Abstract
Aims
Beyond the influence of stimulating devices on cardiac excitation, their use in treating patients with heart failure has positive effects on the myocardium at the molecular level. Electrical signals can induce a wide spectrum of effects in living tissue. Therefore, we sought to determine whether applying electrical microcurrent directly to failing hearts leads to functional improvement.
Methods and results
Sixteen male spontaneously hypertensive rats (SHRs) with heart failure underwent application of a patch electrode to the left ventricular epicardium and placement of a subcutaneous counter electrode. The electrode delivered a 0.35 μA microcurrent to nine of the SHRs for 45 ± 3 days; the other seven SHRs were used as controls. At baseline and before the SHRs were humanely put to death, we measured the left ventricular ejection fraction (LVEF) and the thickness of the LV posterior wall during systole and diastole (LVPWs/d). We used quantitative PCR to determine extracellular matrix parameters [collagen I–III, matrix metalloproteinase (MMP)‐2, MMP‐9, tissue inhibitor of metalloproteinases 3 (TIMP3), TIMP4, connexins (Cxs) 40/43/45, transforming growth factor (TGF)‐β, and interleukin (IL)‐6].
Among SHRs undergoing microcurrent application, LVEF normalized (mean decrease, 22.8%; P = 0.009), and LVPWs decreased (mean, 35.3%; P = 0.001). Compared with the control group, the SHRs receiving microcurrent exhibited a mean decrease in the gene expression of collagen I (10.6%, P = 0.003), TIMP3 (18.5%, P = 0.005), Cx43 (14.3%, P = 0.003), Cx45 (12.7%, P = 0.020), TGF‐β (13.0%, P = 0.005), and IL‐6 (53.7%, P = 0.000). Microcurrent application induced no changes in the expression of collagen III, MMP‐2, MMP‐9, TIMP4, or Cx40.
Conclusions
Applying microcurrent to the LV epicardium of SHRs leads to statistically significant functional improvement and alterations in the levels of inflammatory and extracellular matrix components.
Keywords: Bioelectric, Electrical stimulation, Heart failure, Collagen, Extracellular matrix, Reverse remodelling
Introduction
Heart failure has become a global disease, affecting an estimated 26 million persons worldwide. Despite the progress made in the treatment of heart failure, optimal medical therapy is often not sufficient to save the affected patients from an insidious decline in their cardiac function over the long run.1 Therefore, device‐based therapies are playing an increasing role in the treatment of heart failure. Cardiac resynchronization therapy (CRT) is a well‐established procedure; however, only roughly one‐third of New York Heart Association Class III patients are good candidates for this type of treatment, and ~30% of these do not respond to treatment.2 Interestingly, hearts that improve with CRT frequently exhibit not only an enhancement of excitation propagation but also a recovery of function, as a sign of additional effects of the electrical stimulation.3
Cardiac contractility modulation (CCM) is another method that has achieved divergent results in several clinical studies involving patients with heart failure.4 Devices stimulating the vagus nerve belong to a recently rediscovered group of electroceuticals that aim to improve failing organs by exciting organ‐specific nerves. Moreover, attempts have been made to expand this method to the stimulation of nerves from organs affected by inflammatory diseases such as rheumatoid arthritis, and these attempts have achieved astonishingly promising results among treated patients.5
Our group recently reported that exposing cardiomyocytes to electrical microcurrent under cell‐culture conditions leads to an immediate increase in the proliferation rate of the cells and to modulation of components of the extracellular matrix, such as matrix metalloproteinases (MMPs) and the tissue inhibitors of metalloproteinase (TIMP).6 Because the extracellular matrix is a reservoir for various signalling molecules (cytokines, hormones, and growth factors), its dysfunction plays an important role in the development of heart failure.7
Moreover, electrical current has been used successfully to support several healing processes, albeit without a complete and detailed understanding of the overall complex electrochemical and electrobiological interactions involved. For example, electrical current has been used to stimulate the healing of wounds, complex bone fractures, and fusion of the spine.8 With its use, alterations in both the inflammatory process and gene expression have been described, with resultant clinical improvement.9
The clinical experience with electrophysiological devices and their positive impact on cardiac function, the proven effect of microcurrent applied to cultured cardiomyocytes, and the unmet medical need to improve the treatment of heart failure encouraged us to test the effect of chronically applied non‐excitatory, non‐pulsatile electrical microcurrent to the hearts of spontaneously hypertensive rats (SHRs) at a progressed stage of the disease. Because a failing heart is an inflamed organ and endogenous microcurrent is known to have physiologically anti‐inflammatory effects, we questioned whether artificial microcurrent applied to the epicardium of severely hypertrophic hearts can modify the cardiac function and gene expression of inflammatory components of cardiac tissue, and whether a long‐term objective might be the treatment of heart failure with microcurrent. The recently published paper by Lujan and DiCarlo supports the hypothesis underlying this approach.10
Methods
The SHR model was selected because it is well characterized and has been described comprehensively.11 The rats were provided by the Max Delbrück Center (Berlin, Germany). The provided substrain is characterized by early (5 weeks) development of hypertension and corresponding effects on hypertrophy and diastolic dysfunction. As a criterion for inclusion in the study, a left ventricular ejection fraction (LVEF) higher than 90% was chosen as a surrogate parameter for clinically significant diastolic cardiac dysfunction. A further inclusion parameter was a systolic blood pressure higher than 180 mmHg. Experiments were performed in accordance with the local Institutional Review Board's directives for animal care and use (GZ 66.009/0153‐BrGT/2006 and 66.009/185‐C/GT/2007 Austria). Housing, handling, and experimental procedures followed the written recommendations of the Guide for the Care and Use of Laboratory Animals.12 At the end of the study, all rats were put to death while under anaesthesia by the injection of 100 mg per kg body weight ketamine and the intraperitoneal injection of 10 mg xylazine, according to the guidelines of the local Institutional Animal Care and Use Committee of the Medical University of Vienna.
Surgical procedure
Through a left thoracotomy, a platinum patch electrode (surface area, 1 cm2) was stitched to the left ventricular epicardium of 16 male SHRs. A subcutaneous counter electrode was placed on the contralateral side of the thorax. Both electrode wires converged at the neck. The left ventricular epicardium of nine of the SHRs (mean age, 10.7 ± 4.1 months; mean weight, 293.7 ± 67.7 g) received application of a direct microcurrent of 0.35 μA for 8.5 h daily over a mean period of 45 ± 3 days. SHRs exposed to microcurrent were kept in a large separate cage. They were not anaesthetized or otherwise treated during the procedure. The microcurrent was defined as a direct current that could not excite the cardiomyocytes into inducing contraction. The amplitude of the microcurrent was derived from in vitro experiments with cardiomyocytes. Under culture conditions, the chosen intensity was in the range of optimal survival of cardiomyocytes. Furthermore, the amplitude of the current was chosen such that neither a measurable increase in the temperature of exposed tissue nor corrosion of the electrodes could be detected. The remaining seven SHRs, composing a control group (mean age, 10.9 ± 5.1 months; mean weight, 301.9 ± 37.2 g), were not exposed to the microcurrent.
Echocardiography
At baseline and before the SHRs were humanely put to death, we performed transthoracic echocardiography [Vivid 7 Dimension Cardiovascular Ultrasound System (GE Healthcare, Solingen, Germany) with a 10S sector array probe (4–11.5 MHz)] to determine the two‐dimensional LVEF and the thickness of the LV posterior wall during systole and diastole (LVPWs/d). Rats were placed in the left lateral decubitus position after being anaesthetized with pentobarbital sodium (50 mg/kg) according to established American College of Cardiology/American Heart Association Guidelines and those outlined by Slama and colleagues.13 The same operator performed all echocardiographic examinations and was unaware of the treatment assignment or the timeline of the experiment.
Data analysis
Independent‐samples t‐tests (SPSS Statistics 17.0.2, Chicago, IL, USA) were used to compare variables between the two groups. We measured functional parameters at baseline and at the end of the experiment and compared the two measurements by using the paired‐samples t‐test. Statistical significance was set at the level of P < 0.05. A Bonferroni correction for multiple hypothesis tests was not applied because of the strong dependencies between the tested variables, which had been confirmed by the calculation of Pearson product–moment correlation coefficients.14 Furthermore, because this was an exploratory pilot study, primary endpoints were not established and therefore could not be used to determine the necessary number of animals by power calculations. The number of animals examined was determined on the basis of comparable studies and the authors' experience.
Quantitative PCR analysis
Myocardial specimens were collected from all 16 SHRs. All myocardial specimens were taken from the LVPW, which was in the central flow zone of the electrical current. Quantitative PCR (qPCR) was used to measure the levels of the following components of the extracellular matrix of the myocardial specimens: collagen I, collagen III, MMP‐2, MMP‐9, TIMP3, TIMP4, connexins (Cxs) 40/43/45, transforming growth factor (TGF)‐β, and interleukin (IL)‐6. The qPCR analyses were performed in a certified laboratory according to the rules of good laboratory practice.
Total RNA was isolated from the cardiac specimens (~30 mm3) with TRI Reagent (Sigma‐Aldrich, Vienna, Austria), according to the manufacturer's instructions. RNA was suspended in diethylpyrocarbonate‐treated water (Sigma‐Aldrich), and concentrations were determined by spectrophotometry with a Hitachi U‐2900 spectrophotometer (Hitachi, Tokyo, Japan). Using the SuperScript First Strand Synthesis System (Invitrogen, Life Technologies, Paisley, UK), we reverse transcribed 1 μg of total RNA. For cDNA synthesis, 1 μg of total RNA from each sample was reverse transcribed (SuperScript First‐Strand Synthesis System, Invitrogen). Finally, 1 μL of each cDNA was used for the PCRs. The cDNA was stored at −20 °C until use.
For the PCRs, specific primers for the rat‐specific MMP and TIMP genes (MMP‐2, MMP‐9, TIMP3, and TIMP4), the connexin genes (Cx40, Cx43, and Cx45), and collagen types I and III were designed with Primer Analysis Software (Thermo Fisher Scientific V7, Frankfurt, Germany) and synthesized by VBC‐Genomics Services (Bioscience Research, Vienna, Austria). The rat Gapdh gene was used as a control. The sequences for primers are presented in Table 1. The following PCR conditions were used: initial denaturation for 5 min at 94 °C, denaturation for 1 min at 95 °C, annealing for 1 min at a primer‐specific temperature, extension for 1 min at 72 °C for 30 cycles, and a final extension of 5 min at 72 °C. The reaction mixture was run on a 2% agarose gel stained with ethidium bromide.
Table 1.
Primers used in PCR studies
| Gene | Primer 5′–3′ | Tm (°C) | Product (bp) | Acc. number |
|---|---|---|---|---|
| ratGapdh | F: GCA AGT TCA ACG GCA CAG TCA AGG | 61.6 | 116 | NM_017008 |
| R: AGC ACC AGC ATC ACC CCA TTT G | 60.5 | |||
| ratMMP‐2 | F: CTG ACA TCA TGA TCA ACT TTG GAC G | 55.9 | 322 | NM_031054 |
| R: GCA GAA GCC ATA CTT GCC ATC C | 56.6 | |||
| ratMMP‐9 | F: ACC GCC AAC TAT GAC CAG GAT AAG C | 60.4 | 110 | NM_031055 |
| R: AAG ACG AAG GGG AAG ACG CAC ATC | 61.4 | |||
| ratTIMP3 | F: ACT TGC CTT GCT TTG TGA CCT CC | 58.4 | 166 | NM_012886 |
| R: TTG CTG ATG CTC TTG TCT GGG G | 57.6 | |||
| ratTIMP4 | F: GAG AGC CTG AAT CAT CAC TAC CAC C | 57.0 | 106 | NM_001109393 |
| R: CAG TCA GTC CAG AGA CAC TCA TTG | 57.0 | |||
| ratCx40 | F: GGC TCA CCG TCC TGT TCA TTT TC | 58.5 | 128 | NM_019280 |
| R: GGT CGT AGC AGA CAT TTT GGC AAC | 58.7 | |||
| ratCx43 | F: TCC TTG GTG TCT CTC GCT TTG AAC | 58.5 | 130 | NM_012567 |
| R: AGT CTT TTG ATG GGC TCA GTG GG | 58.5 | |||
| ratCx45 | F: AGA GCA GAG CCA ACC AAA ACC C | 58.3 | 114 | NM_001085381 |
| R: AAG CCC ACC TCA AAC ACA GTC CTG | 60.2 | |||
| ratCol type 1 | F: TGG AGA AGA AGG AAA ACG AGG AGC | 59.0 | 115 | NM_053304 |
| R: AAC ACC ATC AGC ACC AGG GAA AC | 58.8 | |||
| ratCol type 3 | F: TCC TGG TGC TAT TGG TCC ATC TGG | 60.8 | 164 | NM_032085 |
| R: TGC GTC CAT CAA AGC CTC TGT GTC | 62.1 | |||
| ratTGF‐β | F: TCA AGT CAA CTG TGG AGC AAC ACG | 58.8 | 177 | NM_021578 |
| R: AGC GAA AGC CCT GTA TTC CGT C | 56.8 | |||
| ratIL‐6 | F: ACT TCC AGC CAG TTG CCT TCT TG | 58.5 | 107 | NM_012589 |
| R: TCT GTT GTG GGT GGT ATC CTC TGT G | 58.8 |
Acc, accession; Col, collagen; Cx, connexin; Gapdh, glycerinaldehyde‐3‐phosphate dehydrogenase; IL, interleukin; MMP, matrix metalloproteinase; TGF, transforming growth factor; TIMP, tissue inhibitors of metalloproteinase; Tm, melting temperature.
Results
At baseline, all SHRs exhibited systolic blood pressure higher than the inclusion criterion of 180 mmHg (range, 192–201 mmHg). At the end of the study, systolic blood pressure of all SHRs had increased to 198 mmHg or higher (range, 198–209 mmHg). At termination, the mean body weight of the treated SHRs had increased to 302.4 ± 42.4 g, and that of the non‐treated group had decreased slightly to 299.6 ± 31.8 g; the differences were not statistically significant either between or within the groups. At termination, the mean weight of the hearts of the treated group was 1.43 ± 0.11 g, and that of the untreated group was 1.70 ± 010 g. This difference was statistically significant (P = 0.0003).
Comparison of the echocardiographic variables of the SHRs at baseline and at the end of the experiment showed that the nine SHRs exposed to the microcurrent exhibited a statistically significant normalization of the LVEF (mean decrease, 22.8%; P = 0.009) and a significant decrease in the thickness of the LVPWs (mean decrease, 35.3%; P = 0.001) (Figure 1, A and B; Table 2). The thickness of LVPWd decreased by 20%, but this difference did not reach statistical significance. The seven SHRs not exposed to the microcurrent exhibited no significant changes in echocardiographic variables (baseline LVEF, 96.4% ± 2.0%; termination LVEF, 97.6% ± 2.8%; baseline LVPWs, 0.49 ± 0.1 cm; termination LVPWs, 0.52 ± 0.08 cm; baseline LVPWd, 0.32 ± 0.07 cm; termination LVPWd, 0.35 ± 0.09 cm).
Figure 1.
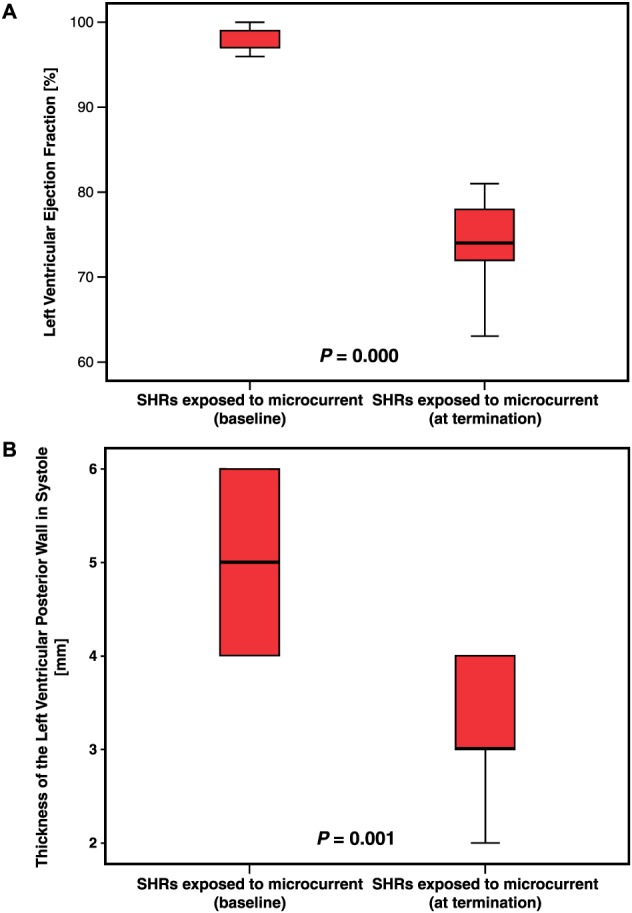
Left ventricular ejection fraction (A) and thickness of the left ventricular wall in systole (B). Measurements were taken at baseline before microcurrent application (left) and at termination just before the end of the experiment after 45 days of microcurrent application (right). Statistically significant differences were found between baseline and termination in A (P = 0.000) and in B (P = 0.001).
Table 2.
Numeric and statistical results of echocardiographic measurements at baseline and at termination
| Baseline (n = 9) | At termination (n = 9) | Difference of (n = 9) | P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Means | Standard error | ||
| EF (%) | 97.9 | 1.45 | 74.9 | 7.49 | 23.0 | 2.77 | 0.000 |
| LVPWs (cm) | 0.51 | 0.09 | 0.33 | 0.07 | 0.18 | 0.04 | 0.001 |
| LVPWd (cm) | 0.30 | 0.05 | 0.24 | 0.05 | 0.06 | 0.03 | 0.095 |
EF, ejection fraction; LVPWd, left ventricular posterior wall thickness in diastole;
LVPWs, left ventricular posterior wall thickness in systole. Bold means statistically significant.
A comparison of the measurements of the components of the extracellular matrix showed that the myocardial specimens from the SHRs that received the microcurrent exhibited a statistically significant down‐regulation in the expression of collagen type I (10.6%; P = 0.003), TIMP3 (18.4%; P = 0.005), Cx43 (14.3%; P = 0.003), Cx45 (12.7%; P = 0.020), TGF‐β (13.0%; P = 0.005), and IL‐6 (53.7%; P = 0.000) (Figure 2, A–F; Table 3). No statistically significant differences induced by the microcurrent could be detected in the down‐regulation of collagen type III (1.4%), MMP‐2 (1.7%), MMP‐9 (2.8%), TIMP4 (5.2%), or Cx40 (4.7%).
Figure 2.
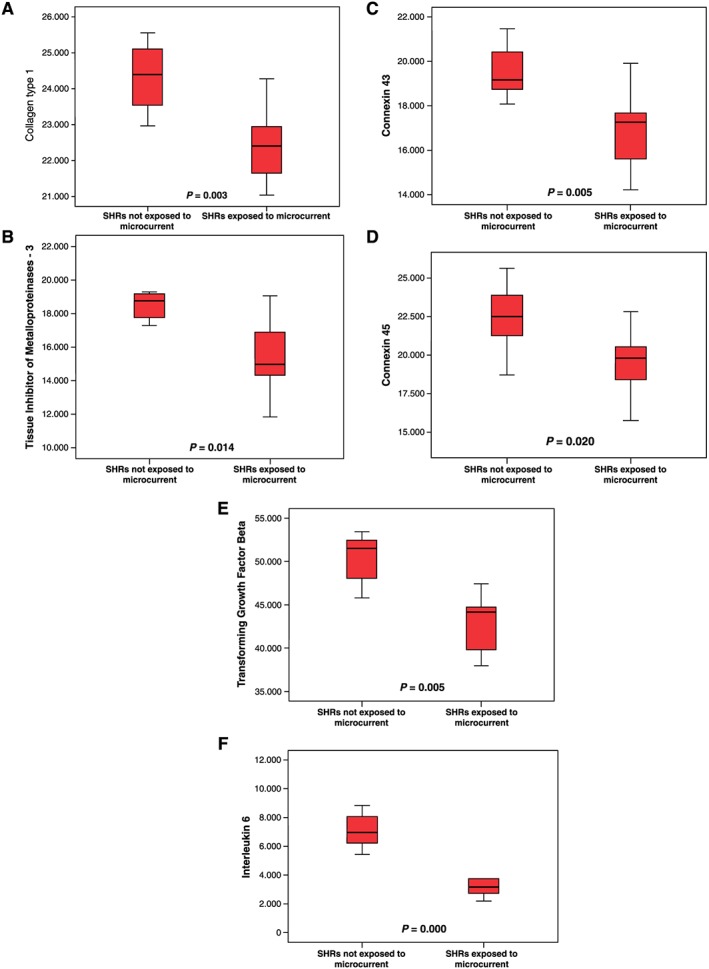
Myocardial mRNA expression of collagen type I (A), tissue inhibitors of metalloproteinases‐3 (B), connexin 43 (C), connexin 45 (D), transforming growth factor‐β (E), and interleukin 6 (F). Myocardial specimens were taken from SHRs (sham) not exposed to microcurrent (left) and after 45 days of microcurrent exposure (right) at termination of the experiment. A significant difference between the specimens was found in collagen type I (P < 0.003), tissue inhibitors of metalloproteinases‐3 (P < 0.014), connexin 43 (P < 0.005), connexin 45 (P < 0.020), transforming growth factor‐β (P < 0.005), and interleukin 6 (P < 0.000).
Table 3.
Numeric and statistical results of myocardial mRNA expression
| SHRs not exposed to microcurrent (n = 7) | SHRs exposed to microcurrent (n = 9) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Difference of the means | Standard error difference | P value | |
| Age (months) | 10.9 | 5.1 | 10.7 | 4.1 | 0.2 | 2.3 | 0.935 |
| Col‐3, re | 30.392 | 1.637 | 30.805 | 1.898 | −0.413 | 902 | 0.654 |
| MMP‐2, re | 746 | 111 | 860 | 50 | −115 | 61 | 0.109 |
| MMP‐9, re | 7.955 | 9.463 | 10.141 | 13.024 | −2.186 | 6.669 | 0.749 |
| TIMP4, re | 21.806 | 2.714 | 20.682 | 4.662 | 1.124 | 1.989 | 0.581 |
| Cx40, re | 16.034 | 1.786 | 15.282 | 1.934 | 0.752 | 943 | 0.439 |
Col‐3, collagen type 3; Cx, connexin; MMP, matrix metalloproteinase; re, relative expression; SHR, spontaneously hypertensive rat; TIMP, tissue inhibitor of metalloproteinases.
The numbers present fold change compared with housekeeping gene (Gapdh) measured as light intensity (pixel/cm2).
Discussion
This pilot study, which, for the first time, applied constant electrical signals chronically to a beating heart with the intention of treating impaired cardiac function, achieved extremely beneficial results that raise more questions than they answer. Our intent with this experiment was to confirm our hypothesis that microcurrent can influence the inflammation of the components of the extracellular matrix and can affect organ function. We focused on certain components of the extracellular matrix, because existing evidence from studies of mechanical circulatory support and left ventricular unloading has shown that normalization of the extracellular matrix is an important precursor to a reverse remodelling of cardiac function.15, 16 The duration of application of the microcurrent was derived (i) from our own clinical experience of a reverse remodelling process induced by mechanical unloading of the heart, a process that achieved optimal improvement of function within 4 to 8 weeks; (ii) from studies using cardiomyocytes exposed to microcurrent under culture conditions, with findings showing an alteration in extracellular matrix components within days; and (iii) from published data about collagen or protein turnover, data indicating that the degradation/synthesis rates of collagen range from 3% to 6% per day.17 Indeed, we found that long‐term and direct in vivo application of microcurrent to the epicardium of beating hearts of SHRs with pronounced heart failure leads to statistically significant alterations in the gene expression of extracellular matrix components, including collagen type I, TIMP3, Cx43, and Cx45. Furthermore, application of current reduces the levels of the cytokines IL‐6 and TGF‐β. Our results and those of other groups indicate that improvement in these variables is paralleled by an impressive improvement in heart function.18
Application of electrical signal
Numerous studies have reported the application of various types of electrical energy to animals and humans, but, to our knowledge, none describes the chronic direct application of electrical signals to an inner organ by a patch electrode. Because microcurrent works multifactorially, it was not the intention of this study to elucidate its molecular mechanism of action.
Thus, the main question generated by this study is whether the induced alterations in the myocardial tissue of SHRs are in accordance with what is known about the effect of the application of electrical energy to biological systems and the effect of gene expression on heart failure. It is widely known that electrical potential gradients and currents in endogenous tissue govern many biological properties of living organisms.19, 20 Ionic channels control the plasma membrane potential and ion movement across membranes, thereby regulating various biological functions, including cellular volume status and some endogenous current‐controlled, voltage‐gated channels of the plasma membrane.21 Because particular cell types are determined by their membrane potential, any alteration in the membrane potential could then modify cell behaviour.22, 23
Clinical experience
Indications that electrical signals applied to the myocardium exert favourable effects on cardiac function have already been derived from the clinical use of certain electrophysiological devices, all aiming to reduce the severity of heart failure. One is the CRT device, the primary intention of which is to resynchronize asynchronous electrical excitation and, concurrently, the asynchronous pump mechanics of the right and left ventricles. CRT has achieved long‐term improvement in cardiac function beyond that which can be achieved solely by eliminating the conduction disturbance. Obviously, the electrical signal also induces anti‐inflammatory effects at the molecular level, which in turn affect the extracellular matrix and cardiomyocyte function.24
Another approach is treating heart failure by applying a high‐energy electrical stimulus during the refractory phase of the cardiac cycle, as is intended with the CCM device. One relevant observed effect is an increase in the level of myocyte calcium in the cytosol during systole, which accompanies moderate clinical improvement.25
Vagus nerve stimulation by electroceuticals is an additional approach. This long‐known method has recently been rediscovered for the treatment of heart failure.26 Stimulating the vagus nerve is intended to balance the aggravated activity of the sympathetic nervous system and the reduced activity of the vagus nerve, which accompanies an increase in the mortality rate among patients with decreased cardiac function. Initial clinical results with these devices are promising and reflect the aim of the recently intensified efforts in bioelectronics to use electrical signals rather than drugs to treat diseases, with the aim of dampening inflammatory processes and repairing functional losses.5, 27
Effects in cell culture
Under cell‐culture conditions, analysis of hypertrophic myocytes taken from the myocardium of 15‐month‐old SHRs has shown that exposure to microcurrent can significantly reduce cell size by 38%. In contrast, compared with H9c2 cells that were not exposed to microcurrent, regular H9c2 cardiomyocytes exposed to microcurrent for 5 days exhibited no changes in cell size (P = 0.5979). Furthermore, the microcurrent appeared to prevent H9c2 cells from increasing in size if they were simultaneously stimulated by the α1‐agonist phenylephrine (P = 0.4277). Without microcurrent exposure, phenylephrine stimulation leads to a statistically significant increase in cell size (P = 0.0000).28
Effects of endogenous electrical current
In diseased hearts, the endogenous direct current gradient of the myocardium may be disarranged by fibrotic remodelling, and, therefore, its function may be disabled.29 Thus, it is conceivable that the external application of a microcurrent could normalize the endogenous electrical gradient, either by influencing the existing current gradient or by triggering protein activation to induce favourable cellular responses.30 Initial evidence showing that electrical signals can modulate kinase activity was reported in a paper published by Zhao and colleagues in Nature.31 They found that electrical signals control wound healing in injured skin by phosphoinositide‐3 kinase isoform γ (PI3Kγ), which can be seen as a transformer of electrical cues into biological cues. The absence of endogenous electrical cues leads to an up‐regulation in the expression of phosphatase and tensin homologue (PTEN), an important antagonist of PI3K. In turn, this up‐regulation disturbs wound healing.
PTEN, PI3K, IL‐6, and TGF‐β
The PI3Kγ also plays an important role in the heart. The PI3K–protein kinase B (PI3K–Akt) pathway regulates the size, survival, angiogenesis, and inflammation of cardiomyocytes.32 PI3K signalling can promote proinflammatory cytokine production by activating nuclear factor‐κB (NFκB) downstream of AKT and can mediate IL‐6 expression, which, in our study, was down‐regulated by microcurrent.33 TGF‐β plays a central role in the induction of fibrosis by stimulation of the Smad system, which in turn increases the expression of collagen types I, III, and VI and accelerates the production of extracellular matrix proteins.31 Microcurrent‐induced down‐regulation of TGF‐β is expected to up‐regulate the expression of PTEN, with the consequence of inhibiting PI3Kγ, which exerts an antifibrotic and contractility‐enhancing effect.34
The migration of T and B cells and other immune cells (e.g. monocytes and natural killer cells) is controlled by electrical fields.35 Given that monocytes are an important source of IL‐6, the downstream effect of a microcurrent on IL‐6 expression is conceivable.
External microcurrent
The direct electrical current has no immediate effect inside the cells (the cell membranes act as capacitors); however, several channels allow the current to penetrate the cell membrane, if only temporarily. Therefore, because the synthesis of adenosine triphosphate (ATP) requires a high mitochondrial membrane potential, the microcurrent may modify the membrane potential to the necessary level. Indeed, we have found that cell cultures of cardiomyocytes from SHRs exhibit an increase (34%; P = 0.01) in mitochondrial membrane potential and in the synthesis rate of ATP (40%; P = 0.036) after a 72 hour exposure to microcurrent (unpublished data).
Cx40, Cx43, and Cx45
Because IL‐6 is considered to be a mediator of inflammatory disorders, a consequential decrease in inflammation could be the initial point for the reduction in collagen expression and, in turn, for a decrease in the expression of Cx43 and TGF‐β.36, 37 In addition, Cx43 can modulate TGF‐β signalling, a finding that indicates a mutual influence on the various signalling pathways.38 Because the transcription of Cx43 is up‐regulated by the chronic mechanical load caused by hypertension, unloading should lead to down‐regulation. However, we found only a slight down‐regulation in Cx45 expression and no significant down‐regulation of Cx40 expression, a finding consistent with the observation that Cx40 and Cx45 are regulated differently and are found mainly in the cells of specific conduction pathways, not in the left ventricular wall.
MMPs and TIMPs
In diastolic heart failure, the expression of MMPs is up‐regulated, but the behaviour of TIMPs has not been fully defined, probably because their behaviour is time course dependent (i.e. compensated vs decompensated stages of heart failure). Although TGF‐β stimulates the synthesis of MMPs, it is surprising that, in the context of normalization of myocardial function, MMP expression remains unchanged. Mediators such as IL‐6 and TGF‐β up‐regulate the synthesis of TIMPs; in addition, fibroblasts, macrophages, and monocytes are counted among the TIMP‐synthesizing cells. Considering our findings of a down‐regulation of the proinflammatory components of the extracellular matrix, a concomitant reduction in TIMP3 expression appears plausible, especially because TIMP3 may be involved in regulating inflammation.39
Functional improvement
The results of the current study show that direct application of microcurrent to diseased hearts leads to improvements in function, even though the application is non‐specific and does not target particular receptors or molecules. Interestingly, the microcurrent appears to induce a self‐healing process, which is activated by a bulk current application of a determined amount. Neither we nor others have found that healthy cells are negatively influenced by the microcurrent.40
Although it is unlikely because of the demonstrated decrease in proinflammatory components, it is possible that the observed functional improvements are induced by increases in myosin synthesis, augmentation of vascular endothelial growth factor levels, increases in mRNA expression (increased capillary density), and enhancements in vagal or inhibited sympathetic innervation.41, 42, 43
Limitations
The limitations of this study include those related to any pilot study. First, even though several anti‐heart failure effects have been observed after the application of microcurrent, the ultimate mechanism of action is still unclear. Although possible mechanisms have been suggested, none could be definitively verified. Second, the number of components examined was limited, and their relationships to changes in the protein level are indirect. Likewise, the number of markers showing inflammatory effects can be explicitly increased. Finally, additional studies are necessary to substantiate our observations, because we found effects on ion channel activity, signal transduction, oxidative stress, key inflammatory regulators (including IL‐6 receptors), lymphocyte infiltration, and fibroblasts.
Furthermore, the intensity of the applied microcurrent was derived from in vitro experiments; we cannot exclude the possibility that a microcurrent with lower or higher intensity could lead to more pronounced effects. Because the SHRs were selected according to their ejection fraction, the age distribution is rather wide. Although there was no statistically significant difference between the groups, it is possible that the age distribution could have weakened the observed effects.
Conclusions
Even if their functional cardiac effect is only moderate, electrical signals from the devices in use have been shown to improve the severity of heart failure. In this study, we supported this approach by in vivo application of direct electrical microcurrent to the left ventricular myocardium of SHRs over several weeks. This application led to a pronounced improvement in cardiac function and to significant alterations in the inflammatory process and in certain components of the extracellular matrix at the mRNA level. Down‐regulation of the measured components of the extracellular matrix is consistent with our observation of improved heart function. It was not our intention to examine the molecular mechanism of the effect of the microcurrent. Although observational data cannot establish causality, our findings are consistent with the hypothesis that the application of minimal electrical energy is associated with biological alterations. We speculate that microcurrent therapy may be beneficial in other heart failure models related to chronic inflammation and may eventually be the basis of a disruptive heart failure treatment that would be in line with current efforts to treat diseases with implantable electroceuticals. Currently, however, we must limit ourselves to the basics of discovering the what before we can learn the why.
Conflict of interest
None declared.
Macfelda, K. , Kapeller, B. , Holly, A. , Podesser, B. K. , Losert, U. , Brandes, K. , Goettel, P. , and Mueller, J. (2017) Bioelectrical signals improve cardiac function and modify gene expression of extracellular matrix components. ESC Heart Failure, 4: 291–300. doi: 10.1002/ehf2.12169.
Contributor Information
Karin Macfelda, Email: karin.macfelda@meduniwien.ac.at.
Johannes Mueller, Email: jmueller@berlinheals.de.
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL, Resynchronization‐Defibrillation for Ambulatory Heart Failure Trial Investigators . Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med 2010; 363: 2385–2395. [DOI] [PubMed] [Google Scholar]
- 3. Melman YF, Shah R, Danielson K, Xiao J, Simonson B, Barth A, Chakir K, Lewis GD, Lavender Z, Truong QA, Kleber A, Das R, Rosenzweig A, Wang Y, Kass DA, Singh JP, Das S. Circulating microRNA‐30d is associated with response to cardiac resynchronization therapy in heart failure and regulates cardiomyocyte apoptosis: a translational pilot study. Circulation 2015; 131: 2202–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borggrefe MM, Lawo T, Butter C, Schmidinger H, Lunati M, Pieske B, Misier AR, Curnis A, Böcker D, Remppis A, Kautzner J, Stühlinger M, Leclerq C, Táborsky M, Frigerio M, Parides M, Burkhoff D, Hindricks G. Randomized, double blind study of non‐excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J 2008; 29: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 5. Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A 2016; 113: 8284–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kapeller B, Mueller J, Losert U, Bruno K, Podesser BK, Macfelda K. Microcurrent stimulation promotes reverse remodeling in cardiomyocytes. ESC Heart Failure 2016; 3: 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol 2010; 48: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gan JC, Glazer PA. Electrical stimulation therapies for spinal fusions: current concepts. Eur Spine J 2006; 15: 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang ET, Zhao M. Regulation of tissue repair and regeneration by electric fields. Chin J Traumatol 2010; 13: 55–61. [PubMed] [Google Scholar]
- 10. Lujan HL, DiCarlo SE. Mimicking the endogenous current of injury improves post‐infarct cardiac remodeling. Med Hypotheses 2013; 81: 521–523. [DOI] [PubMed] [Google Scholar]
- 11. Kurdi M, Randon J, Cerutti C, Bricca G. Increased expression of IL‐6 and LIF in the hypertrophied left ventricle of TGR(mRen2)27 and SHR rats. Mol Cell Biochem 2005; 269: 95–101. [DOI] [PubMed] [Google Scholar]
- 12. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals, 8th ed. Washington, D.C.: National Academies Press; 2011. https://grants.nih.gov/grants/olaw/guide‐forthe‐care‐and‐use‐of‐laboratory‐animals.pdf (April 11, 2017). [Google Scholar]
- 13. Slama M, Ahn J, Varagic J, Susic D, Frohlich ED. Long‐term left ventricular echocardiographic follow‐up of SHR and WKY rats: effects of hypertension and age. Am J Physiol Heart Circ Physiol 2004; 286: H181–H185. [DOI] [PubMed] [Google Scholar]
- 14. Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- 15. Liang H, Müller J, Weng YG, Wallukat G, Fu P, Lin HS, Bartel S, Knosalla C, Pregla R, Hetzer R. Changes in myocardial collagen content before and after left ventricular assist device application in dilated cardiomyopathy. Chin Med J (Engl) 2004; 117: 401–407. [PubMed] [Google Scholar]
- 16. Müller J, Wallukat G, Weng YG, Dandel M, Spiegelsberger S, Semrau S, Brandes K, Theodoridis V, Loebe M, Meyer R, Hetzer R. Weaning from mechanical cardiac support in patients with idiopathic dilated cardiomyopathy. Circulation 1997; 96: 542–549. [DOI] [PubMed] [Google Scholar]
- 17. Bishop JE, Laurent GJ. Collagen turnover and its regulation in the normal and hypertrophying heart. Eur Heart J 1995; 16(Suppl C): 38–44. [DOI] [PubMed] [Google Scholar]
- 18. Mayer B, Holmer SR, Hengstenberg C, Lieb W, Pfeifer M, Schunkert H. Functional improvement in heart failure patients treated with beta‐blockers is associated with a decline of cytokine levels. Int J Cardiol 2005; 103: 182–186. [DOI] [PubMed] [Google Scholar]
- 19. McCaig CD, Zhao M. Physiological electrical fields modify cell behaviour. Bioessays 1997; 19: 819–826. [DOI] [PubMed] [Google Scholar]
- 20. Song B, Zhao M, Forrester J, McCaig C. Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J Cell Sci 2004; 117(Pt 20): 4681–4690. [DOI] [PubMed] [Google Scholar]
- 21. Tai G, Reib B, Cao L, Zhao M. Electrotaxis and wound healing: experimental methods to study electric fields as directional signal for cell migration In Jin T., Hereld D., eds. Chemotaxis: Methods and Protocols, 2nd ed. New York: Humana Press; 2016. [Google Scholar]
- 22. Glaser R. The influence of membrane electric field on cellular function In Glaser R., Gingell D., eds. Biophysics of the Cell Surface, Springer Series in Biophysics New York: Springer; 1990. [Google Scholar]
- 23. Levin M. Endogenous bioelectrical networks store non‐genetic patterning information during development and regeneration. J Physiol 2014; 592: 2295–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belperio J, Horwich T, Abraham WT, Fonarow GC, Gorcsan J 3rd, Bersohn MM, Singh JP, Sonel A, Lee LY, Halilovic J, Kadish A, Shalaby AA. Inflammatory mediators and clinical outcome in patients with advanced heart failure receiving cardiac resynchronization therapy. Am J Cardiol 2016; 117: 617–625. [DOI] [PubMed] [Google Scholar]
- 25. Giallauria F, Vigorito C, Piepoli MF, Stewart Coats AJ. Effects of cardiac contractility modulation by non‐excitatory electrical stimulation on exercise capacity and quality of life: an individual patient's data meta‐analysis of randomized controlled trials. Int J Cardiol 2014; 175: 352–357. [DOI] [PubMed] [Google Scholar]
- 26. Peters TK, Koralewski HE, Zerbst E. The principle of electrical carotid sinus nerve stimulation: a nerve pacemaker system for angina pectoris and hypertension therapy. Ann Biomed Eng 1980; 8: 445–458. [DOI] [PubMed] [Google Scholar]
- 27. Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: a jump‐start for electroceuticals. Nature 2013; 496: 159–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pilecky M. The influence of 3‐hydroxybutyrate and microcurrent treatment on cardiomyocytes during stimulated hypertrophy [Dissertation]. Vienna: University of Applied Sciences; 2010.
- 29. Piek A, de Boer RA, Silljé HH. The fibrosis‐cell death axis in heart failure. Heart Fail Rev 2016; 21: 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev 2005; 85: 943–978. [DOI] [PubMed] [Google Scholar]
- 31. Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol‐3‐OH kinase‐gamma and PTEN. Nature 2006; 442: 457–460. [DOI] [PubMed] [Google Scholar]
- 32. Aoyagi T, Matsui T. Phosphoinositide‐3 kinase signaling in cardiac hypertrophy and heart failure. Curr Pharm Des 2011; 17: 1818–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stark AK, Sriskantharajah S, Hessel EM, Okkenhaug K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr Opin Pharmacol 2015; 23: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E. PI3Kγ modulates the cardiac response to chronic pressure overload by distinct kinase‐dependent and ‐independent effects. Cell 2004; 118: 375–387. [DOI] [PubMed] [Google Scholar]
- 35. Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 2000; 287: 1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brasier AR. The nuclear factor‐kappaB‐interleukin‐6 signalling pathway mediating vascular inflammation. Cardiovasc Res 2010; 86: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang XL, Topley N, Ito T, Phillips A. Interleukin‐6 regulation of transforming growth factor (TGF)‐beta receptor compartmentalization and turnover enhances TGF‐beta1 signaling. J Biol Chem 2005; 280: 12239–12245. [DOI] [PubMed] [Google Scholar]
- 38. Asazuma‐Nakamura Y, Dai P, Harada Y, Jiang Y, Hamaoka K, Takamatsu T. Cx43 contributes to TGF‐beta signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp Cell Res 2009; 315: 1190–1199. [DOI] [PubMed] [Google Scholar]
- 39. Smookler DS, Mohammed FF, Kassiri Z, Duncan GS, Mak TW, Khokha R. Tissue inhibitor of metalloproteinase 3 regulates TNF‐dependent systemic inflammation. J Immunol 2006; 176: 721–725. [DOI] [PubMed] [Google Scholar]
- 40. Zhang P, Liu ZT, He GX, Liu JP, Feng J. Low‐voltage direct‐current stimulation is safe and promotes angiogenesis in rabbits with myocardial infarction. Cell Biochem Biophys 2011; 59: 19–27. [DOI] [PubMed] [Google Scholar]
- 41. Amaral SL, Linderman JR, Morse MM, Greene AS. Angiogenesis induced by electrical stimulation is mediated by angiotensin II and VEGF. Microcirculation 2001; 8: 57–67. [PubMed] [Google Scholar]
- 42. Li L, El‐Hayek YH, Liu B, Chen Y, Gomez E, Wu X, Ning K, Li L, Chang N, Zhang L, Wang Z, Hu X, Wan Q. Direct‐current electrical field guides neuronal stem/progenitor cell migration. Stem Cells 2008; 26: 2193–2200. [DOI] [PubMed] [Google Scholar]
- 43. Lu MC, Tsai CC, Chen SC, Tsai FJ, Yao CH, Chen YS. Use of electrical stimulation at different current levels to promote recovery after peripheral nerve injury in rats. J Trauma 2009; 67: 1066–1072. [DOI] [PubMed] [Google Scholar]


