Abstract
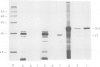
Gene 4 from the T-region of Ti plasmids is responsible for cytokinin effects in crown gall cells; we investigated whether it codes for an enzyme of hormone biosynthesis. In a first set of experiments, gene 4 from octopine plasmid pTiAch5 and nopaline plasmid pTiC58 was expressed in Escherichia coli, and the gene products were identified by reaction with antiserum raised against a decapeptide derived from the DNA sequence of the gene. Extracts from cells expressing the gene contained high isopentenyl-transferase activity catalyzing the formation of N6-(△2-isopentenyl)adenosine from 5'-AMP and △2-isopentenylpyrophosphate. The cytokinin was identified by sequential h.p.l.c. chromatography and mass spectrometry. In a second set of experiments it was shown that crown gall cells contained isopentenyltransferase activity and a protein of mol. wt. 27 000 which was identified as the product of gene 4 by reaction with the antiserum. Isopentenyltransferase activity was specifically inhibited by the antiserum. No comparable enzyme activity or immunoreactive protein was detected in cytokinin-autotrophic, T-DNA free tobacco cells. The results establish that gene 4 from the T-region of octopine and nopaline Ti plasmids codes for an enzyme of cytokinin biosynthesis.
Keywords: cytokinin biosynthesis, isopentenyltransferase, peptide antiserum, crown gall, Ti plasmids
Full text
PDF
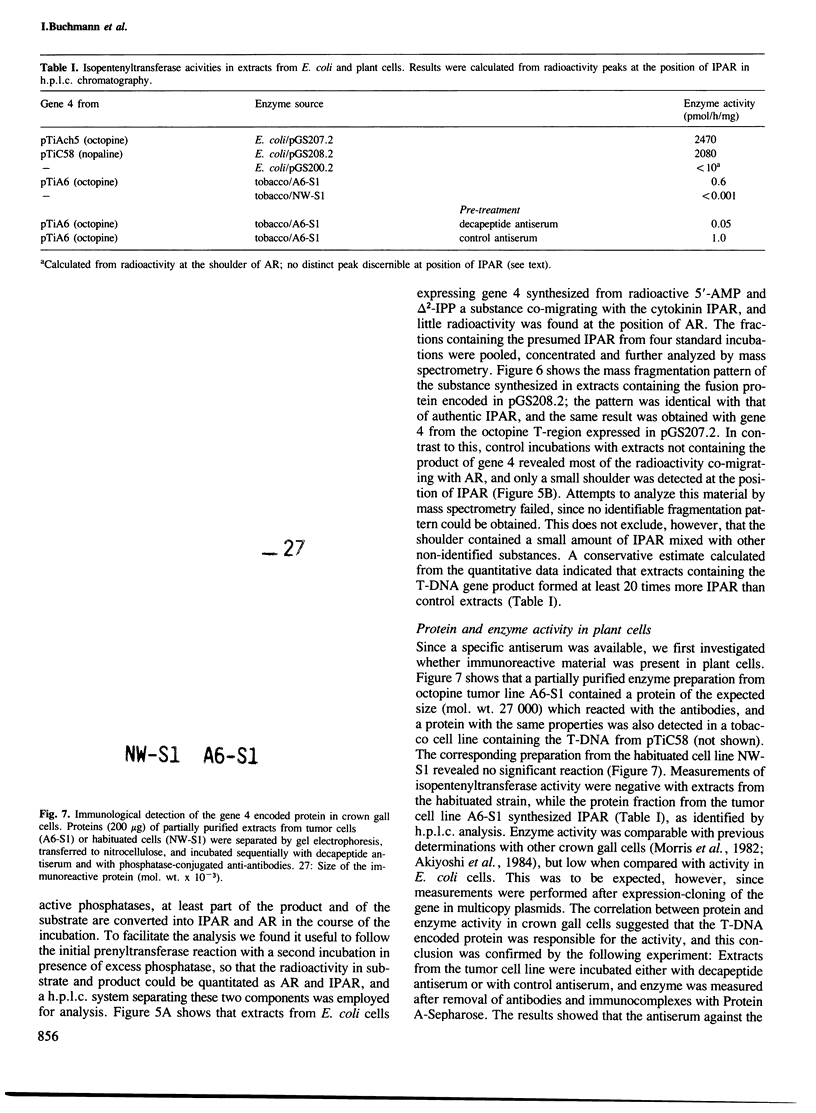



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Klee H., Amasino R. M., Nester E. W., Gordon M. P. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi D. E., Morris R. O., Hinz R., Mischke B. S., Kosuge T., Garfinkel D. J., Gordon M. P., Nester E. W. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci U S A. 1983 Jan;80(2):407–411. doi: 10.1073/pnas.80.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Barry G. F., Rogers S. G., Fraley R. T., Brand L. Identification of a cloned cytokinin biosynthetic gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4776–4780. doi: 10.1073/pnas.81.15.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Melitz D. K. Cytokinin biosynthesis in a cell-free system from cytokinin-autotrophic tobacco tissue cultures. FEBS Lett. 1979 Nov 1;107(1):15–20. doi: 10.1016/0014-5793(79)80452-4. [DOI] [PubMed] [Google Scholar]
- Engler G., Depicker A., Maenhaut R., Villarroel R., Van Montagu M., Schell J. Physical mapping of DNA base sequence homologies between an octopine and a nopaline Ti plasmid of Agrobacterium tumefaciens. J Mol Biol. 1981 Oct 25;152(2):183–208. doi: 10.1016/0022-2836(81)90239-4. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Gielen J., De Beuckeleer M., Seurinck J., Deboeck F., De Greve H., Lemmers M., Van Montagu M., Schell J. The complete nucleotide sequence of the TL-DNA of the Agrobacterium tumefaciens plasmid pTiAch5. EMBO J. 1984 Apr;3(4):835–846. doi: 10.1002/j.1460-2075.1984.tb01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. B., Flick J. S., Rogers S. G. Nucleotide sequence of the tmr locus of Agrobacterium tumefaciens pTi T37 T-DNA. Nucleic Acids Res. 1984 Jun 11;12(11):4665–4677. doi: 10.1093/nar/12.11.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidekamp F., Dirkse W. G., Hille J., van Ormondt H. Nucleotide sequence of the Agrobacterium tumefaciens octopine Ti plasmid-encoded tmr gene. Nucleic Acids Res. 1983 Sep 24;11(18):6211–6223. doi: 10.1093/nar/11.18.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos H., Inzé D., Caplan A., Sormann M., Van Montagu M., Schell J. Genetic analysis of T-DNA transcripts in nopaline crown galls. Cell. 1983 Apr;32(4):1057–1067. doi: 10.1016/0092-8674(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L. Visualization of antigenic proteins on Western blots. Anal Biochem. 1984 Jan;136(1):180–184. doi: 10.1016/0003-2697(84)90321-x. [DOI] [PubMed] [Google Scholar]
- Leemans J., Deblaere R., Willmitzer L., De Greve H., Hernalsteens J. P., Van Montagu M., Schell J. Genetic Identification of functions of TL-DNA transcripts in octopine crown galls. EMBO J. 1982;1(1):147–152. doi: 10.1002/j.1460-2075.1982.tb01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein C., Klee H., Montoya A., Garfinkel D., Fuller S., Flores C., Nester E., Gordon M. Nucleotide sequence and transcript mapping of the tmr gene of the pTiA6NC octopine Ti-plasmid: a bacterial gene involved in plant tumorigenesis. J Mol Appl Genet. 1984;2(4):354–362. [PubMed] [Google Scholar]
- Ream L. W., Gordon M. P., Nester E. W. Multiple mutations in the T region of the Agrobacterium tumefaciens tumor-inducing plasmid. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1660–1664. doi: 10.1073/pnas.80.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum N., Gefter M. L. Delta 2 -isopentenylpyrophosphate: transfer ribonucleic acid 2 -isopentenyltransferase from Escherichia coli. Purification and properties of the enzyme. J Biol Chem. 1972 Sep 25;247(18):5675–5680. [PubMed] [Google Scholar]
- Schaefer K. P., Altman S., Söll D. Nucleotide modification in vitro of the precursor of transfer RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3626–3630. doi: 10.1073/pnas.70.12.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder G., Klipp W., Hillebrand A., Ehring R., Koncz C., Schröder J. The conserved part of the T-region in Ti-plasmids expresses four proteins in bacteria. EMBO J. 1983;2(3):403–409. doi: 10.1002/j.1460-2075.1983.tb01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder G., Waffenschmidt S., Weiler E. W., Schröder J. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem. 1984 Jan 16;138(2):387–391. doi: 10.1111/j.1432-1033.1984.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Packman L. C., Burleigh B. D., Dell A., Morris H. R., Hartley B. S. Primary structure of a chloramphenicol acetyltransferase specified by R plasmids. Nature. 1979 Dec 20;282(5741):870–872. doi: 10.1038/282870a0. [DOI] [PubMed] [Google Scholar]
- Taya Y., Tanaka Y., Nishimura S. 5'-AMP is a direct precursor of cytokinin in Dictyostelium discoideum. Nature. 1978 Feb 9;271(5645):545–547. doi: 10.1038/271545a0. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Reeves S., Thomashow M. F. Crown gall oncogenesis: evidence that a T-DNA gene from the Agrobacterium Ti plasmid pTiA6 encodes an enzyme that catalyzes synthesis of indoleacetic acid. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5071–5075. doi: 10.1073/pnas.81.16.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Scheidtmann K. H., Carbone A., Laudano A. P., Doolittle R. F. Antibodies specific for the carboxy- and amino-terminal regions of simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5197–5200. doi: 10.1073/pnas.77.9.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]