Abstract
Primary cardiac involvement in systemic sclerosis is common, often subclinical, and is associated with significant mortality. We report the case of a patient who developed autoimmune myocarditis at an early stage of systemic sclerosis, who completely recovered from cardiac dysfunction under optimal medical therapy for heart failure and immunosuppression. This challenging case aims at increasing awareness around the fact that the heart is a target organ of scleroderma disease. It also highlights the importance of screening and early diagnosis of cardiac involvement, because a timely treatment may impact the quality of life of these patients and improve their prognosis.
Keywords: Systemic sclerosis, Scleroderma heart disease, Autoimmune myocarditis, Myocardial fibrosis, Prognosis
Introduction
Systemic sclerosis (SSc) is characterized by diffuse vascular damage, abnormal activation of immune system, and fibrosis of the skin and many internal organs.1 Primary cardiac involvement (CI) is a common complication of the disease (though frequently underdiagnosed) with major adverse prognostic impact that can be potentially treatable when timely diagnosed.2
Here, we describe the case of a patient who developed acute myocarditis at an early stage of SSc, with threatening complications related to low cardiac output and thromboembolic phenomena, who evolved with remarkable cardiac recovery under heart failure therapy and immunosuppression.
Case report
A 33‐year‐old Caucasian woman with no relevant medical records presented at the hospital with symptoms of increased fatigue and dyspnoea for 2 weeks [New York Heart Association (NYHA) functional class III] and multiple skin lesions. The physical examination revealed confluent erythematous macules in her back, neck, and face that had been evolving in outbreaks for 2 years. She also had thickening of the fingertips' skin with associated Raynaud's phenomenon, thoracic telangiectasia, jugular turgescence, pulmonary crackles, normal heart sounds, and lower limbs oedema. She had no proximal muscle weakness. Blood tests revealed subclinical autoimmune hypothyroidism (with positive anti‐thyroid peroxidase and anti‐thyroglobulin antibodies) and positive anti‐nuclear (ANA) and anti‐Scl70 antibodies. Renal function was normal, as was troponin I and creatine kinase serum levels. The brain natriuretic peptid level was markedly elevated (1603 pg/mL). Skin biopsy was consistent with diffuse SSc (Figure 1). Electrocardiogram revealed sinus rhythm, right bundle branch block, and left anterior fascicular block. Echocardiogram showed four‐chamber dilatation with biventricular systolic dysfunction (left ventricle ejection fraction of 15% and tricuspid annular plane systolic excursion of 14 mm), and two large left ventricular apical thrombus (Figure 2). There were no signs of LV diastolic dysfunction and estimated pulmonary artery systolic pressure was normal. The coronary angiography was normal, and the cardiac magnetic resonance showed myocardial oedema of the LV, subepicardial and pericardial late gadolinium enhancement, and a pericardial effusion (Figure 3). Endomyocardial biopsy of the right ventricle (RV) revealed interstitial and perivascular lymphocytic infiltrate with positive immunostaining for C4d, without fibrosis (Figure 4). SSc with cutaneous, thyroid, and CI was determined as the diagnosis (gastrointestinal and pulmonary studies were unrevealing). Medical therapy included vitamin K antagonist, loop diuretics, angiotensin‐converting‐enzyme (ACE) inhibitor, beta‐blocker, digoxin, and aldosterone receptor antagonist, and also cyclophosphamide, azathioprine and prednisolone. Apart from a cardio embolic transient ischaemic attack, from which the patient completely recovered, hospitalization was otherwise uneventful. By the 12th day, she was discharged in NYHA II, with brain natriuretic peptid serum level of 330 pg/mL and left ventricle ejection fraction of 39%, with no evidence of ventricular thrombi.
Figure 1.
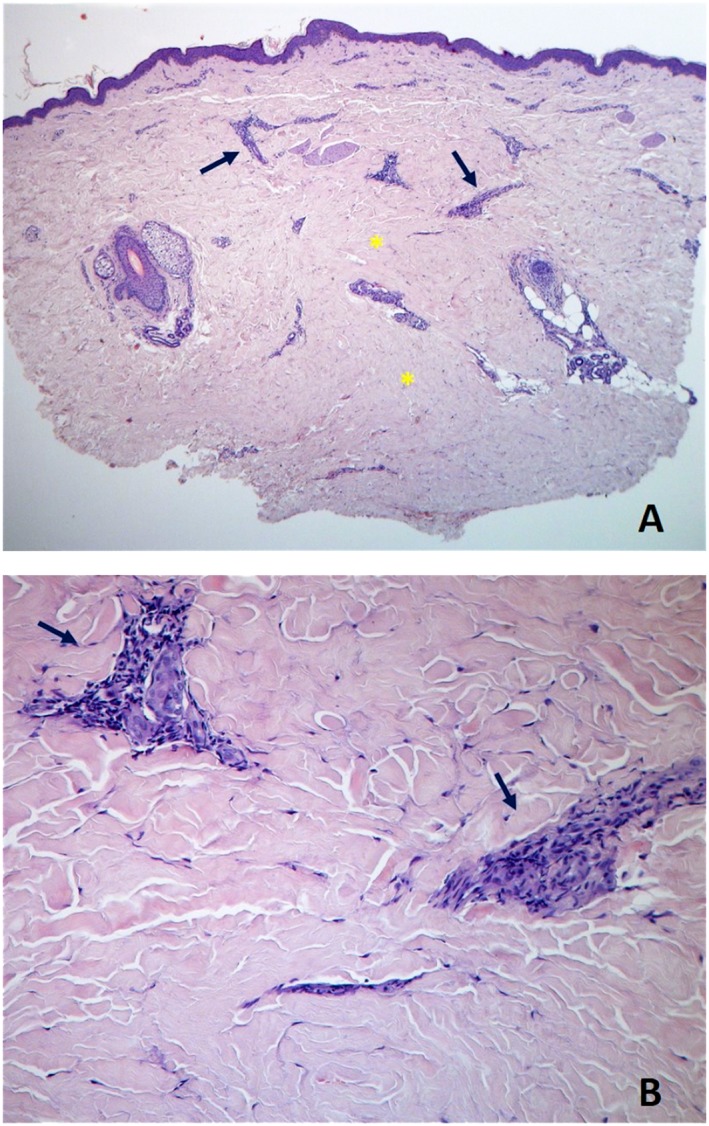
Skin biopsy specimen from right forearm demonstrates dense sclerotic collagen in the reticular and deep dermis (asterisks), sparse lymphocytic infiltrate (arrows), and relative preservation of some cutaneous annexes, compatible with early skin involvement [haematoxylin‐eosin, ×40 panel (A), ×200 panel (B)].
Figure 2.
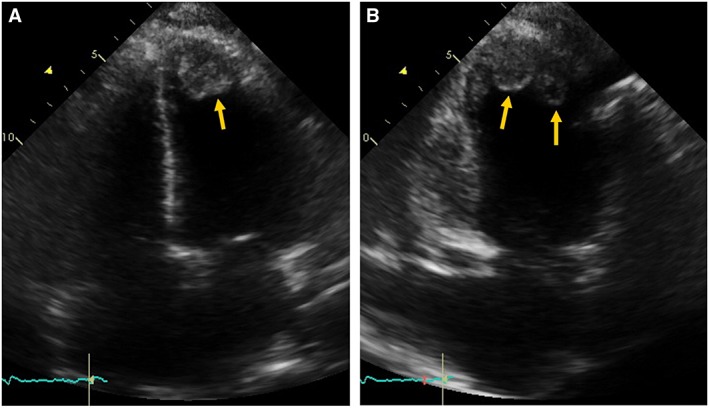
Transthoracic echocardiography. Apical four chambers view (A) and two chambers view (B) showing dilated cardiac chambers and two apical left ventricular thrombus (arrows).
Figure 3.
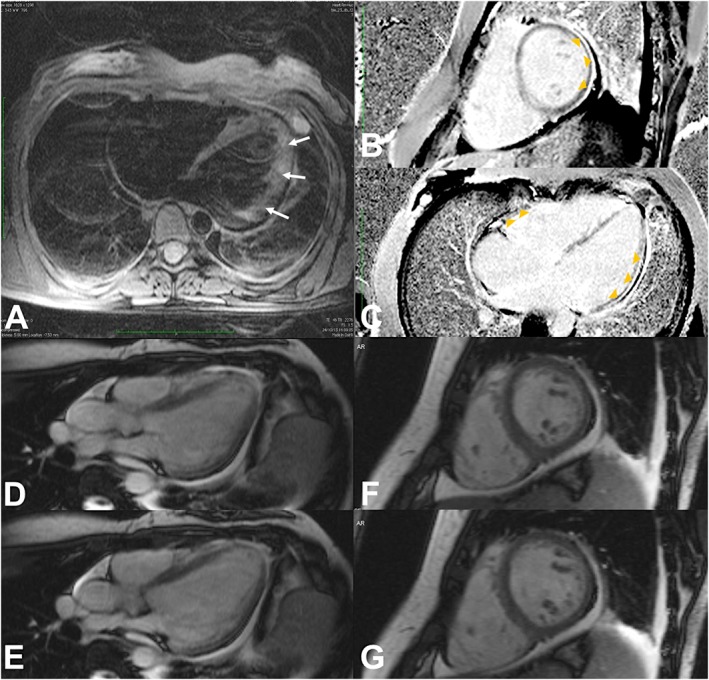
Cardiac Magnetic Resonance. Patchy intramural hyperintensity of the lateral wall of the left ventricular on axial T2‐weighted image [arrows, (A)] suggests myocardial oedema. Inversion‐recovery sequences after intravascular gadolinium administration [short axis and long axis four chambers view, (B) and (C), respectively] show subepicardial delayed enhancement (arrowheads) of the inferior, infero‐lateral and lateral walls of the left ventricular, free wall and septal myocardial of the right ventricular, and pericardium. Steady‐state free precession sequences [long axis three chambers view (D–E), and short axis (F–G) during systole (D and F), and during diastole (E and G)] show pericardial effusion, as well as left sided pleural effusion. Moving images (Movies S1 and S2, Supporting Information) reveal diffuse hypokinesia of both ventricles (more severe at the inferior and infero‐lateral walls of the left ventricle).
Figure 4.
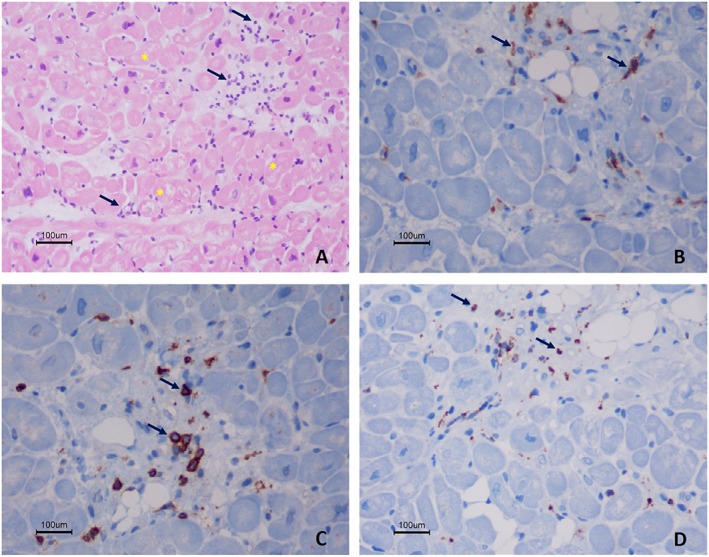
Histology and immunohistochemistry findings from right ventricular endomyocardial biopsy, of active autoimmune myocarditis. Haematoxylin and eosin staining (×200) shows inflammatory infiltrates (arrows) associated with necrosis of adjacent myocytes (cytoplasmic vacuolation and nuclear atypia of myocytes—asterisks) (A). The immunohistochemistry (original magnification of 400×) shows the presence of CD4 positive cells (B), CD8 positive cells (C), and scattered macrophages [CD68 positive cells, (D)]. There is immunostaining for C4d.
At the 12‐month follow‐up, she was in NYHA I with signs of cutaneous involvement progression and pulmonary involvement. Echocardiogram showed complete recovery of systolic function of both ventricles and RV dilation presumably related to the initial adaptation to the interstitial lung disease.
Discussion
Scleroderma heart disease (SHD) can virtually affect any structure of the heart and is frequently associated with diffuse forms of SSc (estimated prevalence of 32%).3 It is usually developed during the first year after diagnosis in patients with other organ‐specific alterations and can account for 20–30% of all premature deaths.4
A broad variety of clinical and subclinical manifestations of SSc has been related to primary CI, a direct consequence of the disease (which may range from left ventricular systolic and/or diastolic dysfunction, to pericarditis with or without pericardial effusion, conduction alterations, autonomic dysfunction, and valvular dysfunction), or to secondary CI due to cor pulmonale resulting from pulmonary interstitial and/or vascular disease.5
The pathogenesis of primary CI is related to myocardial fibrosis, the result of autoimmune myocarditis and/or repeated focal ischaemic injuries due to microvascular coronary abnormalities (functional coronary vasospasm—the so‐called ‘myocardial Raynaud's phenomenon’—in the beginning, with subsequent morphological vascular damage.6) Historically, autopsy series of CI in SSc patients described hypertrophy and/or fibrosis primarily of the myocardium; however, diffuse fibrosis of the endocardium, epicardium, and pericardium has also been reported.7, 8
Two distinct patterns of inflammatory infiltrates were recognizable at endomyocardial biopsy of this patient, which may account for the dual nature of cardiac damage in SSc: ischaemic myocyte necrosis (which ultimately leads to replacement fibrosis) and interstitial/perivascular inflammation that better mirrors the fibrotic progression closely related to the natural history of the disease.
Depressed LV contractility is among the rarest findings in SSc patients, with an estimated prevalence between 1–5%,9 and its prognostic significance has never been evaluated. More often (in about 40% of the cases), patients present with relaxation abnormalities, valvular regurgitation, and RV pathology.10 The earliest, most frequent and characteristic echocardiographic feature of SHD is the left ventricular diastolic dysfunction, and multiple symptoms of SSc patients (including decreased exercise capacity, dyspnoea, and decompensation) are strongly related to LV filling pressure. Its prevalence ranges from 17.7% in a large cohort using traditional Doppler echocardiography11 to 63% in the latest studies performing tissue Doppler echocardiography.2 Left ventricular diastolic dysfunction is related to the degree of myocardial fibrosis and is inversely and independently associated to the survival of the patients.12
Cardiovascular Magnetic Resonance (CMR) is a useful imaging tool for cardiac functional assessment that also provides excellent tissue characterization, enabling the degree and extension of myocardial inflammation and fibrosis to be readily and non‐invasively appreciated, without the need of radiation. In the present case, CMR showed patchy areas of intramural hyperintensity on T2‐weighted images, suggesting myocardial oedema, and areas of late gadolinium enhancement (LGE) with subepicardial distribution along both ventricles and pericardium, suggestive of regional inflammation and fibrosis. Meanwhile, conventional CMR techniques have some drawbacks and T2‐weighted imaging has been recently challenged (for its modest sensitivity to detect myocardial oedema and proneness for artefacts) by newer T2 mapping sequences that correlate closely with free tissue water content associated with active myocardial inflammation.13 On the other hand, LGE has its strengths in detecting focal cardiac fibrosis/scarring based on the distribution difference of gadolinium in between healthy and diseased myocardium, but has limited value for detection of diffuse fibrotic processes. Quantitative assessment of myocardial T1 values (‘T1 mapping’) has recently re‐emerged as an additional and promising technique in cardiac imaging. It provides more detailed tissue characterization than LGE alone and better defines the degree of diffuse fibrosis, which is highly prevalent among SSc patients.8 Native T1 values have been reported to have increased accuracy in diagnosis of acute myocarditis when applied together with any other criteria (like Lake Louise criteria, T2 ratio, or LGE), being the single most accurate one.14 In this regard, the concept of multiparametric CMR including mapping techniques is promising and might be useful in early detection and characterization of myocardial involvement in SSc patients.
Cardiac conduction system was also involved in this patient, and she had electrocardiographic evidence of intraventricular conduction disturbance with bifascicular block pattern. Conduction system alterations are present in about 20% of patients,15 and right bundle branch block has been recently described to be an independent predictor of mortality in this group.16
Prognosis of SSc patients with CI seems worse, particularly when it becomes clinically evident.17 According to previous reports, CI doubles the mortality rate of SSc patients and is considered to be an independent mortality risk factor along with interstitial lung disease, pulmonary hypertension, scleroderma renal crisis, and age.18
Our patient developed an unusual form of SHD, with acute myocarditis and severe dysfunction of both ventricles. Because of its rarity, the best therapeutic approach and prognostic impact of this type of presentation are not known. In 1992, Clemson et al.19 reported a case where premortem biopsy documentation of myocarditis and myocardial fibrosis was made in a 23‐year‐old SSc patient, who developed acute myocarditis and died of heart failure within 3 weeks, despite the heart failure and SSc specific therapy. It is important to note that in such a case, the heart failure therapy only included ACE inhibitor and digoxin, whereas for SSc corticoid was the only therapy provided. More recently, out of a 181 cohort, seven patients with newly developed symptoms and signs of CI were found to have biopsy‐proven myocarditis (with interstitial and perivascular fibrosis in six patients and areas of replacement fibrosis in two).1 Those patients were treated with immunosuppressants (steroids, cyclophosphamide, and azathioprine) along with diuretics, beta‐blockers, and ACE inhibitors. At the 12‐month follow‐up, all the patients had shown a significant clinical and laboratory improvement, although only one patient had experienced complete recovery of contractile function. The best results were obtained in patients with lower degrees of fibrosis, suggesting that early diagnosis is crucial for modifying the natural history of SHD. This study also highlighted that there are some associated factors, like early disease, skeletal myositis, c‐ANCA positivity, and pericardial effusion, which should be carefully addressed as ‘red flags’ in SSc for the recognition of patients with myocardial inflammation.
Our patient was quickly diagnosed and treated with optimal heart failure therapy and immunosuppressant, which resulted in complete recovery of systolic function of both ventricles. Optimized and timely prescribed therapy (before myocardial fibrosis could develop) may have been decisive for this good outcome.
Conflicts of interest
None declared.
Supporting information
Movies S1 and S2. Short axis and long axis three chambers views cine‐MRI showing difuse hypokinesia of both ventricles and global compromise of systolic biventricular function.
Movies S1 and S2. Short axis and long axis three chambers views cine‐MRI showing difuse hypokinesia of both ventricles and global compromise of systolic biventricular function.
Ramalho, A. R. , Costa, S. , Silva, F. , Donato, P. , Franco, F. , and Pêgo, G. M. (2017) Autoimmune myocarditis in systemic sclerosis: an unusual form of scleroderma heart disease presentation. ESC Heart Failure, 4: 365–370. doi: 10.1002/ehf2.12139.
References
- 1. Pieroni M, De Santis M, Zizzo G, Bosello S, Smaldone C, Campioni M, De Luca G, Laria A, Meduri A, Bellocci F, Bonomo L, Crea F, Ferraccioli G. Recognizing and treating myocarditis in recent‐onset systemic sclerosis heart disease: potential utility of immunosuppressive therapy in cardiac damage progression. Semin Arthritis Rheum 2014; 43: 526–535. [DOI] [PubMed] [Google Scholar]
- 2. Faludi R, Kolto G, Bartos B, Csima G, Czirjak L, Komocsi A. Five‐year follow‐up of left ventricular diastolic function in systemic sclerosis patients: determinants of mortality and disease progression. Semin Arthritis Rheum 2014; 44: 220–227. [DOI] [PubMed] [Google Scholar]
- 3. Ferri C, Valentini G, Cozzi F, Sebastiani M, Michelassi C, La Montagna G, Bullo A, Cazzato M, Tirri E, Storino F, Giuggioli D, Cuomo G, Rosada M, Bombardieri S, Todesco S, Tirri G, Systemic Sclerosis Study Group of the Italian Society of Rheumatology (SIR‐GSSSc) . Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81: 139–153. [DOI] [PubMed] [Google Scholar]
- 4. Komócsi A, Vorobcsuk A, Faludi R, Faludi R, Pintér T, Lenkey Z, Költo G, Czirják L. The impact of cardiopulmonary manifestations on the mortality of SSC: a systematic review and meta‐analysis of observational studies. Rheumatol (United Kingdom) 2012; 51: 1027–1036. [DOI] [PubMed] [Google Scholar]
- 5. Boueiz A, Mathai SC, Hummers LK, Hassoun PM. Cardiac complications of systemic sclerosis: recent progress in diagnosis. Curr Opin Rheumatol 2010; 22: 696–703. [DOI] [PubMed] [Google Scholar]
- 6. Lambova S. Cardiac manifestations in systemic sclerosis. World J Cardiol 2014; 6: 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oram S, Stokes W. The heart in scloeroderma. Br Heart J 1961; 23: 243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Rai AB, Matthews PM, Robson MD, Moon J, Wordsworth PB, Neubauer S, Karamitsos TD. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis—a clinical study using myocardial T1‐mapping and extracellular volume quantification. J Cardiovasc Magn Reson 2014; 16: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, Riemekasten G, Cozzi F, Beretta L, Derk CT, Komócsi A, Farge D, Balbir A, Riccieri V, Distler O, Chialà A, Del Papa N, Simic KP, Ghio M, Stamenkovic B, Rednic S, Host N, Pellerito R, Zegers E, Kahan A, Walker UA, Matucci‐Cerinic M, EUSTAR co‐authors . Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69: 218–221. [DOI] [PubMed] [Google Scholar]
- 10. Allanore Y, Avouac J, Kahan A. Systemic sclerosis: an update in 2008. Jt Bone Spine 2008; 75: 650–655. [DOI] [PubMed] [Google Scholar]
- 11. de Groote P, Gressin V, Hachulla E, Carpentier P, Guillevin L, Kahan A, Cabane J, Francès C, Lamblin N, Diot E, Patat F, Sibilia J, Petit H, Cracowski JL, Clerson P, Humbert M, ItinerAIR‐Scleroderma Investigators . Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67: 31–36. [DOI] [PubMed] [Google Scholar]
- 12. Hinchcliff M, Desai CS, Varga J, Shah SJ. Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol 2012; 30 DOI:10.1016/j.biotechadv.2011.08.021. [PMC free article] [PubMed] [Google Scholar]
- 13. Mayr A, Kitterer D, Latus J, Steubing H, Henes J, Vecchio F, Kaesemann P, Patrascu A, Greiser A, Groeninger S, Braun N, Alscher MD, Sechtem U, Mahrholdt H, Greulich S. Evaluation of myocardial involvement in patients with connective tissue disorders: a multi‐parametric cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2017; 18: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luetkens JA, Doerner J, Thomas DK, Dabir D, Gieseke J, Sprinkart AM, Fimmers R, Stehning C, Homsi R, Schwab JO, Schild H, Naehle CP. Acute myocarditis: multiparametric cardiac MR imaging. Radiology 2014; 273: 383–392. [DOI] [PubMed] [Google Scholar]
- 15. Parks JL, Taylor MH, Parks LP, Silver RM. Systemic sclerosis and the heart. Rheum Dis Clin North Am 2014; 40: 87–102. [DOI] [PubMed] [Google Scholar]
- 16. Draeger HT, Assassi S, Sharif R, Gonzalez EB, Harper BE, Arnett FC, Manzoor A, Lange RA, Mayes MD. Right bundle branch block: a predictor of mortality in early systemic sclerosis. PLoS ONE 2013; 8: e78808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernández‐Codina A, Simeón‐Aznar CP, Pinal‐Fernandez I, Rodríguez‐Palomares J, Pizzi MN, Hidalgo CE, Del Castillo AG, Prado‐Galbarro FJ, Sarria‐Santamera A, Fonollosa‐Plà V, Vilardell‐Tarrés M. Cardiac involvement in systemic sclerosis: differences between clinical subsets and influence on survival. Rheumatology International 2015; 37: 75–84. [DOI] [PubMed] [Google Scholar]
- 18. Rubio‐Rivas M, Royo C, Simeón CP, Corbella X, Fonollosa V. Mortality and survival in systemic sclerosis: systematic review and meta‐analysis. Semin Arthritis Rheum 2014; 44: 1–12. [DOI] [PubMed] [Google Scholar]
- 19. Clemson BS, Miller WR, Luck JC, Feriss JA. Acute myocarditis in fulminant systemic sclerosis. Chest 1992; 101: 872–874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movies S1 and S2. Short axis and long axis three chambers views cine‐MRI showing difuse hypokinesia of both ventricles and global compromise of systolic biventricular function.
Movies S1 and S2. Short axis and long axis three chambers views cine‐MRI showing difuse hypokinesia of both ventricles and global compromise of systolic biventricular function.


