHHL synthase (YpeI) could only be maintained in a ΔyenR background and this strain strongly inhibited the growth of adjacent WT and ΔypeR (but not ΔyenR) strains (Figure 3B).
Figure 3.
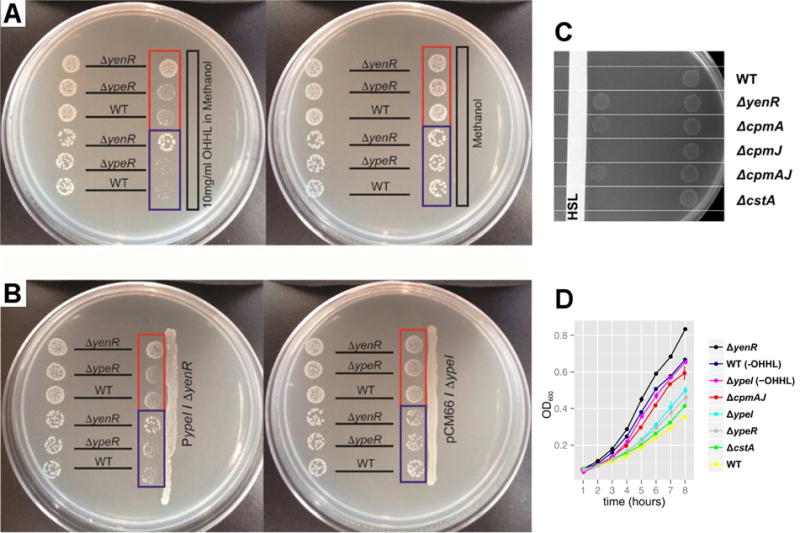
QS Induces Growth Suppression in S. praecaptivus. (A & B) Each bacterial strain (labeled according to genotype) was spotted in two positions on the plate. Panel A shows spots placed either distal (left) or proximal (right) to (A) a strip of sterile paper that was impregnated with exogenous OHHL in methanol (left plate) or methanol alone (right plate). Panel B shows spots placed either distal (left) or proximal (right) to a streak of the S. praecaptivus ΔyenR strain maintaining plasmid pCM66 overexpressing the ypeI gene (left plate) or a streak of the S. praecaptivus ΔypeI strain maintaining plasmid pCM66 lacking ypeI (right plate). The spots highlighted in the red boxes have a 10-fold higher concentration of cells than their counterparts highlighted in blue. (C) S. praecaptivus strains (labeled according to genotype) were spotted in two positions on a plate, either proximal (left) or distal (right) to a strip of sterile paper impregnated with exogenous OHHL in methanol (labeled “HSL”). Note that deletion of cpmA alone relieves QS-mediated growth suppression. Deletion of either cpmJ or cstA (another gene whose transcription is increased under quorum), has no effect on growth rate. (D) Assays of growth performed in liquid media containing 1 mg/ml OHHL, or no HSL as indicated by the “-OHHL” suffix. Data were obtained from three biological replicates and error bars show standard errors. Note that growth is enhanced by deletion of yenR or cpmAJ.
In order to identify the gene(s) responsible for this growth suppression in vitro, we generated mutant strains of S. praecaptivus lacking genes identified in the transcriptomic analysis (Table S1) and screened for relief of growth suppression. This led to the discovery that cmpA (but not the adjacent cpmJ gene) is largely responsible for the growth suppression phenotype observed in this study (Figures 3C & 3D). Curiously, while cpmA and cpmJ share significant sequence identity with components of an antibiotic (carbepenem) biosynthesis pathway found in other Gram-negative bacteria (Coulthurst et al., 2005; Derzelle et al., 2002), the majority of genes in this pathway are absent in Sodalis spp, and their CpmA homologs lack a critical region of a β-lactam synthase, indicating that they cannot make this antibiotic. Conservation of an asparagine synthase domain in CpmA suggests that its function involves amidohydrolase activity (Miller et al., 2003), which has wide-ranging functions in nature. Notably, the transcriptomic data (Table S1) shows that cpmA is the 12th most abundant protein-coding transcript in the presence of OHHL and since cpmA has been retained under the control of QS in two insect symbionts, (Pontes et al., 2008), we conclude that it likely has an important function in symbiotic interactions.
QS controls a weevil killing phenotype
In order to examine the role of QS in vivo, we microinjected WT and mutant strains of S. praecaptivus into newly emerged adult grain weevils (S. zeamais), pre-treated to remove their native symbiont (SOPE), which is a very close relative of S. praecaptivus (Oakeson et al., 2014). The use of aposymbiotic weevils ensures that the results obtained in the current study only reflect the interaction between the weevil, S. praecaptivus and its endogenous QS system. In addition, symbiont-free weevils are considerably easier to microinject because Ca. S. pierantonius str. SOPE provides the weevil with nutrients (aromatic amino acids) that harden the cuticle (Vigneron et al., 2014). Following microinjection, weevil mobility and survival was monitored daily (Figure S1, Table S2). At one week following injection, weevils injected with the ΔypeI strain became lethargic (Figure S1) and died over the course of the next two weeks (Figure 4A). Weevils injected with the wild type strain suffered no lethargy or death aside from a small percentage of early deaths resulting from injuries sustained as a consequence of microinjection (<5%).
Figure 4.
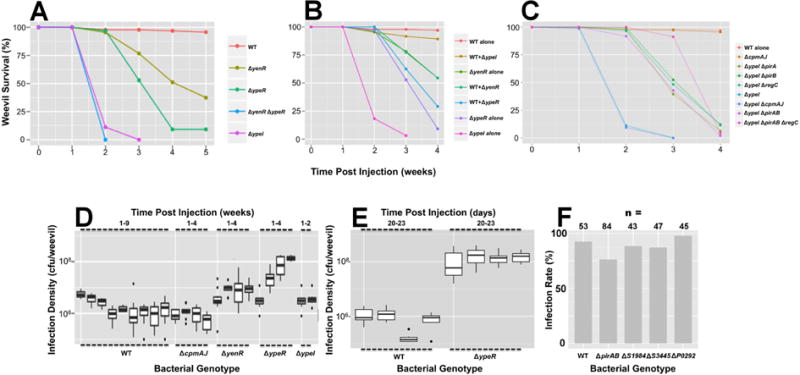
Weevil Survival Following Injection of WT and Mutant S. praecaptivus. (A) Inactivation of genes involved in the S. praecaptivus QS system results in weevil killing. Inactivation of both ypeR and yenR in a double mutant strain yields the most potent killing phenotype. (B) The WT strain complements the killing phenotype of the ΔypeI mutant strain.following co-injection. Note that the “+” symbol indicates injection of a 1:1 mixture of WT and respective mutant strain. (C) Identification of genes that mediate weevil killing. Inactivation of pirA, pirB and regC genes produce a significant delay in weevil killing in a ΔypeI genetic background, with PirAB and RegC having a synergistic effect. Data and results of statistical tests related to panels A–C are reported in Tables S2 & S3. (D & E) Bacterial infection densities are depicted as box plots for a range of different S. praecaptivus strains at various time intervals following microinjection. Note that the infection densities of the ΔyenR and ΔypeR strains increase over time relative to the WT and ΔcpmAJ strains (D), particularly in the latter stages of infection, concomitant with insect death (E). (F) Weevil infection rates observed 7 days after injection of a reduced number of bacterial cells (1:10 dilution of overnight culture). Statistical analysis of the data (likelihood ratio test) shows that the ΔpirAB strain has a reduced infection rate relative to the other strains tested (p = 0.0084).
Injection of the ΔypeR and ΔyenR mutant strains yielded a delayed/reduced killing effect relative to the ΔypeI strain and a ΔyenR ΔypeR double mutant killed as effectively and rapidly as the ΔypeI mutant (Figure 4A). Further confirmation of the role of QS in killing was obtained by injection with a 1:1 mixture of WT and mutant strains. WT bacteria suppressed the killing effect of the ΔypeI mutant, by providing a source of exogenous OHHL (Figure 4B). However, WT bacteria did not mitigate the killing of the ΔypeR or ΔyenR mutant strains because they lack the means to respond to OHHL. These results show that YpeR and YenR act synergistically to repress expression of genes involved in killing, with YpeR (ΔyenR) having a more potent effect. This contrasts with the in vitro results and suggests that the regulatory interactions between YenR, YpeR and Ypel function in a different way in vivo likely as a consequence of distinct physical conditions or the influence of additional regulatory elements. Nonetheless, the results clearly show that strains of S. praecaptivus lacking any of the three genic components of the QS system have a potent weevil killing phenotype that is suppressed in WT bacteria.
Identification of the genic effectors of killing
In order to identify the QS-regulated genes that are responsible for weevil killing, we identified differentially expressed genes that might play a role in killing based on their predicted functions. These genes (highlighted in yellow in Table S2) were then knocked out in a ΔypeI mutant background and the resulting double mutants were injected into weevils to assess their killing capability. None of the double mutants tested completely relieved their weevil killing phenotype. However, the ΔypeI ΔregC, ΔypeI ΔpirA and ΔypeI ΔpirB double mutant strains all demonstrated a statistically significant delay in killing (Figure 4C; Tables S2 & S3). Comparative analysis of ΔpirA, ΔpirB and ΔpirAB mutant strains yielded results that are consistent with the notion that PirA and PirB constitute a binary toxin complex, requiring both components for activity. The highest level of suppression was observed with a ΔypeI ΔpirAB ΔregC quadruple mutant, suggesting that RegC and PirAB function independently to impact killing. However, even this strain was not completely suppressed in terms of killing.
We also tested the effect of a ΔypeI ΔSant_1962 double mutant, because this protein shares high sequence identity with insecticidal endotoxins and is also differentially regulated by OHHL (adjusted p = 0.09; S1 Table). However, weevils injected with this strain showed no suppression of killing relative to the ΔypeI single mutant (Table S3). All other genes encoding putative toxins (Sant_0919, Sant_1053, Sant_2840) displayed no significant reduction in expression in the presence of OHHL in the transcriptomic analyses (Table S1). Thus, we conclude that there are other effectors of killing or combinatorial effects that have not been identified in our limited screen.
In order to examine the role of CpmA-mediated growth suppression in killing, we also injected weevils with ΔcpmAJ mutant strains. However, deletion of cpmAJ had no effect on killing either when the mutation was maintained alone or in a ΔypeI background (Figure 4C). Bacterial infection densities remained constant for the WT strain throughout the course of weevil infection whereas the ΔypeR and ΔyenR strains demonstrated a >10-fold and ~2-fold increase in infection density, respectively, consistent with their enhanced virulence properties (Figures 4D & 4E; Table S2). However, the ΔcpmAJ strain maintained lower numbers in the insect, especially at the onset of infection, consistent with the notion that CpmA has an adaptive role in vivo.
Loss of PirAB impairs the ability of S. yraecaytivus to infect weevils
To determine if the virulence factors identified in this study have an adaptive role during the initial stages of S. praecaptivus infection, we injected reduced numbers of WT and mutant strains of S. praecaptivus into weevils and screened for the presence of bacteria at 7 days post infection. Only the ΔpirAB mutant strain was found to have a statistically significant infection defect (Figure 4F). It should be noted however that virulence is clearly a polygenic trait in S. praecaptivus and many of the putative virulence factors identified in our transcriptomic screen have redundant functions (e.g. chitinases). In addition, microinjection is not a natural route of infection and might obviate the requirement for bacteria to utilize virulence factors to degrade the host integument.
Discussion
In this study, we show that S. praecaptivus maintains a QS system with complex regulatory dynamics that utilizes the same signaling molecule (OHHL) and is genetically orthologous to the system previously characterized in the tsetse fly symbiont, S. glossinidius (Pontes et al., 2008). However, transcriptomic analyses show that these bacteria differ markedly in the target genes that are regulated by QS. Indeed, the only component of QS-mediated regulation that is shared between S. praecaptivus and S. glossinidius involves upregulation of cpmA and cpmJ transcripts in response to OHHL. The work outlined here shows that CpmA mediates the suppression of S. praecaptivus growth in vitro in response to the QS signal, OHHL. This OHHL-mediated growth suppression is eliminated in mutant strains lacking cpmA, ypeI (encoding the OHHL synthase) or yenR (a QS response regulator).
The transcriptomic analyses in this study shows that the S. praecaptivus QS system has some unique properties that have been lost in S. glossinidius as a consequence of genome degeneration in the obligate insect-associated lifestyle (Clayton et al., 2012). Principal among these is the observation that QS reduces the expression of virulence factors that are anticipated to target insects, including insecticidal toxins and enzymes that degrade the insect integument. We compared the effects of injecting WT and QS-mutant strains of S. praecaptivus into a weevil host. This led to the striking finding that QS-deficient strains have a potent weevil killing phenotype. Thus it appears that the QS system has evolved to maintain a benign and long lasting infection in the weevil. Subsequent experiments revealed that killing is mediated by virulence factors rather than (CpmA-mediated) growth suppression.
The binary insecticidal Pir toxins were initially identified as key virulence factors in the insect pathogen Photorhabdus luminescens (Waterfield et al., 2005). They were subsequently found in other pathogenic bacteria including Vibrio parahaemolyticus, in which they play an important role in shrimp pathogenesis (Sirikharin et al., 2015). It was shown that the crystal structure of the V. parahaemolyticus Pir toxin complex resembles those of the Bacillus thuringiensis Cry toxins, suggesting that they may have a conserved mode of action that ultimately causes destabilization of the host cell cytoskeleton, leading to cell death (Lee et al., 2015). Another gene identified to impact weevil killing by S. praecaptivus (regC) shares substantial sequence and structural homology with a bacteriophage P2 transcriptional repressor. It may serve as a secondary transcriptional regulator in the QS circuit, modulating the transcription of additional gene(s) that enhance the killing phenotype that were not identified in our limited screen. This is supported by the observation that the deletion of pirAB and regC has an additive effect in suppressing the killing mediated by the QS-defective ΔypeI strain.
Since QS reduces the expression of genes (includingpirAB and regC) under conditions of high bacterial population density, it stands to reason that these genes should provide a beneficial function when their expression is elevated at low bacterial population density. Since infections must commence from low density, the functions of these virulence genes are expected to be important in mediating entry of host tissues and cells. Consist with this interpretation, the recently derived insect symbionts, S. glossinidius and Ca. S. pierantonius, have already lost many of the QS-regulated virulence genes, including pirAB. This is anticipated to occur as a consequence of the transition to permanent insect association, because endosymbionts do not have to undertake cyclical infection of hosts. Furthermore, our results show that a mutant strain of S. praecaptivus lacking PirAB is deficient in its ability to establish infection in weevils via microinjection.
Notably, the attenuation of virulence (towards the vector) is predicted to be an important adaptation for a vector-borne pathogen because virulence decreases host fitness and therefore reduces the likelihood of successful (vector-borne) pathogen dispersal (Elliot et al., 2003; Ewald, 1987). It is also one of the two criteria, along with a switch from horizontal to vertical transmission, predicted to be of key importance in the evolutionary transition from parasitism to mutualism (Ewald, 1987). To this end, the QS system in S. praecaptivus acts to enhance the ability of this bacterium to maintain a longstanding infection in an insect host. This mechanism of gene expression control mitigates one side of the double-edged sword of virulence by limiting the harm inflicted upon the host once a successful infection has been established. At one level, this distinguishes S. praecaptivus from a wide variety of pathogenic bacteria, including the entomopathogen, Bacillus thuringiensis (Dubois et al.. 2012), that utilize QS to enhance the expression of virulence factors at high infection densities, in order to facilitate a concerted and debilitating attack on the host (Winzer and Williams, 2001; von Bodman et al., 2003; Antunes et al., 2010; Rutherford and Bassler; 2012; Bassler and Papenfort, 2016). At another level, it presents an interesting parallel to the pathoadaptive microniche evolution observed in chronic cystic fibrosis lung infections involving Pseudomonas aeruginosa, in which mutations accumulate in regulatory systems and genes responsible for the production of virulence factors that facilitate acute infection (Smith et al., 2006). This has been proposed to be adaptive because these particular virulence factors are predicted to be major drivers of inflammatory responses (Smith et al., 2006). However, it is also conceivable that such adaptations arise simply as a consequence of relaxed selection on the utility of these virulence factors following initiation of infection (Winstanley et al., 2016). Of course, since multiple null mutations cannot readily be reverted, the resulting strains are expected to be attenuated in terms of their capability to initiate de novo acute infections. Thus, one advantage of utilizing QS to control the activity of virulence factors, as highlighted in our study, is that regulatory control can be maintained over recurrent cycles of infection, until a stable mutualistic association becomes established and the virulence genes are then inactivated and lost in the process of genome degeneration.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Colin Dale (colin.dale@utah.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Insects
Sitophilus zeamais weevils (derived from a colony maintained by USDA, Manhattan, KS, U.S.A) were reared on organic whole yellow maize (Purcell Mountain Farms) in an incubator at 27 °C and 60 % relative humidity. Symbiont-free (aposymbiotic) weevils were generated by rearing on rifampicin treated corn prepared by hydrating dried corn with a 3 % (w/v) solution of rifampicin (1 mg/ml), prior to addition of insects. After three generations on this antibiotic supplemented diet, loss of symbionts was confirmed by microscopic examination of 100 larvae (revealing absence of bacteriomes) and by negative PCR assays on whole weevil DNA using Taq Polymerase MasterMix (Thermo Fisher Scientific) using symbiont specific primers (1675 and 1676 in Table S4), with 35 cycles of PCR (95°C 15s, 60°C, 30s, 72°C 30s). Aposymbiotic insects were subsequently maintained on a normal diet and checked periodically to confirm the absence of bacteriomes. Newly emerged adult weevils were assigned to experimental treatments groups randomly with respect to sex.
Bacteria
All experiments in the manuscript were conducted using the type strain of S. praecaptivus described in Clayton et al. (2012) and deposited at the American Type Culture Collection (ATCC) as product BAA-2554. All genetic mutants of S. praecaptivus described in this study were derived from this strain and cultured in LB media with appropriate antibiotics, at 30°C, unless otherwise described. S. glossinidius was cultured in MM medium at 25°C. AHL bioassays were conducted using two bacterial reporter strains: A. tumefaciens strain, KYC55 (Zhu et al., 2003) (pJZ372)(pJZ384)(pJZ410), was cultured in AT medium at 30°C and Chromobacterium violaceum strain CV026 (McClean et al., 1997) was cultured on LB agar at 30°C.
METHOD DETAILS
Genetic Modification
Gene disruptions/deletions in S. praecaptivus were achieved using the lambda Red recombineering procedure, as described previously (Datsenko and Wanner, 2000). Constructs were obtained using three step PCR reactions, as follows: Approximately 150 nucleotides of the 5′ and the 3′ of target genes were amplified by PCR with Phusion High Fidelity DNA Polymerase (Thermo Fisher Scientific) using the oligonucleotides and annealing temperatures listed in Table S4. Identical PCR conditions were employed for all construction steps unless otherwise indicated. For example, to disrupt ypeI with a Spectinomycin resistance cassette (ΔypeI::Spc), primer pairs 232/233 and 234/235 were used. The PCR was performed for 35 cycles (98°C 5s, 62°C, 30s, 72°C 20s) in identical reactions with and without 5% DMSO. The resulting PCR products were then purified with Agencourt AMPure XP beads (Beckman Coulter). The 5′ and 3′ flanking sequences were joined to antibiotic resistance cassettes cassettes (KanR, SpcR, or GenR) using Taq Polymerase MasterMix (Thermo Fisher Scientific), using the oligonucleotides and annealing temperatures listed in Table S4, in two steps. The first joining step was performed with only the three dsDNA template fragments in 10 cycles of PCR (95°C 15s, 45°C, 30s, 72°C 60s) to facilitate hybridization. The second assembly step was performed with primer pairs as indicated in Table S4. For example, to create ΔypeI::Spc, primer pairs 232/235 were used in a 35 cycle reaction (95°C 15s, 58°C, 30s, 72°C 60s). The PCR products were again purified with Agencourt AMPure XP beads. The resulting dsDNA fragments were electroporated into an S. praecaptivus strain harboring the lambda Red-Gamm plasmid, pRed/Gamm (CAT). For transformation, S. praecaptivus cultures were grown in 25 ml 2YT5.8 media (20 g/l Tryptone, 8 g/l Yeast Extract, 10 g/l NaCl, adjusted to pH 5.8) until OD600 reached ~0.4 units. Expression of lambda Red-Gamm proteins was then induced by addition of 0.4 % arabinose for 30 minutes. Cells were harvested and washed twice in ice-cold sterile water. DNA was electroporated into cells using an Eppendorf electroporator model 2510, at 1600 V/s. Following a six-hour recovery in LB medium, recombinant clones were then selected by plating on LB agar with the appropriate antibiotic(s). Gene disruptions were verified by PCR with Taq Polymerase MasterMix (Thermo Fisher Scientific) with primers pairs and annealing temperatures listed in Table S4. For example ΔypeI::Spc was verified with primer pairs 236/138 and 237/139 in a 35 cycle reaction (95°C 15s, 58°C, 30s, 72°C 30s). The resulting PCR products were purified using Agencourt AMPure XP beads and sequenced using the Sanger method to confirm the integrity of junctions between the antibiotic resistance marker and the bacterial chromosome.
OHHL extraction and TLC assay
OHHL extraction was performed on cultures of S. glossinidius (grown in liquid MM medium) and S. praecaptivus (grown in LB medium) were pelleted by centrifugation (8,000g, 20 min., 4°C) once their turbidities reached an O.D600 of ~0.4 units. The resulting culture supernatants were filtered through 0.22 micron pore-size membrane filters (Argos Technologies) and then extracted by shaking with an equal volume of ethyl acetate for 30 minutes. The ethyl acetate extracts were dried with anhydrous magnesium sulfate and filtered. The solvent was then evaporated using a vacuum centrifuge at 60°C and the OHHL-containing residue was resuspended in 100 μl methanol. These extracts were then subjected to TLC on a C18 reverse-phase plate (Whatman) using methanol:water (60:40) as the solvent/carrier. Following development, the plate was dried and overlaid with a live culture of A. tumefaciens strain KYC55 (Zhu et al., 2003). For the C. violaceum AHL reporter assay, strain CV026 was streaked alongside S. praecaptivus on LB agar and incubated at 30 °C for 48 hours.
OHHL bioassays
The S. praecaptivus strains were cultured overnight in 4.5 ml LB liquid media. Bacteria from these cultures were pelleted, washed twice in 25 ml LB and resuspended in 20 ml fresh media to yield OD600 of 0.025 units. During a 12 hour incubation with shaking at 200 rpm and 30 °C, the cultures were sampled every two hours to determine cell densities and to assay for the presence of OHHL with the β-galactosidase activity assay using an A. tumefaciens acyl-HSL reporter strain, as described previously (Zhu et al., 2003). Specifically, the culture was cleared of cells by centrifugation at 16,000 × g for 2 min. 20 μl of 10-fold serial dilutions of the supernatant were added to 180 μl of the preinduced reporter strain and incubated at 30°C for 16 h without shaking. The β-galactosidase assays were performed on 100 μl of the reporter cell cultures. To estimate the concentration of OHHL in WT S. praecaptivus culture media at OD600 of 0.4, OHHL extraction was performed from total culture supernatant into 200 μl of methanol, which was then used as substrate in the A. tumefaciens β-galactosidase assay. Solutions of 50, 500 and 1250 nM OHHL (Sigma Aldrich, K3255) were prepared in methanol for use as standards. The concentration of OHHL in the OD600 = 0.4 culture was determined to be ~1 μg OHHL/ml.
Transcriptomics
For transcriptomics, triplicate (biological replicate) 20 ml cultures of S. praecaptivus were grown in LB media, at 200 rpm shaking and 30 °C, with or without supplementation with OHHL (10 μg/ml). Total nucleic acid was extracted from each culture using Triton X-100, following four hours of exposure (or lack of exposure) to OHHL for the comparison involving the ΔypeI strain, or after five hours of growth for the comparisons involving the WT strain (supplemented with OHHL) and the ΔypeR and ΔyenR strains. RNA was then purified using the Purelink RNA minikit (Ambion). The resulting RNA was then analyzed using an Agilent bioanalyzer to ensure that it lacked DNA contamination and was of sufficient quality for cDNA preparation. Sequencing libraries were prepared using the Illumina TruSeq method and sequencing on a HiSeq instrument (Illumina). The resulting quality fitered 50-base single reads were mapped to the reference genome using bowtie2 (Langmead and Salzberg, 2012) employing the –very-sensitive preset. Read counts mapping to individual genes were generating using HTSeq (Anders et al., 2014) by running htseq-count using the options -s no -a 0 -m intersection-nonempty -t gene. Next, using custom Perl scripts, counts from three biological replicate cultures were combined and subject to statistical analysis. After normalization, the combined counts files were compared using DESeq2 (Love et al., 2014).
Chitinase assay
Chitinase activities in the S. praecaptivus culture supernatants were measured using a fluorimetric assay (Sigma-Aldrich, CS1030) according to the manufacturer’s instructions. Cells were grown in LB media (15 ml volume), from a starting OD600 of 0.05, with 200 rpm shaking at 30 °C until O.D 600 reached ~1 unit. 10 μl of culture supernatant was added to 90 μl of the substrate working solution (containing 4-methylumbelliferyl β-D-N, N-diacetylchitobioside hydrate) in a 96-well fluorescence plate (Greiner Bio-One, 655096) and incubated for 60 min at 37 °C in the dark. The reaction was stopped by adding 200 μl of sodium carbonate buffer, and the fluorescence was measured with a SynergyMx plate reader (BioTek Instruments Inc.) within 15 minutes of the reaction end point. The chitinase activity was calculated using a 4-methyl umbelliferone standard.
Growth suppression assays
Plate growth assays were performed by spotting appropriate dilutions of overnight cultures on LB agar plates (lacking NaCl to inhibit swarming motility). OHHL was dissolved in methanol and added to sterile paper strips or pipetted directly onto plates. One recombinant S. praecaptivus strain, maintaining a pCM66 plasmid expressing ypeI in a ΔyenR background, overproduces OHHL without undergoing growth suppression and was also used as a source of OHHL by streaking it onto plates. A control strain was also utilized that maintains an empty pCM66 plasmid in a ΔypeI background and therefore does not produce any OHHL. All plates were maintained at 30 °C and observed for growth at daily intervals. Liquid assays were performed by maintaining triplicate cultures of WT and mutant strains of S. praecaptivus in LB media at 30°C with shaking at 200 rpm in the presence of 1 mg/ml OHHL and recording turbidity (OD600nm) at hourly intervals.
Weevil microinjection
Newly emerged adult aposymbiotic weevils were microinjected with capillary tubes (3.5′ Drummond # 3-000-203-G/X, Drummond Scientific Company) that were pulled to create a short sharp tip (Bee-Stinger needle) with Micropipette puller P-97 (Sutter Instruments). The settings on the micropipette puller were as follows: Heat=292, Pull=100, Velocity=60, Time=250, Pressure=500. Weevils were immobilized by a brief incubation on ice and the needle was immersed in 50 μl of overnight bacterial culture to collect bacteria by capillary action. The needle was then used to pierce the weevil in the thoracic region between the middle and hind legs. This resulted in a median 2800 bacterial cells being transferred into each weevil, as determined by plating weevil homogenates directly after injection. Following injection, weevils were then transferred to hydrated maize grains and maintained in an incubator at 27 °C and 60 % relative humidity.
Weevil motility assay
Individual weevils were placed in the center of an empty petri dish and the time taken for them to move from the center of the dish to the periphery was recorded. Weevils that displayed no sign of movement during the assay were excluded.
Bacterial isolation from weevils
Surface contaminants were removed from weevils by immersing the adult insects in a solution of 10 % bleach for 5 minutes with agitation. Weevils were then air dried and homogenized in 100 μl of sterile water. A bacterial dilution series was plated on LB agar (without NaCl) containing 50 μg/ml polymyxin B to select for S. praecaptivus.
QUANTIFICATION AND STATISTICAL ANALYSIS
Transcriptomics
RNA-Seq experiments were carried out in triplicate using independent bacterial cultures. Complete datasets derived from DESeq2-based analysis of RNAseq data are presented in Table S1.
Statistical analyses
All statistical analyses were performed using the R software package. Data from chitinase assays were analyzed by ordinary one-way ANOVA comparisons. The numbers of insects used in the weevil microinjection experiments are reported in Table S2, along with their median lifespan. Pairwise log-rank tests were performed to compare lifespan of weevils injected with different strains of S. praecaptivus using the data summarized in Table S2. The results of these statistical analyses are presented in Table S3. The box and whisker plots presented in Figure 4 (panels D and E) were obtained using the geom_boxplot function in R using the ggplot2 package. Statistical analysis of data relating to weevil infection at 7 days post microinjection (Figure 4F) was performed using a likelihood ratio test under the log-linear model in R. The numbers of insects sampled for this analysis are presented at the top of the figure panel. The numbers of insects sampled for bacterial quantification (Figure 4D; for data presented from left to right) were 10, 10, 10, 10, 10, 10, 10, 10, 12, 10, 10, 10, 12, 12, 12, 12, 9, 10, 10, 10, 2, 10, 9. For Figure 4E (left to right) the numbers were 8, 8, 8, 8, 7, 6, 8, 5.
DATA AND SOFTWARE AVAILABILITY
Sequence reads relating to the transcriptomics experiments in the study can be obtained at the NCBI Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE97720.
Supplementary Material
Table S1, related to Figure 2. Data from DESeq2 analysis of the transcriptomes of several S. praecaptivus strains, ranked according to log2fold change in transcript numbers. Note that the spreadsheet contains three pages detailing the results of three separate comparisons each involving three biological replicates. On page 1, the columns containing count data are labeled ct_ypeI+OHHL1-3 (for cultures with OHHL) or ct_ypeI-OHHL1-3 (for cultures lacking OHHL), for the comparisons involving the (OHHL-induced and non-induced) ΔypeI mutant strain. On page 2, the columns containing count data are labeled ct_WT1-3 or ct_ypeR1-3 for the comparisons involving the WT and ΔypeR mutant strains. On page 3, the columns containing count data are labeled ct_WT1-3 or ct_yenR1-3 for the comparisons involving the WT and ΔyenR mutant strains.
Table S2, related to Figure 4. Data from weevil infection experiments involving S. praecaptivus.
Table S3, related to Figure 4. Data obtained from pairwise log-rank tests on weevil survival.
Table S4, related to Key Resources Table. PCR oligonucleotides, uses and annealing temperatures.
Figure S1, related to Figure 4. Weevil Movement Following Injection of S. praecaptivus WT and ΔypeI Strains. Histograms depict the average time taken by a group of weevils to complete a movement assay, on days 9, 10, 11 and 12 following injection with WT and ΔypeI strains of S. praecaptivus. Any weevils that did not complete the movement assay within the total observation time of 100 seconds were binned into the >95 second category.
Highlights.
Quorum sensing negatively regulates virulence in Sodalis praecaptivus.
Mutant strains defective in quorum sensing have an insect killing phenotype.
Killing occurs as a result of the production of insecticidal toxins.
Limiting bacterial toxin production may facilitate a stable and benign infection.
Acknowledgments
This work was supported by the National Institutes of Health (grant number 1R01AI095736 awarded to CD.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
S.E. and C.D. designed experiments. S.E., A.L.C and A.C. performed experiments. C.D., A.C. and S.E. wrote the paper.
References
- Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2014:btu638. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes LC, Ferreira RB, Buckner MM, Finlay BB. Quorum sensing in bacterial virulence. Microbiology. 2010;156:2271–2282. doi: 10.1099/mic.0.038794-0. [DOI] [PubMed] [Google Scholar]
- Bassler BL, Papenfort K. Quorum sensing signal–response systems in Gram-negative bacteria. Nat Rev Microbiol. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bodman SB, Bauer WD, Coplin DL. Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol. 2003;41:455–482. doi: 10.1146/annurev.phyto.41.052002.095652. [DOI] [PubMed] [Google Scholar]
- Clayton AL, Oakeson KF, Gutin M, Pontes A, Dunn DM, von Niederhausern AC, Weiss RB, Fisher M, Dale C. A novel human-infection-derived bacterium provides insights into the evolutionary origins of mutualistic insect-bacterial symbioses. PLoS Genet. 2012;8:e1002990. doi: 10.1371/journal.pgen.1002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulthurst SJ, Barnard AM, Salmond GP. Regulation and biosynthesis of carbapenem antibiotics in bacteria. Nat Rev Microbiol. 2005;3:295–306. doi: 10.1038/nrmicro1128. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derzelle S, Duchaud E, Kunst F, Danchin A, Bertin P. Identification, characterization, and regulation of a cluster of genes involved in carbapenem biosynthesis in Photorhabdus luminescens. Appl Environ Microbiol. 2002;68:3780–3789. doi: 10.1128/AEM.68.8.3780-3789.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AE. The microbial dimension in insect nutritional ecology. Funct Ecol. 2009;23:38–47. [Google Scholar]
- Douglas AE. Symbiosis as a general principle in eukaryotic evolution. Cold Spring Harb Perspect Biol. 2014;6:a016113. doi: 10.1101/cshperspect.a016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois T, Faegri K, Perchat S, Lemy C, Buisson C, Nielsen-LeRoux C, Gohar M, Jacques P, Ramaroa N, Kolstø A, Lereclus D. Necrotrophism Is a Quorum-Sensing-Regulated Lifestyle in Bacillus thuringiensis. PLoS Pathogens. 2012;8:e1002629. doi: 10.1371/journal.ppat.1002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot SL, Adler FR, Sabelis MW. How virulent should a parasite be to its vector? Ecology. 2003;84:2568–2574. [Google Scholar]
- Ewald PW. Transmission Modes and Evolution of the Parasitism-Mutualism Continuum. Ann N Y Acad Sci. 1987;503:295–306. doi: 10.1111/j.1749-6632.1987.tb40616.x. [DOI] [PubMed] [Google Scholar]
- Fuqua C, Parsek MR, Greenberg EP. Regulation of Gene Expression by Cell-to-Cell Communication: Acyl-Homoserine Lactone Quorum Sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- Husnik F, McCutcheon JP. Repeated replacement of an intrabacterial symbiont in the tripartite nested mealybug symbiosis. Proc Natl Acad Sci U S A. 2016;113:E5416–5424. doi: 10.1073/pnas.1603910113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiwa N, Hosokawa T, Kikuchi Y, Nikoh N, Meng XY, Kimura N, Ito M, Fukatsu T. Primary gut symbiont and secondary, Sodalis-allied symbiont of the Scutellerid stinkbug Cantao ocellatus. Appl Environ Microbiol. 2010;76:3486–3494. doi: 10.1128/AEM.00421-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Chen IT, Yang YT, Ko TP, Huang YT, Huang JY, Huang MF, Lin SJ, Chen CY, Lin SS, et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc Natl Acad Sci U S A. 2015;112:10798–10803. doi: 10.1073/pnas.1503129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre C, Charles H, Vallier A, Delobel B, Farrell B, Heddi A. Endosymbiont phylogenesis in the Dryophthoridae weevils: evidence for bacterial replacement. Mol Biol Evol. 2004;21:965–973. doi: 10.1093/molbev/msh063. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:1. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiol. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- Miller MT, Gerratana B, Stapon A, Townsend CA, Rosenzweig AC. Crystal structure of carbapenam synthetase (CarA) J Biol Chem. 2003;278:40996–41002. doi: 10.1074/jbc.M307901200. [DOI] [PubMed] [Google Scholar]
- Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- Nadarasah G, Stavrinides J. Insects as alternative hosts for phytopathogenic bacteria. FEMS Microbiol Rev. 2011;35:555–575. doi: 10.1111/j.1574-6976.2011.00264.x. [DOI] [PubMed] [Google Scholar]
- Oakeson KF, Gil R, Clayton AL, Dunn DM, von Niederhausern AC, Hamil C, Aoyagi A, Duval B, Baca A, Silva FJ, et al. Genome Degeneration and Adaptation in a Nascent Stage of Symbiosis. Genome Biol Evol. 2014;6:76–93. doi: 10.1093/gbe/evt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Degnan PH, Burke GR, Moran NA. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol. 2010;55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- Pontes MH, Babst M, Lochhead R, Oakeson K, Smith K, Dale C. Quorum Sensing Primes the Oxidative Stress Response in the Insect Endosymbiont, Sodalis glossinidius. PLoS One. 2008;3:e3541. doi: 10.1371/journal.pone.0003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs JL, Skophammer RG, Bansal N, Stajich JE. Evolutionary origins and diversification of proteobacterial mutualists. Proc Biol Sci. 2013 doi: 10.1098/rspb.2013.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirikharin R, Taengchaiyaphum S, Sanguanrut P, Chi TD, Mavichak R, Proespraiwong P, Nuangsaeng B, Thitamadee S, Flegel TW, Sritunyalucksana K. Characterization and PCR Detection Of Binary, Pir-Like Toxins from Vibrio parahaemolyticus Isolates that Cause Acute Hepatopancreatic Necrosis Disease (AHPND) in Shrimp. PLoS ONE. 2015;10:e0126987. doi: 10.1371/journal.pone.0126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AK, McMillen CM, Wallenhorst P, Rio RV. The phylogeny of Sodalis-like symbionts as reconstructed using surface-encoding loci. FEMS Microbiol Lett. 2011;317:143–151. doi: 10.1111/j.1574-6968.2011.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toju H, Fukatsu T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: relevance of local climate and host plants. Mol Ecol. 2011;20:853–868. doi: 10.1111/j.1365-294X.2010.04980.x. [DOI] [PubMed] [Google Scholar]
- Tsai TS, Winans SC. LuxR-type quorum-sensing regulators that are detached from common scents. Mol Microbiol. 2010;77:1072–1082. doi: 10.1111/j.1365-2958.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet-Gely I, Lemaitre B, Boccard F. Bacterial strategies to overcome insect defences. Nat Rev Microbiol. 2008;6:302–313. doi: 10.1038/nrmicro1870. [DOI] [PubMed] [Google Scholar]
- Vigneron A, Masson F, Vallier A, Balmand S, Rey M, Vincent-Monégat C, Aksoy E, Aubailly-Giraud E, Zaidman-Rémy A, Heddi A. Insects recycle endosymbionts when the benefit is over. Curr Biol. 2014;24:2267–2273. doi: 10.1016/j.cub.2014.07.065. [DOI] [PubMed] [Google Scholar]
- Waterfield N, Kamita SG, Hammock BD, Ffrench-Constant R. The Photorhabdus Pir toxins are similar to a developmentally regulated insect protein but show no juvenile hormone esterase activity. FEMS Microbiol Lett. 2005;245:47–52. doi: 10.1016/j.femsle.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. QUORUM SENSING: Cell-to-Cell Communication in Bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Winstanley C, O’Brien S, Brockhurst MA. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 2016;24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer K, Williams P. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int J Med Microbiol. 2001;291:131–143. doi: 10.1078/1438-4221-00110. [DOI] [PubMed] [Google Scholar]
- Zhu J, Chai Y, Zhong Z, Li S, Winans SC. Agrobacterium Bioassay Strain for Ultrasensitive Detection of N-Acylhomoserine Lactone-Type Quorum-Sensing Molecules: Detection of Autoinducers in Mesorhizobium huakuii. Appl Environ Microbiol. 2003;69:6949–6953. doi: 10.1128/AEM.69.11.6949-6953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1, related to Figure 2. Data from DESeq2 analysis of the transcriptomes of several S. praecaptivus strains, ranked according to log2fold change in transcript numbers. Note that the spreadsheet contains three pages detailing the results of three separate comparisons each involving three biological replicates. On page 1, the columns containing count data are labeled ct_ypeI+OHHL1-3 (for cultures with OHHL) or ct_ypeI-OHHL1-3 (for cultures lacking OHHL), for the comparisons involving the (OHHL-induced and non-induced) ΔypeI mutant strain. On page 2, the columns containing count data are labeled ct_WT1-3 or ct_ypeR1-3 for the comparisons involving the WT and ΔypeR mutant strains. On page 3, the columns containing count data are labeled ct_WT1-3 or ct_yenR1-3 for the comparisons involving the WT and ΔyenR mutant strains.
Table S2, related to Figure 4. Data from weevil infection experiments involving S. praecaptivus.
Table S3, related to Figure 4. Data obtained from pairwise log-rank tests on weevil survival.
Table S4, related to Key Resources Table. PCR oligonucleotides, uses and annealing temperatures.
Figure S1, related to Figure 4. Weevil Movement Following Injection of S. praecaptivus WT and ΔypeI Strains. Histograms depict the average time taken by a group of weevils to complete a movement assay, on days 9, 10, 11 and 12 following injection with WT and ΔypeI strains of S. praecaptivus. Any weevils that did not complete the movement assay within the total observation time of 100 seconds were binned into the >95 second category.


