Abstract
Angiotensin‐converting enzyme inhibitor induced angioedema commonly involves the head and neck area. We report a case of angiotensin‐converting enzyme inhibitor induced intestinal angioedema in a heart transplant recipient on mTOR immunosuppression. A 36‐year‐old Caucasian woman with history of heart transplantation on sirolimus, tacrolimus and prednisone presented to the Emergency Department with abdominal pain, one day following lisinopril initiation. A computer tomography scan demonstrated diffuse bowel wall thickening consistent with pancolitis and edema. She was subsequently diagnosed with angiotensin‐converting enzyme inhibitor induced angioedema. Patients on mTOR immunosuppression are at higher risk for this potentially life‐threatening side effect. Knowledge of this interaction is critical for providers prescribing mTOR agents.
Keywords: ACE inhibtor, mTOR inhibitor, heart transplantation, intestinal angioedema
Introduction
Angioedema is a swelling of the skin and mucous membranes which typically presents with swelling of the lips, tongue or face; however, it can manifest in any organ system. It is thought to be caused by increases in histamine and bradykinin that cause increased capillary leakage and edema. Angioedema associated with angiotensin‐converting enzyme (ACE) inhibitors is a well‐known phenomenon with incidence rates near 0.4%.1 We report a case of acute intestinal angioedema related to ACE inhibitor administration in an orthotopic heart transplant recipient.
Case report
A 36‐year‐old Caucasian woman presented with severe diffuse abdominal pain one day following initiation of lisinopril for hypertension. She had a history of a tachycardia‐induced cardiomyopathy requiring orthotopic heart transplantation in 2002 and was maintained on sirolimus, tacrolimus, and prednisone for immunosuppression. She was prescribed an ACE inhibitor the day prior to the admission for elevated blood pressures, a class of medications that she had never taken before. On presentation, she endorsed rapid onset of abdominal pain, dizziness, and sweating. She was without fevers, had a heart rate of 79 beats per minute, a blood pressure of 170/105 mmHg that decreased to 74/52 mmHg 8 h after arrival and normalized with administration of intravenous fluids. She had diffuse tenderness to light palpation below the umbilicus and abdominal guarding on exam with clear lung fields notable for absence of bronchospasm and no urticaria or rash on skin exam. Initial laboratory analyses demonstrated a mild increase in white blood cells and liver tests.
A computer tomography of the abdomen and pelvis (Figure 1 A and B) revealed diffuse wall thickening with enhancement affecting both small and large bowels and fat stranding consistent with pancolitis and edema. Given the time of onset and relationship with ACE inhibition, she was diagnosed with intestinal angioedema secondary to ACE inhibitor initiation. Alternative causes for diagnosis were considered; however, there was an absence of risk factors for other causative etiologies, and further lab workup was unremarkable. She was not evaluated for hereditary angioedema. All oral medications were then held. She was monitored with serial abdominal exams and given opiates. All laboratory studies were normalized, and home medications including immunosuppressive agents were restarted the next day except for lisinopril. Over a 72‐h period, her pain resolved, and she was discharged to home with the addition of amlodipine 2.5 mg daily for hypertension.
Figure 1.
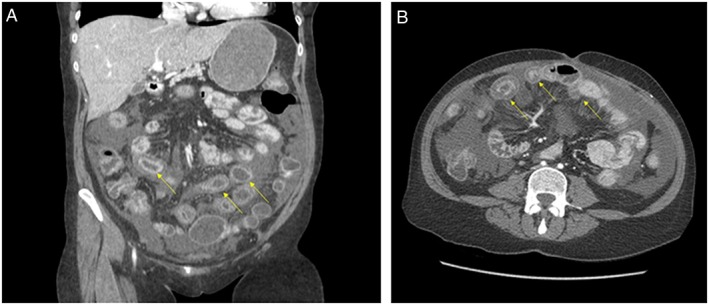
(A) Computed tomography imaging of intestinal angioedema in the coronal view. Arrow demonstrates profound small and large bowel wall edema and fat stranding. (B) Computed tomography imaging of intestinal angioedema in the transverse view. Arrow demonstrates profound small and large bowel wall edema and fat stranding.
Discussion
Use of immunosuppressive medications with ACE inhibitors is a risk factor for angioedema among patients on immunosuppression, including the heart transplant population.2, 3, 4, 5, 6, 7 Isolated involvement of the gastrointestinal tract is a rare presentation of ACE inhibitor induced angioedema. Although not definitively demonstrated in humans, one hypothesis is that immunosuppressive agents increase this risk by decreasing the activity of circulating dipeptidyl peptidase IV (both dipeptidyl peptidase IV and ACE inactivate vasodilatory bradykinin and substance P). This effect is seen with both calcineurin and mTOR inhibitors but is greater with the latter.2, 8 Reports have demonstrated episodes of facial and sublingual angioedema in kidney transplant patients on mTOR inhibitors without concomitant use of an ACE‐inhibitor.4, 9 In September 2015, the Food and Drug Administration (FDA) issued an update on the safety of ACE inhibitors stating that use in combination with mTOR inhibitors may increase the risk for angioedema.10 Despite this, our electronic medical record did not have this as a drug interaction.
In summary, this case and the relevant medical literature suggest that ACE inhibitor‐induced angioedema of the gastrointestinal tract must be considered in the differential diagnosis of both acute and recurrent abdominal pain in patients receiving concomitant ACE inhibitor and immunosuppression with an mTOR inhibitor. Despite the increasing incidence of ACE inhibitor‐induced angioedema in patients taking calcineurin inhibitors,2, 3, 4, 5, 6, 7, 8, 9 extrapolation of basic science studies suggests that mTOR inhibitors pose a greater risk to interact with ACE inhibitors. Physicians prescribing mTOR inhibitors should have knowledge of the potential interaction.
Conflict of interest
None declared.
Acknowledgements
This research was supported by the University of Michigan, Department of Cardiology. We would like to thank the following colleagues who provided their clinical expertise and insight that greatly assisted in the completion of this project: Dr Keith Aaronson, Dr Brahmajee Nallamothu, Dr Todd Koelling, Dr Nour Al‐Hadidi, and Dr Asad Ghafoor.
Srinivasan, D. , Strohbehn, G. W. , and Cascino, T. (2017) ACE inhibitor‐associated intestinal angioedema in orthotopic heart transplantation. ESC Heart Failure, 4: 384–386. doi: 10.1002/ehf2.12161.
References
- 1. Piller LB, Ford CE, Davis BR, Nwachuku C, Black HR, Oparil S, Retta TM, Probstfield JL. Incidence and predictors of angioedema in elderly hypertensive patients at high risk for cardiovascular disease: a report from the antihypertensive and lipid lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens 2006; 8: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Byrd JB, Woodard‐Grice A, Stone E, Lucisano A, Schaefer H, Yu C, Eyler AE, Salloum NE, Brown NJ. Association of angiotensin‐converting enzyme inhibitor‐associated angioedema with transplant and immunosuppressant use. Allergy 2010; 65: 1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stallone G, Infante B, Di Paolo S, Schena A, Grandaliano G, Gesualdo L, Schena FP. Sirolimus and angiotensin‐converting enzyme inhibitors together induce tongue oedema in renal transplant recipients. Nephrol Dial Transplant 2004; 19: 2906–2908. [DOI] [PubMed] [Google Scholar]
- 4. Fuchs U, Zittermann A, Berthold HK, Tenderich G, Deyerling KW, Minami K, Koerfer R. Immunosuppressive therapy with everolimus can be associated with potentially life‐threatening lingual angioedema. Transplantation 2005; 79: 981–983. [DOI] [PubMed] [Google Scholar]
- 5. Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin‐converting enzyme inhibitor‐induced isolated visceral angioedema in a liver transplant recipient. Transplantation 2003; 75: 730–732. [DOI] [PubMed] [Google Scholar]
- 6. Mahé E, Morelon E, Lechaton S, Kreis H, de Prost Y, Bodemer C. Angioedema in renal transplant recipients on sirolimus. Dermatology 2007; 214: 205–209. [DOI] [PubMed] [Google Scholar]
- 7. Jung M, Ranpura VN, Dunbar CE, Tisdale JF, Fitzhugh CD, Hsieh MM. Angioedema in patients treated with sirolimus and ACE inhibitor post hematopoietic SCT. Bone Marrow Transplant 2014; 49: 1448–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K. Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol 2010; 5: 703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wadei H, Gruber SA, El‐Amm JM, Garnick J, West MS, Granger DK, Sillix DH, Migdal SD, Haririan A. Sirolimus‐induced angioedema. Am J Transplant 2004; 4: 1002–1005. [DOI] [PubMed] [Google Scholar]
- 10. FDA . ACE Inhibitors and mTOR Inhibitor Coadministration. 2015. https://www.fda.gov/safety/medwatch/safetyinformation/ucm461227.htm.


