Abstract
Giant cell myocarditis (GCM), a rapidly progressive inflammation of the myocardium, is associated with fulminant heart failure, refractory ventricular arrhythmias, and conduction system abnormalities. Few case reports have noted orbital myositis as the initial clinical presentation. Our case demonstrates a unique presentation of GCM with only ocular symptoms, which unlike prior studies, rapidly progressed to heart failure, tachyarrhythmias, and conduction disease. Our case necessitated quick recognition and treatment with mechanical support making this the first known case of GCM with successful placement of biventricular assist devices and ultimately with heart transplantation.
Keywords: Giant cell myocarditis, Orbital myositis, Endomyocardial biopsy, Impella device, HeartWare ventricular assist device
Introduction
Giant cell myocarditis (GCM) is a rare and rapidly progressive disorder with inflammation of the myocardium often associated with fulminant heart failure, refractory ventricular arrhythmias, conduction system abnormalities, and/or angina‐like symptoms. While this immune‐mediated disease generally carries a poor prognosis, early recognition and diagnosis with endomyocardial biopsy, aggressive treatment with immunosuppressive therapy, and timely involvement of heart failure teams for evaluation of transplant or mechanical assist devices can improve outcomes. Roughly, 20% of cases are associated with systemic autoimmune disease, but only rare case reports1, 2, 3, 4 have noted orbital myositis as the initial clinical presentation. Our case demonstrates a unique presentation of GCM with only ocular symptoms that rapidly progressed to heart failure, tachyarrhythmias, and conduction disease. Prompt recognition allowed early intervention with immunosuppression, placement of biventricular assist devices, and eventual successful heart transplantation.
Case report
A 50‐year‐old man with well‐controlled rheumatoid arthritis on Leflunomide presented to our hospital with sudden onset right‐sided diplopia and 2 weeks of progressive ophthalmoplegia and ptosis. MRI of the orbits showed multifocal extraocular muscle hypertrophy suggestive of orbital inflammation (Figure 1). Presenting vitals were significant for tachycardia (heart rate 110 bpm) and relative hypotension (98/59). His troponin was elevated to 0.68 ng/mL on admission. Initial ECG showed sinus tachycardia with RSR’ pattern in lead V1, inferior Q waves with sub‐millimetre ST‐segment elevation (Figure 2). Echocardiogram showed a non‐dilated left ventricle, reduced left ventricular ejection fraction of 30–35% with global hypokinesis, and concentric left ventricular hypertrophy (Figure 3). Given the lack of significant cardiovascular symptoms, the initial thought was demand‐related troponin elevation; thus, noninvasive risk stratification was obtained. Pharmacologic stress myoview demonstrated no scintigraphic evidence of infarct or stress‐induced ischemia. On hospital day 2, plasmapharesis was initiated for the presumed autoimmune orbital symptoms. On hospital day 3, he complained of diaphoresis and chest heaviness and was found to have high‐grade atrioventricular block with 6 s ventricular pauses. He developed syncope with an asystolic rhythm for 20 s requiring a brief episode of chest compressions with quick recovery of spontaneous circulation. He underwent emergent placement of a transvenous pacing wire. Cardiac catheterization was notable for no significant coronary artery disease; however, because of low cardiac output, mechanical circulatory support with an Impella device was instituted, which resulted in hemodynamic normalization. Intravenous steroids were initiated for a likely inflammatory process, and on day 5, he developed ventricular tachycardia (heart rate 250 bpm) requiring cardioversion and antiarrhythmic therapy. Endomyocardial biopsy was performed that demonstrated numerous nucleated giant cells within an inflammatory infiltrate consistent with GCM (Figure 4). Immunosuppressive therapy including steroids (prednisone 60 mg daily, mycophenolate mofetil (1 g twice daily), and tacrolimus (0.5 g twice daily) were started. The Impella was weaned on day 10; however, the patient continued to require inotropic support. Serial echocardiography showed worsening biventricular systolic function, which prompted elective placement of HeartWare HVAD (HeartWare ventricular assist device) biventricular assist devices on hospital day 23 (Figure 5). Six days later, he was listed for heart transplant and by day 31, he underwent successful heart transplantation. He remained on standard post‐transplant immunosuppression with no evidence of recurrence in the newly transplanted heart and gradual improvement of his ocular symptoms although still with residual deficits at 3 month follow‐up.
Figure 1.
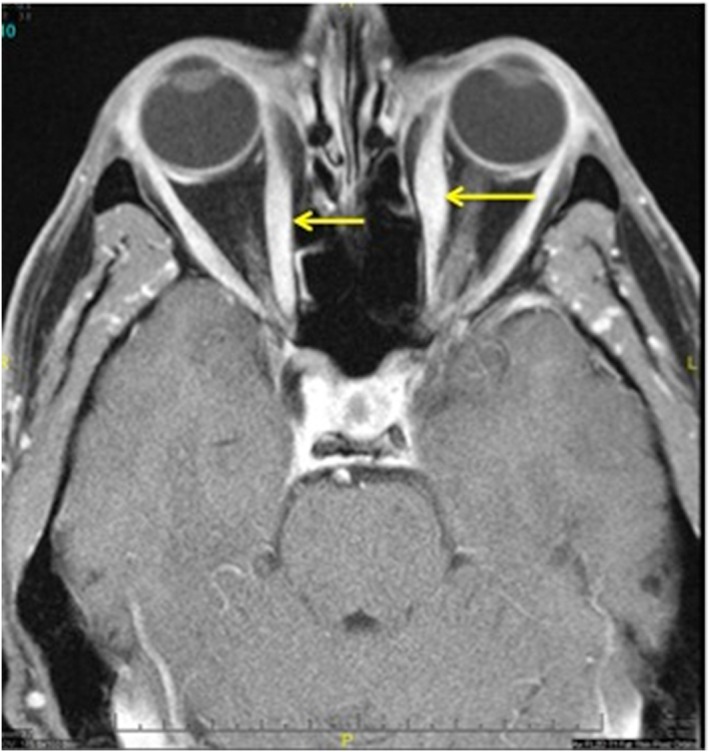
MRI of orbits showing multifocal extraocular muscle enlargement (arrows) and signal abnormality suggestive of orbital inflammatory disease.
Figure 2.
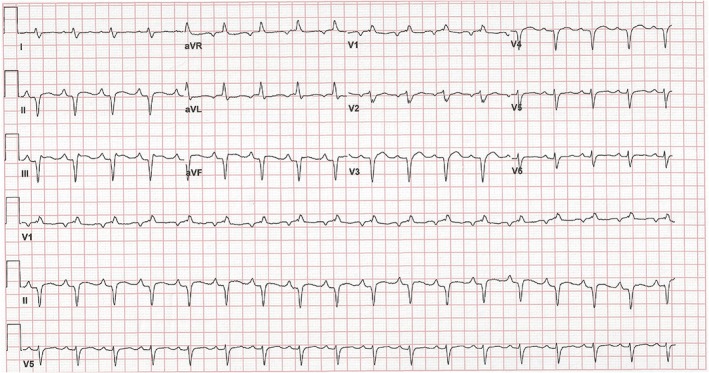
Electrocardiogram showing sinus vs. atrial tachycardia with an incomplete right bundle branch block. There are q waves in the inferior and anterior leads suggestive of infarction.
Figure 3.
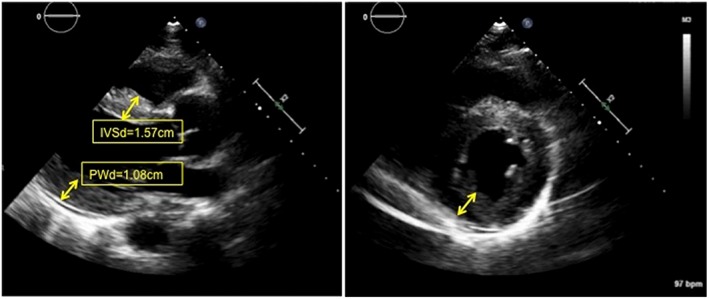
Two‐dimensional echocardiography in the parasternal long (left) and parasternal short (right) axes showing thickened myocardium (arrows). Cine images (see [Link], [Link]) demonstrate globally reduced ejection fraction around 30–35% without significant chamber enlargement. IVSd, interventricular septum at diastole; PWd, posterior wall at diastole.
Figure 4.
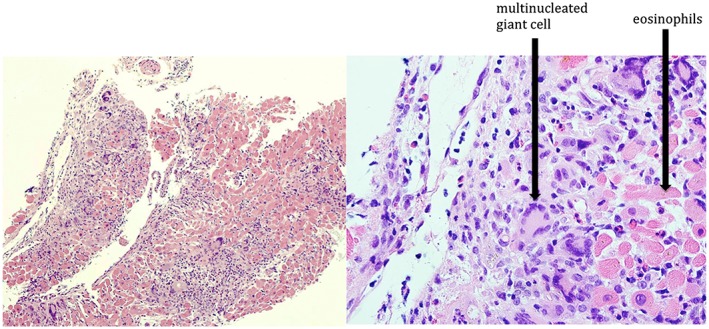
Endomyocardial biopsy demonstrating inflammatory infiltration of cardiac myocardium with a haematoxylin and eosin stain (left). Magnified view (right) demonstrating numerous giant cells within inflammatory infiltrate consisting of lymphocytes, histiocytes, and eosinophils.
Figure 5.
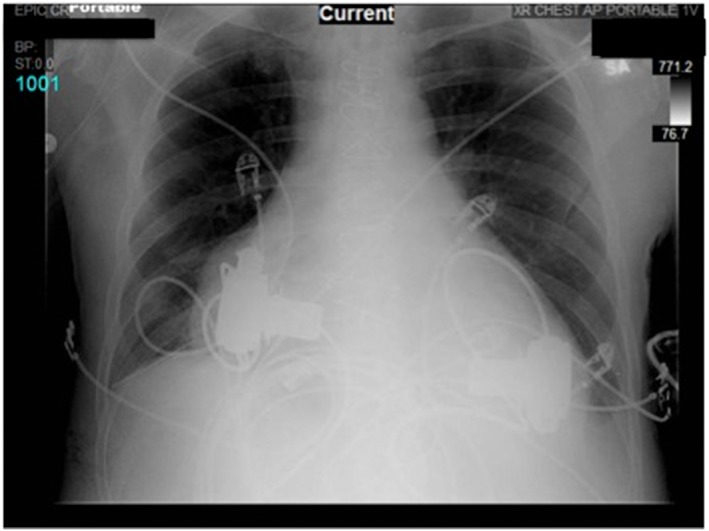
Portable chest X‐ray showing biventricular HeartWare ventricular assist devices.
Discussion
Giant cell myocarditis presents with fulminant heart failure (41–75%), refractory ventricular arrhythmias (14–17%), angina‐like symptoms (6–9%), and/or conduction system abnormalities (5–28%).5, 6 While the exact pathogenesis is unknown, CD‐4 T lymphocyte‐mediated inflammation of the myocardium is suggested.5, 7 There is a 20% incidence of concomitant autoimmune disorder, but only a handful of cases are associated with orbital myositis.
Diagnosis relies on high clinical suspicion and biopsy‐proven evidence of giant cells within cardiac tissue with a cautious realization of the high rate of false‐negative biopsy results (sensitivity 68% with single biopsy).8 Imaging such as cardiac MRI or 18FDG‐PET with SPECT imaging may aide in localization of inflammatory infiltration resulting in targeted biopsies.8 Treatment requires a multimodality approach with pharmacologic interventions and mechanical support devices. Survival without treatment is dismal with a rate of death or cardiac transplantation of 89% and a medial survival of about 5.5 months from symptom onset.5 With combination immunosuppressive agents, survival without need for transplant is drastically improved with a 1 year survival of 77–80% and a 5 year survival of 58%.6, 8 However, most studies have shown that the majority of patients ultimately require transplantation or permanent ventricular support.9 For this reason, timely referral to centres with a heart failure service and capability of advanced heart failure support is essential. Importantly, recurrence in the transplanted heart is also reported and warrants close follow‐up and routine biopsies.5, 8
In our case, the patient presented with ocular symptoms, initially thought to be autoimmune in aetiology although extensive autoimmune and infectious workups, was unrevealing. While GCM was not initially suspected upon presentation, there were critical clues from the onset that suggested cardiac involvement. The initial abnormalities on the electrocardiogram, persistent tachycardia, hypotension, and troponin elevation were important early clinical manifestations. While ocular and/or endomyocardial biopsy was considered, treatment was initiated because of anticipated delay in transferring the patient to our quaternary referral centre. Within 3 days of hospital presentation, the patient showed signs of high‐degree atrioventricular block, cardiomyopathy on echocardiography, and elevated left‐sided filling pressures requiring mechanical support. Prompt initiation of inotropic medications and mechanical support with the Impella device likely avoided hemodynamic compromise. The rapidly accelerating course demanded further cardiac investigation with endomyocardial biopsy with a leading diagnosis of GCM. Previous studies cited steroids, azathioprine, and cyclosporine as immunosuppressive agents for treatment of GCM;5, 6, 8 however, after discussion with experts in GCM, mycophenolate mofetil and tacrolimus were used given the high likelihood of heart transplant in our case.
This case is particularly unique due to the rarity of initial presenting symptom of orbital myositis, use of Impella as a mechanical bridge, and rapidity of cardiovascular decline. Few case reports of orbital myositis in adult patients have been linked to GCM,1, 2, 3, 4 with orbital myositis predating cardiac symptoms by months to years (1 month–3 years). While rare, these case studies demonstrate a substantial link between the two diagnoses. Another unique aspect of our case is the use of a percutaneous mechanical assist device as a temporizing measure. To our knowledge, there is only one previous case report in which a patient with GCM was successfully implanted with an Impella device as a bridge to more permanent left ventricular support.10 Additionally, this is the first reported case of successful implantation of HeartWare biventricular HVAD in a patient with biventricular failure due to GCM.
Patients with autoimmune disease including orbital myositis and any sign of cardiac abnormality should be thoroughly investigated. High index of suspicion and close vigilance of typical cardiac manifestations will give physicians an advantage in diagnosing and treating this time‐sensitive disease. The rapidity of disease acceleration and fulminant course underscore the importance of aggressive treatment including pharmacologic and mechanical assistive support devices and/or orthotopic cardiac transplantation. Early recognition of orbital myositis as a potential early sign of GCM as well as timely goal‐directed therapies can significantly improve outcomes.
Conflict of interest
None declared.
Supporting information
Movie S1. Cine‐echo image in parasternal long axis.
Movie S2. Cine‐echo image in parasternal short axis.
Movie S3. Cine‐echo image in apical four chamber view.
Garg, V. , Tan, W. , Ardehali, R. , Shah, J. , Huynh, T. , and Aksoy, O. (2017) Giant cell myocarditis masquerading as orbital myositis with a rapid, fulminant course necessitating mechanical support and heart transplantation. ESC Heart Failure, 4: 371–375. doi: 10.1002/ehf2.12141.
References
- 1. Ali MS, Mba BI, Husain AN, Ciftci FD. Giant cell myocarditis: a life‐threatening disorder heralded by orbital myositis. BMJ Case Rep 2016; doi:10.1136/bcr-2015-213759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klein BR, Hedges TR 3rd, Dayal Y, Adelman LS. Orbital myositis and giant cell myocarditis. Neurology 1989; 39: 988–990. [DOI] [PubMed] [Google Scholar]
- 3. Stevens AW, Grossman ME, Barr ML. Orbital myositis, vitiligo, and giant cell myocarditis. J Am Acad Dermatol 1996; 35: 310–312. [DOI] [PubMed] [Google Scholar]
- 4. Leib ML, Odel JG, Cooney MJ. Orbital polymyositis and giant cell myocarditis. Ophthalmology 1994; 101: 950–954. [DOI] [PubMed] [Google Scholar]
- 5. Cooper LT Jr, Berry GJ, Shabetai R. Idiopathic giant‐cell myocarditis—natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med 1997; 336: 1860–1866. [DOI] [PubMed] [Google Scholar]
- 6. Ekstrom K, Lehtonen J, Kandolin R, Raisanen‐Sokolowski A, Salmenkivi K, Kupari M. Long‐term outcome and its predictors in giant cell myocarditis. Eur J Heart Fail 2016. [DOI] [PubMed] [Google Scholar]
- 7. Humbert P, Faivre R, Fellman D, Bassand JP, Dupond JL. Giant cell myocarditis: an autoimmune disease? Am Heart J 1988; 115: 485–487. [DOI] [PubMed] [Google Scholar]
- 8. Kandolin R, Lehtonen J, Salmenkivi K, Raisanen‐Sokolowski A, Lommi J, Kupari M. Diagnosis, treatment, and outcome of giant‐cell myocarditis in the era of combined immunosuppression. Circ Heart Fail 2013; 6: 15–22. [DOI] [PubMed] [Google Scholar]
- 9. Maleszewski JJ, Orellana VM, Hodge DO, Kuhl U, Schultheiss HP, Cooper LT. Long‐term risk of recurrence, morbidity and mortality in giant cell myocarditis. Am J Cardiol 2015; 115: 1733–1738. [DOI] [PubMed] [Google Scholar]
- 10. Suradi H, Breall JA. Successful use of the Impella device in giant cell myocarditis as a bridge to permanent left ventricular mechanical support. Tex Heart Inst J 2011; 38: 437–440. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Cine‐echo image in parasternal long axis.
Movie S2. Cine‐echo image in parasternal short axis.
Movie S3. Cine‐echo image in apical four chamber view.


