Abstract
Aims
Chronic kidney disease (CKD) is prevalent and is associated with increased cardiovascular morbidity and mortality. The interaction between diastolic dysfunction (DD) and CKD in subjects with preserved systolic function is not well defined. This study sought to determine the association between renal function and DD in subjects with preserved ejection fraction.
Methods and results
Through the Rochester Epidemiology Project, subjects who underwent echocardiography over 2 years with EF ≥50% were identified and the clinical data were obtained. Glomerular filtration rate (GFR) was estimated using the modification of diet in renal disease equation. Linear regression was used to test for association of GFR and DD. DD was defined as follows: Grade 2 or pseudonormal pattern (0.75 < E/A ≤ 1.5, E/e′ ≥ 10, DT > 140 ms, ΔE/A ≥ 0.5, and PV S < D) or Grade 3+ or restrictive pattern (E/A > 1.5, E/e′ ≥ 10, DT < 140 ms, and PV S < D). Cox regression was used to assess correlation of GFR and DD with time‐to‐event outcomes. A total of 2056 patients were identified. There was significant correlation between worsening GFR and degree of DD assessed by echo Doppler E/e′ ratio (P = 0.005), left ventricular mass index (P = 0.004), and right ventricular systolic pressure (P = 0.01). Worsening GFR was associated with increased mortality, development of heart failure, and hospitalization (P < 0.001). Within each GFR group, abnormal DD was associated with a higher risk of the clinical outcomes. No interaction between GFR and DD was noted, suggesting an increased risk of events associated with abnormal DD across ranges of GFR.
Conclusions
Worsening GFR was associated with a greater degree of diastolic dysfunction and adverse clinical outcomes. Within each GFR group, the presence of DD was associated with increased morbidity and mortality. Further studies are warranted to determine if improving DD in patients with CKD will benefit clinical outcomes.
Keywords: Heart failure, Diastolic dysfunction, Chronic kidney disease, Hospitalization, Death
Introduction
Heart failure is a progressive cardiac condition associated with significant morbidity and mortality, especially in the elderly population. While much is known about the pathophysiology and causes of heart failure with reduced ejection fraction, an understanding of the pathophysiology of heart failure with preserved ejection fraction (HFpEF), which affects approximately 50% of patients with heart failure, is limited.1, 2, 3, 4 Vogel et al. noted a progression from preclinical diastolic dysfunction (DD), defined as DD without symptoms, to symptomatic heart failure that was associated with renal insufficiency [defined as a glomerular filtration rate (GFR) <60 mL/min per 1.73 m2] independent of comorbidities or ejection fraction.5, 6
Chronic renal insufficiency (CRI) is a condition affecting an increasing portion of the US and world population.7, 8 It is broadly defined as impaired renal function lasting greater than 3 months with a GFR <60 mL/min per 1.73 m2. There have been noted associations between reduced estimated GFR and increased risk of death, cardiovascular events, and hospitalization in a large community cohort involving ~1 million patients.9 A better understanding of the possible association between CRI and increasingly common HFpEF is relevant.
The aim of this study was to characterize the relationship between CRI and DD in a general community cohort as measured by diastolic grade and other echocardiographic parameters. We additionally sought to assess whether CRI affected long‐term outcomes associated with DD, namely, all‐cause mortality, all‐cause hospitalization, development of heart failure, and atrial fibrillation. Lastly, we also sought to determine the interaction between CRI and DD on clinical outcomes.
Methods
Study setting
This study was reviewed and approved by both the Mayo Clinic and Olmsted County Institutional Research Board. Population‐based epidemiological research has been made possible through extensive record keeping of the Rochester Epidemiology Project. The Rochester Epidemiology Project captures the healthcare information for the entire population of Olmsted County, MN, USA, which includes roughly 502 820 individuals who have resided in the region between 1966 and 2010.10 The unique geographic isolation of this community cohort means that 90% of its subjects seek care at the Mayo Clinic, the Olmsted Medical Group, or its affiliated facilities.11
We utilized these clinical data from the Rochester Epidemiology Project and the Mayo Clinic to identify patients who were eligible for this retrospective study. A list of subjects from Olmsted County was generated from the echocardiography database of patients who were 18 years or older who underwent echocardiography between 1 January 2006 and 31 December 2008 with a left ventricular ejection fraction ≥50%. Inclusion and exclusion criteria were as follows:
Inclusion criteria:
patients who were 18 years or older who underwent echocardiography between 1 January 2006 and 31 December 2008;
left ventricular ejection fraction ≥50%; and
LV diastolic assessment performed.
Exclusion criteria:
Patients were excluded if they carried a diagnosis of the following:
moderate to severe valvular disease;
hypertrophic cardiomyopathy;
complex congenital disease;
medication or exposure‐induced cardiomyopathy; and
chronic persistent atrial fibrillation.
Data were collected through automated electronic data retrieval from the Rochester Epidemiology Project database. Median follow‐up time was 6 years (interquartile range: 4.5–7.0).
Echocardiographic data
Echocardiography data were performed according to established guidelines set forth for the evaluation of left ventricular DD. DD was assessed via pulsed wave Doppler examination of mitral inflow before and during a Valsalva manoeuvre along with Doppler tissue imaging of the mitral annulus.12 Diastolic function was classified as follows: Grade 1 or impaired relaxation (E/A ≤ 0.75, E/e′ < 10, ΔE/A < 0.5, and S > D), Grade 1a – impaired relaxation (E/A ≤ 0.75, E/e′ ≥ 10, ΔE/A < 0.5, and S > D), Grade 2 or pseudonormal pattern (0.75 < E/A ≤ 1.5, E/e′ ≥ 10, DT > 140 ms, ΔE/A ≥ 0.5, and PV S<D), and Grade 3+ or restrictive pattern (E/A > 1.5, E/e′ ≥ 10, DT < 140 ms, and PV S<D).12 DD was defined as having Grade 2 or Grade 3+ diastolic grade.
We additionally collected data on left atrial volume index, right ventricular systolic pressures, ejection fraction, left ventricular mass index (LVMI), peak early mitral filling velocity (E), late mitral filling velocity at atrial contraction (A), velocity of mitral annulus early diastolic motion (e′), ratio of early and atrial mitral filling velocities (E/A), and ratio of early mitral filling velocity to early diastolic motion mitral annulus velocity (E/e′).
Additional data
In addition to the inclusion echocardiogram that we obtained on all patients, we collected the following additional clinical data: age, weight, gender, body mass index, body surface area, and creatinine within 3 months of the inclusion echocardiogram. GFR was calculated via the revised four‐variable modification of diet in renal disease equation that utilized the patient's age, gender, creatinine, and ethnicity.13 Subjects were then allocated into a subgroup of CRI according to their GFR as follows:
normal: GFR ≥ 90 mL/min/1.73m2;
mild CRI: GFR 60–89 mL/min/1.73m2;
moderate CRI: GFR 30–59 mL/min/1.73m2; and
severe CRI: GFR < 30 mL/min/1.73m2.
Study design
International Classification of Disease and Hospital International Classification of Disease Adaptation codes were used to identify pre‐existing medical conditions in patients at the time of inclusion into the study. Relevant cardiac medications (beta‐blockers, ACE inhibitors, and anticoagulants) were also recorded at the time of the inclusion echocardiogram. Mortality data were obtained through automated data retrieval from the Rochester Epidemiology Project Database and was corroborated through review of 100 randomly selected patients from our selected cohort. Additional outcomes including all‐cause hospitalization, onset of heart failure, development of atrial fibrillation, and pulmonary arterial hypertension were also collected and corroborated via randomized review as well. The beginning of follow‐up was defined as the date of the inclusion echocardiogram. All baseline clinical data that were documented were included within a 3 month window of this echocardiogram. Primary outcomes were death, all‐cause hospitalization, and onset of heart failure. Subjects were then followed for a maximum of 8.5 years from the inclusion echocardiogram. The end‐date for follow‐up was 30 June 2014.
Statistical analysis
Summaries for categorical baseline clinical conditions were demonstrated as percentages within each group of CRI. Continuous variables such as age, weight, body mass index, and various echo parameters were demonstrated as means with standard deviations. Comparison of characteristics between subgroups was performed using Pearson χ2 test for categorical or two‐sample t‐test for continuous variables. Linear regression was used to test association of grade of CRI with DD grade and associated echocardiographic parameters. The progression to time‐to‐event endpoints was illustrated via the Kaplan–Meier methods, and statistically significant differences between curves were tested using log‐rank tests. For time‐to‐event analyses, subjects not known to have events were censored at last known clinical follow‐up. Cox proportional hazards regression was used to test for association of worsening CRI with primary and secondary endpoints. These models were adjusted for age, gender, and pre‐existing comorbidities. Results of these analyses are presented as hazard ratios (HR) with corresponding 95% confidence interval (CI). For differences in effects of DD by CRI to be tested, an interaction term was added to the model and tested and reported (P‐int). Analyses were performed using SAS version 9.4 (Cary, NC, USA), and two‐sided P‐values <0.05 were considered to be statistically significant.
Results
Baseline characteristics
Two thousand fifty‐six patients were included in the study and were classified into four groups based upon GFR: 268 patients had a GFR ≥90 mL/min/1.73 m2 (normal), 1070 patients with GFR 60–89 mL/min/1.73 m2 (mild CRI), 632 patients with GFR 30–59 mL/min/1.73 m2 (moderate CRI), and 86 patients with GFR <30 mL/min/1.73 m2 (severe CRI). This patient sample comprised roughly 0.41% of the total population size of the Rochester Epidemiology Project.10 Baseline characteristics including age, gender, and associated comorbidities are noted in Table 1. Worsening CRI was associated with increased age as well as increased prevalence of chronic medical conditions including diabetes, hypertension, and cerebrovascular disease. A comorbidity index was created using pre‐existing medical conditions including type‐2 diabetes, hyperlipidemia, clinical heart failure (CHF), and stroke in each GFR subgroup. This comorbidity index was used to adjust for the effects that these pre‐existing medical conditions had on clinical outcomes. Relevant cardiovascular medications for patients in each CRI subgroup are also listed. Use of calcium channel blockers and diuretics was higher in patients with severe CRI as compared with patients with normal to mildly impaired renal function. Use of statins was higher in patients in normal to moderate CRI, compared with patients with severe CRI.
Table 1.
Baseline characteristics
| Variable | Normal GFR ≥ 90 (N = 268) | Mild CRI GFR 60–89 (N = 1070) | Moderate CRI GFR 30–59 (N = 632) | Severe CRI GFR < 30 (N = 86) | Normal vs severe P‐value | Mild vs severe P‐value | Moderate vs severe P‐value |
|---|---|---|---|---|---|---|---|
| Age | 64.87 ± 12.72 | 71.99 ± 11.40 | 76.00 ± 9.87 | 76.43 ± 14.57 | <0.001 | <0.001 | 0.72 |
| Female, n (%) | 114 (43%) | 577 (54%) | 414 (66%) | 53 (62%) | 0.002 | 0.17 | 0.48 |
| BMI* | 28.77 ± 7.25 | 28.79 ± 6.11 | 29.15 ± 6.68 | 28.01 ± 7.56 | 0.40 | 0.27 | 0.15 |
| BSA* | 1.94 ± 0.31 | 1.89 ± 0.25 | 1.86 ± 0.26 | 1.82 ± 0.30 | 0.002 | 0.02 | 0.18 |
| Parox Afib*, n (%) | 57 (21%) | 202 (19%) | 157 (25%) | 23 (27%) | 0.29 | 0.08 | 0.70 |
| renal_disease, n (%) | 81 (30%) | 375 (35%) | 370 (59%) | 82 (95%) | <0.001 | <0.001 | <0.001 |
| Dialysis, n (%) | 0 (0%) | 3 (0%) | 5 (1%) | 21 (24%) | <0.001 | <0.001 | <0.001 |
| Chf*, n (%) | 43 (16%) | 183 (17%) | 156 (25%) | 41 (48%) | <0.001 | <0.001 | <0.001 |
| Htn*, n (%) | 212 (79%) | 867 (81%) | 577 (91%) | 84 (98%) | <0.001 | <0.001 | 0.04 |
| Cad*, n (%) | 120 (45%) | 532 (50%) | 358 (57%) | 51 (59%) | 0.02 | 0.09 | 0.64 |
| Diabetes, n (%) | 130 (49%) | 461 (43%) | 346 (55%) | 49 (57%) | 0.17 | 0.01 | 0.70 |
| Hyperlip*, n (%) | 209 (78%) | 859 (80%) | 544 (86%) | 71 (83%) | 0.36 | 0.61 | 0.38 |
| Stroke, n (%) | 44 (16%) | 253 (24%) | 195 (31%) | 48 (56%) | <0.001 | <0.001 | <0.001 |
| Statin, n (%) | 94 (35%) | 395 (37%) | 268 (42%) | 21 (24%) | 0.07 | 0.02 | 0.05 |
| Ccblock*, n (%) | 30 (11%) | 129 (12%) | 95 (15%) | 27 (31%) | <0.001 | <0.001 | 0.001 |
| ace_arb*, n (%) | 83 (31%) | 343 (32%) | 240 (38%) | 23 (27%) | 0.46 | 0.31 | <0.001 |
| alpha_block*, n (%) | 12 (4%) | 55 (5%) | 24 (4%) | 2 (2%) | 0.37 | 0.25 | 0.04 |
| Diuretic, n (%) | 75 (28%) | 354 (33%) | 270 (43%) | 42 (49%) | <0.001 | 0.003 | 0.49 |
| anti_coag*, n (%) | 61 (23%) | 189 (18%) | 134 (21%) | 19 (22%) | 0.90 | 0.30 | 0.28 |
| anti_arrhy*, n (%) | 4 (1%) | 27 (3%) | 19 (3%) | 2 (2%) | 0.60 | 0.91 | 0.85 |
| Vasodilator, n (%) | 47 (18%) | 159 (15%) | 118 (19%) | 13 (15%) | 0.60 | 0.95 | 0.73 |
| lv_dys_grade*, n (%) | 0.004 | 0.003 | 0.41 | ||||
| Grade 1 | 133 (50%) | 496 (46%) | 240 (38%) | 25 (29%) | |||
| Grade 1a | 41 (15%) | 208 (19%) | 154 (24%) | 24 (28%) | |||
| Grade 2 | 84 (31%) | 345 (32%) | 213 (34%) | 32 (37%) | |||
| Grade 3+ | 10 (4%) | 21 (2%) | 25 (4%) | 5 (6%) |
ace_arb, ACE inhibitor or angiotensin receptor blocker; afib, Paroxysmal atrial fibrillation; alpha_block, alpha‐blocker; anti_coag, anti‐coagulant; BMI, body mass index; BSA, body surface area; cad, coronary artery disease; ccblock, calcium channel blocker; chf, clinical heart failure; GFR, glomerular filtration rate; hyperlip, hyperlipidemia; htn, hypertension; lv dys grade, left ventricular diastolic grade.
Mean + SD or number (%).
Baseline echocardiographic parameters
Table 2 provides the mean values of various echo parameters traditionally associated with DD. There was a significant correlation between worsening GFR and degree of DD assessed by echo Doppler E/e′ ratio (P‐trend = 0.005), LVMI (P‐trend = 0.004), and right ventricular systolic pressure (P‐trend = 0.01) after adjustment for age, gender, and comorbidities. Diastolic grade was expressed as an ordinal variable and was found to correlate with worsening GFR after adjustment for age, gender, and comorbidity (P‐trend = 0.05).
Table 2.
Baseline echocardiographic characteristics
| Variable | Norm GFR ≥ 90 mL/min/1.73 m2 (N = 268) | Mild CRI GFR 60–89 mL/min/1.73 m2 (N = 1070) | Mod CRI GFR 30–59 mL/min/1.73 m2 (N = 632) | Severe CRI GFR < 30 mL/min/1.73 m2 (N = 86) | Trend P‐value | Trend P‐value (age/sex adj) | Trend P‐value (age/sex and comorb adj) |
|---|---|---|---|---|---|---|---|
| EF (%) | 62.15 ± 6.26 | 62.87 ± 6.14 | 63.49 ± 6.30 | 62.66 ± 6.16 | 0.02 | 0.39 | 0.36 |
| LV Dys grade* (ordinal) | 1.89 ± 0.98 | 1.90 ± 0.93 | 2.04 ± 0.95 | 2.22 ± 0.97 | <0.001 | 0.008 | 0.05 |
| la_volume* (cc) | 68.54 ± 24.69 | 67.00 ± 21.06 | 67.77 ± 21.34 | 70.01 ± 26.63 | 0.69 | 0.49 | 0.72 |
| la_vol_index* (cc/m2) | 35.33 ± 11.70 | 35.68 ± 10.80 | 36.69 ± 11.27 | 38.37 ± 13.75 | 0.01 | 0.63 | 0.50 |
| RV_P_s_CWD* (mmHg) | 34.86 ± 11.73 | 34.89 ± 10.50 | 36.81 ± 11.69 | 45.24 ± 17.11 | <0.001 | 0.007 | 0.01 |
| lv_mass_index* (g/m2) | 97.84 ± 29.02 | 98.09 ± 25.45 | 99.55 ± 24.75 | 108.60 ± 37.07 | 0.01 | 0.002 | 0.004 |
| mitral_E* (m/s) | 0.74 ± 0.24 | 0.73 ± 0.23 | 0.76 ± 0.25 | 0.85 ± 0.28 | <0.001 | 0.03 | 0.10 |
| mitral_a* (m/s) | 0.85 ± 0.23 | 0.87 ± 0.24 | 0.90 ± 0.24 | 0.9 ± 0.29 | <0.001 | 0.57 | 0.58 |
| mitral_ea_ratio* | 0.94 ± 0.55 | 0.90 ± 0.43 | 0.92 ± 0.47 | 0.97 ± 0.63 | 0.88 | 0.09 | 0.17 |
| E/e′ ratio* | 12.28 ± 5.75 | 12.89 ± 5.85 | 14.37 ±6.65 | 16.4 ± 6.33 | <0.001 | <0.001 | 0.005 |
E/e′, ratio of early diastolic mitral inflow velocity and early diastolic mitral annular velocity; EF, ejection fraction; lv_dys_grade, left ventricular diastolic grade; lv_mass_index, left ventricular mass index; la_volume, left atrial volume; mitral_ea_ratio, ratio of mitral early and atrial components of mitral inflow; mitral_a, mitral atrial component of mitral inflow; mitral_e, mitral early companant of inflow; RV_P_s_CWD, right ventricular systolic pressure.
Mean + SD.
Long‐term outcomes and renal function
All‐cause mortality
Figure 1 A illustrates cumulative survival free of all‐cause mortality for various CRI subgroups over the course of follow‐up through Kaplan–Meier curves. Univariate analysis showed a statistically significant trend between all‐cause mortality and worsening GFR. Mortality at 6 years for patients was 25% for normal CRI, 24% for mild CRI, 36% for moderate CRI, and 72% for severe CRI. Cox regression analysis was used to estimate the risk of death in CRI subgroups relative to normal renal function. This analysis showed a 2.3‐fold increased risk of all‐cause mortality for the severe CRI group after adjustment for age, gender, and the comorbidity index (HR: 2.31, 95% CI: 1.62–3.30, P < 0.0001).
Figure 1.
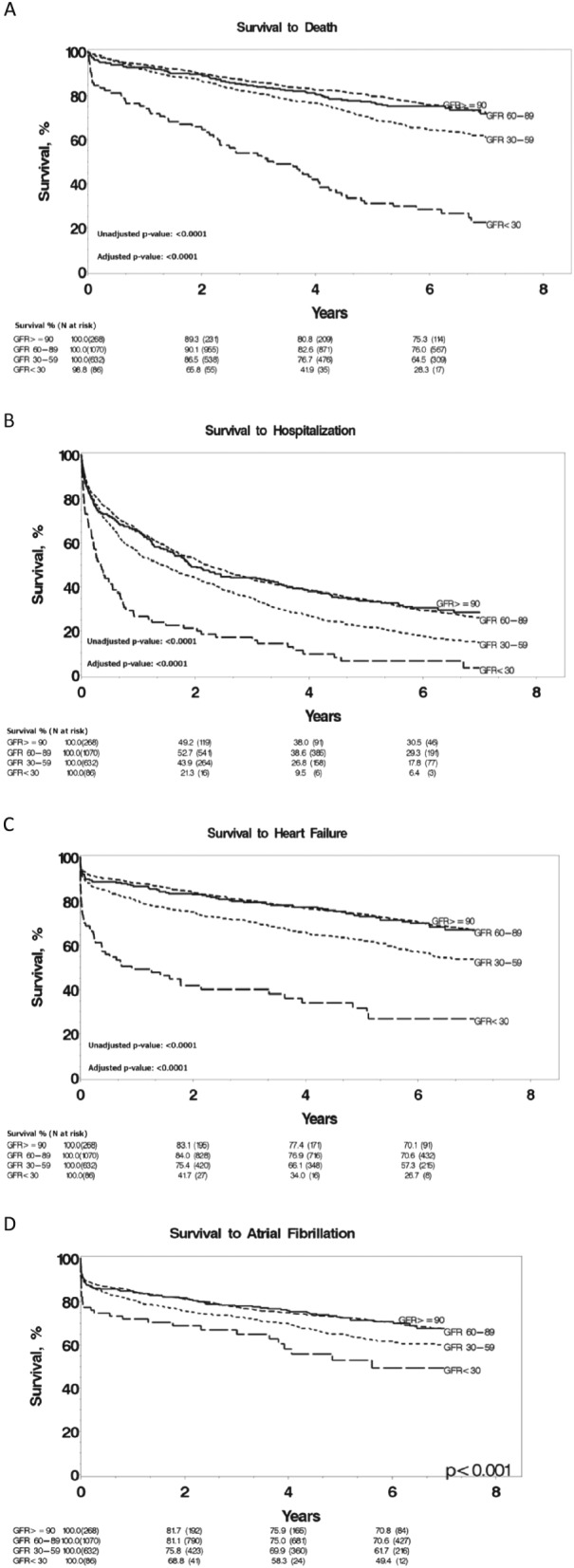
Kaplan–Meier survival curves for all‐cause mortality (A), hospitalization (B), heart failure (C), and atrial fibrillation (D) as a function grouped by glomerular filtration rate. GFR, glomerular filtration rate. P‐values adjusted for age, gender, and comorbidities.
All‐cause hospitalization
Figure 1 B demonstrates time to first hospitalization for patients in all CRI subgroups. Hospitalization within the first 3 months of initial echocardiograms was not included. First time hospitalization rates at 6 years were 70% for normal CRI, 71% for mild CRI, 82% for moderate CRI, and 94% for severe CRI. Cox regression analysis showed a 1.9‐fold increased risk of hospitalization for severe CRI relative to normal renal function after adjustment for age, gender, and the comorbidity index (HR: 1.91, 95% CI: 1.43–2.54, P < 0.0001). The risk of hospitalization in the other CRI subgroups was similar to those with normal renal function.
Development of clinical heart failure
Figure 1 C demonstrates documented development of CHF amongst various GFR subgroups. Percentage of patients who developed clinically documented heart failure at 6 years was as follows: 30% for normal CRI, 29% for mild CRI, 43% for moderate CRI, and 73% for severe CRI. Results of Cox regression analysis showed a 2.2‐fold increased risk of CHF for severe CRI compared with normal renal function after adjustment for age, gender, and the comorbidity index (HR: 2.20, 95% CI: 1.52–3.17, P < 0.0001).
Development of atrial fibrillation
Figure 1 D demonstrates documented development of atrial fibrillation amongst various GFR subgroups. Percentage of patients who developed clinically documented atrial fibrillation at 6 years was as follows: 29% for normal CRI, 29% for mild CRI, 38% for moderate CRI, and 51% for severe CRI. Results of Cox regression analysis showed a 1.4‐fold increased risk of atrial fibrillation for severe CRI compared with normal renal function after adjustment for age, gender, and the comorbidity index (HR: 1.36, 95% CI: 0.89–2.07, P < 0.0001).
Renal function was additionally evaluated as a continuous variable in Cox regression analysis for death, CHF, and all‐cause hospitalization. Every 20 mL/min/1.73 m2 decrease in GFR was associated with increased risk of death (HR: 1.14, 95% CI: 1.06–1.23, P = 0.002), CHF (HR: 1.18, 95% CI: 1.09–1.27, P < 0.0001), and hospitalization (HR: 1.10, 95% CI: 1.04–1.16, P = 0.0006) adjusted for age, gender, and comorbidities.
Long‐term outcomes and diastolic dysfunction
All‐cause mortality
Subjects were additionally grouped according to normal and abnormal diastolic function to assess differences in risk of all‐cause mortality (Figure 2 A). Abnormal diastolic function was associated with progression to death over the course of follow‐up and a 1.3‐fold increased risk of mortality after correction for CRI group, age, gender, and comorbidities (HR: 1.32, 95% CI: 1.11–1.55, P = 0.001).
Figure 2.
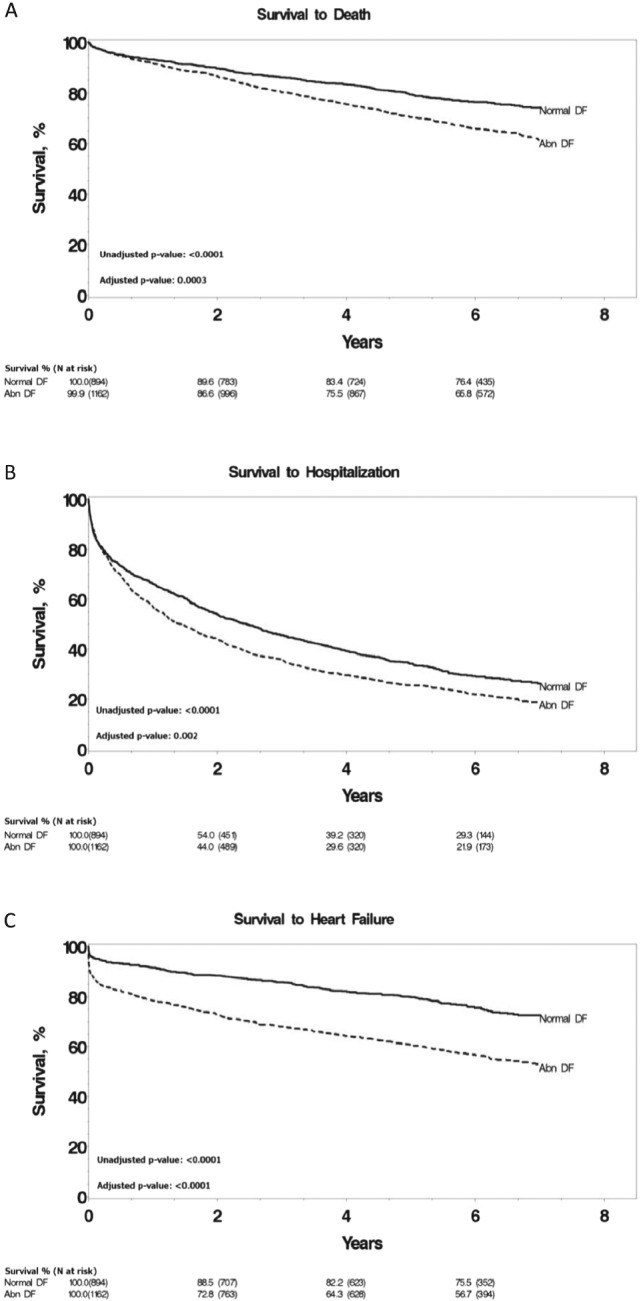
Kaplan–Meier survival curves for all‐cause mortality (A), hospitalization (B), and heart failure (C) grouped by normal and abnormal diastolic function. Normal DF, normal diastolic function; Abnormal DF, abnormal diastolic function. P‐values adjusted for age, gender, and comorbidities.
All‐cause hospitalization
Similar to all‐cause mortality, subjects were additionally segregated according to normal and abnormal diastolic function to assess for differences in risk of all‐cause hospitalization (Figure 2 B). Abnormal diastolic function was associated with a 1.2‐fold increased risk of hospitalization during the follow‐up after adjustment for CRI group, age, gender, and comorbidities (HR: 1.16, 95% CI: 1.05–1.29, P = 0.005).
Development of clinical heart failure
Subjects with abnormal diastolic function were found to have an increased progression to CHF over the course of follow‐up (Figure 2 C). The risk of progression was increased to 1.7‐fold in patients with abnormal DD after adjustment for CRI group, age, gender, and comorbidities (HR: 1.74, 95% CI: 1.48–2.05, P < 0.0001).
Diastolic dysfunction was defined as a continuous variable using the E/e′ ratio. Every standard deviation increase in E/e′ ratio (6.2 units) was associated with increased risk of death (HR: 1.17, 95% CI: 1.10–1.25, P < 0.0001), CHF (HR: 1.34, 95% CI: 1.26–1.42, P < 0.0001), and hospitalization (HR: 1.11, 95% CI: 1.06–1.17, P < 0.0001) after adjustment for age, gender, and comorbidities.
Interaction between GFR and DD
We additionally tested for interactions between DD and CRI for each endpoint. Cox regression analysis did not reveal significant interactions between both disease processes that could be associated with multiplicative increases in long‐term morbidity and mortality. Interaction analysis revealed P‐values between 0.20 and 0.88.
Discussion
Our study resulted in three major findings. Firstly, there is a graded correlation between worsening renal function and worsening DD, namely, LVMI, right ventricular systolic pressures, E/e′, and left atrial volume index. Secondly, increasing severity of CRI was associated with long‐term morbidity and mortality. Thirdly, the presence of DD was associated with long‐term morbidity and mortality. However, modified Cox regression analysis did not demonstrate an interaction between DD and GFR, revealing that DD carries an increased risk of cardiac morbidity and mortality across all ranges of GFR.
Our results substantiate previous work that has demonstrated an association between renal dysfunction and DD as measured by serial echocardiography.14, 15 Cai et al. illustrated an increased progression of left ventricular hypertrophy and left atrial mass index and reduction in peak systolic and diastolic mitral annular velocity in a cohort of 300 patients with CKD stage 3‐5.15 In a sub‐study of 217 patients from the Paramount study who had echo‐confirmed HFpEF with concurrent measures of kidney function, there was an association between abnormal renal function (GFR < 60 mL/min/1.73 m2 or albuminuria) and greater LV wall thickness and LV mass.14 Our study extends these findings by demonstrating a graded association between worsening CRI, as measured by the modification of diet in renal disease equation, and echocardiographic markers of DD. Further studies should focus on assessing progression of DD in CRI patients in a community cohort through serial echocardiography to further characterize the progression over time for DD.
Our cohort‐based study also reveals that worsening renal insufficiency is associated with increased cardiovascular morbidity and mortality. Previous population‐based studies support our findings. In a study of 754 heart failure patients, survival was significantly tied to renal insufficiency with increased mortality associated with worsening renal dysfunction (P = 0.002).16 Additionally, reduced GFR was associated with an increased risk of cardiovascular death and heart failure hospitalizations in 2680 North American patients with systolic and DD.17 Our study supports these findings and expands on these results by demonstrating that the presence of DD was associated with worse long‐term outcomes independent of renal function.
Redfield et al. have illustrated in a large community cohort that mild DD, without a clinical diagnosis of heart failure, was associated with increased mortality (HR: 8.31, 95% CI: 3.00–23.1, P < 0.001) consistent with the findings of our studies.12 Further population‐based studies have additionally confirmed an increased progression of symptomatic heart failure in patients with preclinical DD and renal dysfunction.5 Our results not only confirm these findings but also show that worsening DD is associated with an increased risk of progression to death, heart failure, and all‐cause hospitalization independent of renal function.
Interestingly, adjusted Cox regression analysis did not demonstrate a significant interaction between DD and GFR in our cohort. This does not imply that the two disease processes are independent, but rather that there is no multiplicative or synergistic effect between DD and renal dysfunction in leading to morbidity and mortality. This may imply that reversal in renal function may not improve cardiovascular morbidity and mortality associated with DD. Previous clinical studies have revealed that improvement in renal function yielded only limited cardiovascular benefits. In a group of 143 patients on hemodialysis for end‐stage renal disease who underwent renal transplantation, there was a decrease in LVMI at 2 years post‐transplantation. Further regression in LVMI was not noted at Years 3 and 4 post‐transplantation.18
The role that CRI plays in the progression of DD must still be explored further in future studies. We demonstrated the graded association in a community cohort between worsening renal insufficiency and DD as well as cardiovascular morbidity and mortality. Interestingly, we report for the first time that abnormal diastolic function was associated with increased progression to death, heart failure, and hospitalization irrespective of GFR. Future studies should focus on monitoring CRI patients with serial echocardiography to assess progression of DD as well as investigate potential underlying pathophysiological mechanisms that contribute to the progression of both CRI and DD.
Limitations
There remains the risk of selection bias in our community cohort, because our retrospective study only selected patients who had undergone echocardiography at the start of the study for clinical indications and not for research related purposes. Given the retrospective nature of our study, it was not possible to obtain serial echocardiograms on patients at well‐defined intervals. While the Rochester Epidemiology Project offers an unprecedented glimpse into the longitudinal nature of certain diseases, the lack of diversity of the cohort may remain an issue. Recent reports indicate that the Olmsted County community, which encompasses the Rochester Epidemiology Project, is predominantly (>80%) Caucasian.19 Further studies may need to be performed to assess for the interactions of DD and CRI on clinical endpoints in other ethnic groups.
Conclusions
In summary, this population‐based study demonstrates that DD and CRI were both associated with increased risk for all‐cause mortality, all‐cause hospitalization, and development of heart failure over the follow‐up period. The subgroup analysis confirms a graded association between CRI and worsening DD. Our study is unique in that statistical analysis revealed no interaction between DD and CRI, suggesting no synergistic effect of both conditions in progression to cardiac morbidity and mortality. It will be necessary to further assess the impact that renal insufficiency has on diastolic function via serial echocardiography in a community cohort. It is also possible that CRI and DD are both manifestations of an underlying pathophysiological process. Further studies should focus on the elucidation of this potential underlying process.
Conflicts of interest
H.H.C. has filed patents for chimeric natriuretic peptides. Mayo Clinic has licenced patents to Capricor Therapeutics and Anexon with other patents pending at the US patent office. H.H.C. has received royalties from Capricor Therapeutics, Anexon Inc., and UpToDate and is the co‐founder of Zumbro Discovery Inc.
Funding
This research was supported by grants from the National Institutes of Health [PO1 HL 76611, R01 HL‐84155]; the Rochester Epidemiology Project from the National Institute on Aging [grant number R01 AG034676]; and Mayo Foundation.
H.H.C. has received research grants from NIH; Scios Inc; and Mayo Clinic.
Acknowledgements
Initial results of this manuscript were presented as an abstract poster at the American College of Cardiology Scientific Session in 2015. Further data collection and analysis has been provided in this manuscript.
Jain, A. , Scott, C. , and Chen, H. H. (2017) The renal–cardiac connection in subjects with preserved ejection fraction: a population based study. ESC Heart Failure, 4: 266–273. doi: 10.1002/ehf2.12143.
References
- 1. Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 2001; 87: 413–419. [DOI] [PubMed] [Google Scholar]
- 2. Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 2003; 41: 217–223. [DOI] [PubMed] [Google Scholar]
- 3. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 4. Tsukamoto Y, Mano T, Sakata Y, Ohtani T, Takeda Y, Tamaki S, Omori Y, Ikeya Y, Saito Y, Ishii R, Higashimori M, Kaneko M, Miwa T, Yamamoto K, Komuro I. A novel heart failure mice model of hypertensive heart disease by angiotensin II infusion, nephrectomy, and salt loading. Am J Physiol Heart Circ Physiol 2013; 305: H1658–H1667. [DOI] [PubMed] [Google Scholar]
- 5. Vogel MW, Slusser JP, Hodge DO, Chen HH. The natural history of preclinical diastolic dysfunction: a population‐based study. Circ Heart Fail 2012; 5: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wan, S.‐H. , Vogel M.W., and Chen H.H., Preclinical Diastolic Dysfunction . Journal of the American College of Cardiology, 2013. [Google Scholar]
- 7. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047. [DOI] [PubMed] [Google Scholar]
- 8. Levey AS, Coresh J. Chronic kidney disease. The Lancet in press; 379: 165–180. [DOI] [PubMed] [Google Scholar]
- 9. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 10. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: the Rochester Epidemiology Project (REP) medical records‐linkage system. Int J Epidemiol 2012; 41: 1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Melton Iii LJ. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996; 71: 266–274. [DOI] [PubMed] [Google Scholar]
- 12. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289: 194–202. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254. [DOI] [PubMed] [Google Scholar]
- 14. Gori M, Senni M, Gupta DK, Charytan DM, Kraigher‐Krainer E, Pieske B, Claggett B, Shah AM, Santos AB, Zile MR, Voors AA, McMurray JJ, Packer M, Bransford T, Lefkowitz M, Solomon SD; PARAMOUNT Investigators . Association between renal function and cardiovascular structure and function in heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3442–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai Q‐Z, Lu X‐Z, Lu Y, Wang AY‐M. Longitudinal Changes of Cardiac Structure and Function in CKD (CASCADE Study). J Am Soc Nephrol, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 2004; 109: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 17. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ; Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators . Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678. [DOI] [PubMed] [Google Scholar]
- 18. Rigatto C, Foley RN, Kent GM, Guttmann R, Parfrey PS. Long‐term changes in left ventricular hypertrophy after renal transplantation. Transplantation 2000; 70: 570–575. [DOI] [PubMed] [Google Scholar]
- 19. Bureau, U.S.C . Census Data for Olmsted County, Mn. U.S: Census; 2010. [Google Scholar]


