Abstract
Aims
Matrix metalloproteinase (MMP) is up‐regulated during heart failure (HF) and influences ventricular remodeling. We hypothesized that disparity between MMP‐9 and tissue inhibitors of MMP‐1 (TIMP‐1) results in clinical manifestations and is related to prognostic risk in patients with chronic HF.
Methods and results
Plasma levels of MMP‐9, TIMP‐1, and brain natriuretic peptide (BNP) were measured in 173 patients with chronic HF. Combined endpoints of worsening HF events were assessed during follow‐up (median 109 months). MMP‐9 and TIMP‐1 levels and the MMP‐9/TIMP‐1 ratio increased with increasing severity of the New York Heart Association class (P for trend = 0.003, 0.011, and 0.005, respectively). Patients with HF events (n = 35) had significantly higher MMP‐9 than those without HF events (P = 0.004). Kaplan–Meier analysis demonstrated a higher probability of HF events with high MMP‐9 values (>23.2 ng/mL; P = 0.005). A multivariate Cox proportional hazard model showed that high MMP‐9 values were an independent predictor of HF events (hazard ratio, 3.73; 95% confidence interval (CI), 1.03–13.46; P = 0.043). In patients with lower BNP levels (≤210 pg/mL), the adjusted hazard ratio for HF events was 3.63 (95% CI, 1.20–11.02; P = 0.023) among patients with high MMP‐9 values compared with patients with low BNP and low MMP‐9 values.
Conclusions
MMP‐9 and TIMP‐1 levels correlate with the severity of chronic HF. MMP‐9 is a strong predictor of HF events, suggesting that a disparity between MMP‐9 and TIMP‐1 levels and increased MMP‐9 levels may help predict HF events.
Keywords: Matrix metalloproteinase‐9, Tissue inhibitors of matrix metalloproteinase‐1, Chronic heart failure, Brain natriuretic peptide
Introduction
Cardiac remodeling is accompanied by an extensive reorganization of the cardiac extracellular matrix. Metalloproteinases (MMPs) are zinc‐dependent endopeptidases that catalyze degradation of extracellular matrix proteins, thereby controlling such processes as development and tissue remodeling.1, 2, 3
MMPs play an important role in many pathophysiological processes like angiogenesis and in diseases with a degradation component such as heart failure (HF), myocardial infarction and atherosclerotic plaque. The activity of MMPs is controlled by regulation of expression and proteolytic activation of pro‐enzymes and by the Tissue Inhibitors of Metalloproteinases (TIMPs). TIMP‐1 binds to the inactive pro‐MMP‐9, forming a complex in which TIMP‐1 retains its ability to inhibit the activity of another active MMP. Imbalance of MMP and TIMP activity may correlate with pathological conditions in patients with HF. MMPs and TIMPs are matrix‐degrading enzymes that are up‐regulated in the failing heart and influence left ventricular remodeling.2, 3, 4 Recently, several biomarkers have been identified that can predict an adverse prognosis in patients with HF. Of these, elevated plasma concentrations of brain natriuretic peptide (BNP) show notable associations with development of left ventricular dysfunction and HF.5 Although elevated BNP concentrations have diagnostic and prognostic importance,6, 7, 8 whether intense therapy for HF guided by BNP values is superior to therapy guided by clinical symptoms is unclear.9, 10, 11 BNP play important roles in maintaining cardiorenal homeostasis under physiological and pathological conditions. BNP is synthesized by cardiomyocytes and fibroblasts, and their production is stimulated in pathologic conditions such as myocardial infarction and HF. Additionally, BNP inhibits collagen synthesis and increases MMP expression.12 Proinflammatory cytokines such as IL‐6 and TNF‐alpha also play an important role in cardiac remodeling.13, 14 Induction or stimulation of MMP expression is mediated by a variety of inflammatory cytokines, including IL‐6 and TNF‐alpha.15 During the progression of left ventricular dysfunction, increased sympathetic stimulation and levels of norepinephrine were observed. Following chronic neurohormonal system activation, increased elaboration and release of MMPs into the extracellular matrix of the left ventricle occurs.16 Therefore, the sympathetic nerve system may modulate MMP productions in patients with HF. Thus, we evaluated the significance of circulating MMP and TIMP levels and comprehensive neurohormonal profiles in patients with chronic HF.
We tested the hypothesis that an imbalance between MMP‐9 and TIMP‐1 levels is related to prognostic risk in patients with chronic HF. We examined whether circulating MMP molecules are related to the prognostic risk of chronic HF and determined the prognostic risk of HF events associated with circulating levels of MMP‐9, TIMP‐1, and other biomarkers.
Methods
Study subjects and design
Patients were recruited from the Department of Cardiovascular Medicine of the University of Fukui Hospital. Patients with chronic HF (n = 202) were enrolled in this study. Symptoms and signs of HF were defined according to the Framingham criteria.17 Twenty‐nine patients were excluded due to exclusion criteria as stated as follows and/or because their blood samples were not appropriate for measuring. We also excluded patients with a history of neoplastic, hepatic, infectious, or autonomic disease; peripheral atherosclerotic disease; or any surgical procedure in the preceding 6 months. No patients had inflammatory signs at the time of evaluation. Thus, 173 inpatients who were admitted between June 1, 1992 and August 31, 2002 were enrolled. The investigation conformed to the principles outlined in the Declaration of Helsinki. Ethics committee approval and informed consent from all patients were obtained.
To obtain follow‐up information and screen for the occurrence of HF events, all patients were reevaluated after discharge from the hospital by reviewing their medical records including clinical visits, conducting telephone interviews, and obtaining clinical information from the in‐charge cardiologists; the last follow‐up was on December 31, 2009. We observed the HF events that occurred during the follow‐up period (mean ± SD, 88 ± 49 months) and looked for a correlation between disease severity and events, and between TIMP‐1, BNP, noradrenaline (NA), interleukin [IL]‐6 and tumor necrosis factor [TNF]‐alpha and MMP levels during follow‐up. HF events were defined as cardiac death (including sudden cardiac death and death from HF), lethal arrhythmia (ventricular tachycardia/ventricular fibrillation), and admission due to worsening HF. Patients were classified into two groups: patients with HF events (n = 35) and patients without HF events (n = 138). HF with reduced ejection fraction (HFREF) was defined as a left ventricular ejection fraction ≤45%, whereas HF with preserved ejection fraction (HFPEF) was defined as a left ventricular ejection fraction >45%.
Blood collections and plasma separation
All patients were treated with standard regimens for HF, and all drugs continued to be administered before blood collection. Overnight fasting blood samples were obtained from the forearm or femoral vein and kept on ice. Plasma and serum samples were separated by centrifugation within 30 min, frozen, and stored at −80°C until use. BNP and NA were assayed rapidly after blood collection. MMP family members and cytokines were assayed within 1 year after blood collection. Samples used for analysis had not been previously thawed. On the day of use, the samples were thawed at room temperature and analysed immediately.
Measurements of plasma MMP‐9 and serum TIMP‐1 levels
Sandwich enzyme immunoassays using commercially available kits with monoclonal antibodies against each molecule were performed to measure the concentrations of plasma MMP‐9 and serum TIMP‐1 according to the manufacturer's instructions (Fuji Chemical Industries Ltd., Takaoka, Japan).18
Measurements of plasma brain natriuretic peptide and noradrenaline
Plasma levels of BNP were measured using a commercially available radioimmunoassay kit (Shionogi, Osaka, Japan), and plasma levels of NA were measured with high‐performance liquid chromatography.6 Plasma NA concentration provides prognosis utility in congestive HF patients.19
Measurement of plasma levels of cytokines
Sandwich enzyme immunoassays using commercially available kits with monoclonal antibodies against each molecule were performed to measure concentrations of plasma cytokines (IL‐6 and TNF‐alpha) according to the manufacturer's instructions (Quantikine HS, R&D Systems, Minneapolis, MN, USA). The same trained researcher, who was blinded to the patient's clinical status, performed all tests throughout the study. Detailed methods are as previously reported.20 TNF‐alpha and IL‐6 are elevated in patients with HF, and these cytokines are correlated with both the severity and development of HF. Thus, these cytokines may have both pathophysiological importance and utility as clinically predictive biomarkers.
Statistical analysis
Continuous values are expressed as the mean ± SD or median [interquartile range]. The chi‐square test was used to compare categorical data. Comparisons between two groups of continuous measurements were performed with Mann–Whitney U tests. Correlations between two variables were assessed with the Spearman rank test. Comparisons of MMP‐9 and TIMP‐1 levels, and the MMP‐9/TIMP‐1 ratio across New York Heart Association (NYHA) functional classes were performed with the Jonckheere‐Terpstra trend test and Kruskal‐Wallis analysis of variance. Receiver operating characteristic (ROC) curve analysis was used to determine the ability of the variables to distinguish between patients with and without HF events. Optimal cut‐off values for each variable were calculated to provide the greatest combined sensitivity and specificity. A Cox proportional hazards regression model was used to explore the effect of other variables on event‐free survival. Variables included in the model were age, sex, calcium channel blockers, BNP, NA, TNF‐alpha, MMP‐9, TIMP‐1, IL‐6, and ejection fraction. Hazard ratios (HR) and 95% confidence intervals (CI) were determined. With regard to sample size estimation, we planned a study with an accrual interval of 120 months, and additional follow‐up after the accrual interval of 84 months. The median survival time on the low MMP group was estimated as 84 months. If the true hazard ratio (relative risk) of low MMP subjects relative to high MMP subjects is 2, we will need to study 61 experimental subjects and 61 control subjects to be able to reject the null hypothesis that the experimental and control survival curves are equal with probability (power) 80%. The Type I error probability associated with this test of this null hypothesis is 0.05.
We generated Kaplan–Meier curves to describe the association between MMP‐9 values and time to incident HF events. Log rank tests were used to compare event‐free survival between different groups. We assessed the model performance of the modified model, which was made by incorporating MMP‐9 information into the model. Variables included in the model were age, sex, calcium channel blockers and BNP. Additive information of MMP‐9 was evaluated by category‐free net reclassification improvement (NRI) and integrated discrimination improvement (IDI). Statistical significance was set at P < 0.05 (two‐tailed). Data were analysed with R software V.3.0.1 (http://www.r‐project.org) and SPSS version 20 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Baseline demographics, medications, and etiology of HF did not differ between the group of patients with and without HF events. Compared with the non‐HF events group, the HF events group included patients with higher MMP‐9, TIMP‐1, IL‐6, and TNF‐alpha levels. Patients with HF events also had a lower ejection fraction and higher prescription rate of calcium channel blockers. During the follow‐up period, 35 patients experienced HF events. Patients who had HF events had higher MMP‐9 levels than patients without HF events (28.0 [17.4–50.5] ng/mL vs. 20.0 [14.3–33.6] ng/mL, P = 0.004) (Table 1). When patients were classified into two groups: patients with symptomatic HF (classified as NYHA class II, III, and IV) (n = 104) and patients without symptomatic HF (classified as NYHA class I) (n = 69), we observed higher MMP‐9 levels in patients with symptomatic HF than in patients without symptomatic HF (23.1 [15.7–45.8] ng/mL vs. 20.1 [14.0–26.5] ng/mL, P = 0.04). No significant differences were found between symptomatic HF and asymptomatic HF in levels of TIMP‐1 (134.5 [67.5–174.0] ng/mL vs. 116.0 [45.0–159.7] ng/mL, P = 0.08).
Table 1.
Patient characteristics
| Chronic HF (n = 173) | HF events (+) (n = 35) | HF events (−) (n = 138) | P value | |
|---|---|---|---|---|
| Age, years | 64.4 ± 12.4 | 65.0 ± 16.2 | 64.3 ± 11.4 | 0.27 |
| Sex (M/F), n (%) | 114 (65.8)/59 (34.1) | 22 (62.9)/13 (37.1) | 92 (66.7)/46 (33.3) | 0.69 |
| Hypertension, n (%) | 93 (53.7) | 15 (42.9) | 78 (56.5) | 0.18 |
| DM, n (%) | 52 (30.0) | 9 (25.7) | 44 (31.9) | 0.35 |
| HL, n (%) | 67 (38.7) | 11 (31.4) | 59 (42.8) | 0.25 |
| Beta‐blocker, n (%) | 26 (15.0) | 8 (22.9) | 18 (13.0) | 0.18 |
| ARB/ACE‐I, n (%) | 110 (63.5) | 24 (68.6) | 76 (55.1) | 0.18 |
| Statin, n (%) | 31 (17.9) | 3 (8.6) | 28 (20.3) | 0.14 |
| Calcium channel blocker, n (%) | 83 (47.9) | 10 (28.6) | 73 (52.9) | 0.01 |
| Nitrate, n (%) | 110 (63.5) | 25 (71.4) | 85 (61.6) | 0.32 |
| Digitalis, n (%) | 18 (10.4) | 6 (13.6) | 12 (9.3) | 0.21 |
| ICM/non‐ICM, n (%) | 107 (61.8)/66 (38.1) | 18 (51.4)/17 (48.6) | 89 (64.5)/49 (35.5) | 0.17 |
| MMP‐9, ng/mLa | 21.1 [15.0–39.3] | 28.0 [17.4–50.5] | 20.0 [14.3–33.6] | <0.01 |
| TIMP‐1, ng/mLa | 122.7 [48.6–165.1] | 138.0 [49.6–182.9] | 116.5 [45.7–161.2] | 0.02 |
| MMP‐9/TIMP‐1 ratioa | 0.198 [0.118–0.385] | 0.343 [0.135–0.553] | 0.177 [0.115–0.334] | 0.14 |
| ANP, pg/mLa | 42.2 [20.4–105.0] | 73.5 [31.9–115.0] | 38.7 [18.8–92.0] | 0.08 |
| BNP, pg/mLa | 112.0 [41.9–353.5] | 225.0 [44.0–620.2] | 101.0 [41.9–250.5] | 0.08 |
| NA, ng/mLa | 0.29 [0.17–0.37] | 0.29 [0.20–0.92] | 0.30 [0.14–0.36] | 0.12 |
| IL‐6, pg/mLa | 8.7 [4.4–14.6] | 8.8 [5.3–19.4] | 7.5 [4.4–14.4] | 0.02 |
| TNF‐alpha, pg/mLa | 4.2 [1.7–17.9] | 11.6 [3.4–18.7] | 3.1 [1.3–7.9] | <0.01 |
| EF, %a | 48.6 [30.2–64.1] | 40.3 [17.2–55.9] | 50.9 [34.3–65.1] | <0.01 |
ACE‐I, angiotensin converting enzyme inhibitor; ANP, atrial natriuretic peptide; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide; DM, diabetes mellitus; EF, ejection fraction; HF, heart failure; HL, hyperlipidemia; ICM, ischemic cardiomyopathy; IL, interleukin; MMP, matrix metalloproteinase; NA, noradrenaline; TIMP, tissue inhibitor of MMP; TNF, tumor necrosis factor.
median value (interquartile range).
Sixty‐four patients were classified as HFREF, and 109 patients were classified as HFPEF. We observed higher plasma levels of BNP in HFREF than in HFPEF (278.0 [9.6–769.7] pg/mL vs. 81.6 [5.0–217.7] pg/mL, P < 0.01) and a higher MMP‐9/TIMP‐1 ratio in HFREF than in HFPEF (0.242 [0.073–0.665] vs. 0.179 [0.041–0.421], P = 0.03). No significant differences were found between HFPEF and HFREF in levels of MMP‐9 (21.7 [4.5–41.6] ng/mL vs. 25.7 [1.0–68.2] ng/mL, P = 0.09) or TIMP‐1 (138.0 [66.6–209.4] ng/mL vs. 160.0 [89.1–230.9] ng/mL, P = 0.06) (see Supporting Information Figure S1 ). There were no significant interactions between MMP‐9 and TIMP‐1 levels and following drugs administration; beta‐blocker, ARB/ACE‐I, statin, calcium channel blocker, nitrate, digitalis.
NYHA functional class and MMP molecules
Sixty‐nine patients (39.8%), 73 patients (42.1%), 23 patients (13.2%), and 8 patients (4.6%) were classified as NYHA class I, II, III, and IV, respectively. Values of MMP‐9, TIMP‐1, and the MMP‐9/TIMP‐1 ratio were positively related to the NYHA functional class (Figure 1).
Figure 1.
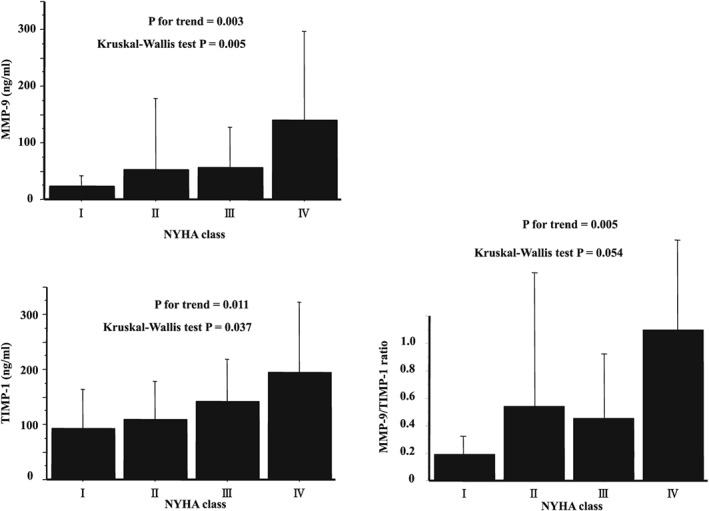
Relationships between the NYHA classification and MMP‐9, TIMP‐1, and the MMP‐9/TIMP‐1 ratio. MMP, matrix metalloproteinase; NYHA, New York Heart Association class; TIMP, tissue inhibitor of MMP.
Correlations between MMP‐9 and neurohormonal factors
Correlations between MMP‐9 levels and various factors, including neurohormonal factors and other biomarkers, are presented in Supporting Information Table S1 . MMP‐9 levels showed weak but significantly positive correlations with TIMP‐1 (rs = 0.395; P < 0.001), NA (rs = 0.159; P = 0.036), IL‐6 (rs = 0.184; P = 0.015), and TNF‐alpha (rs = 0.248; P = 0.001). No correlation was found between the MMP‐9 level and BNP value (rs = 0.071; P = 0.35).
Receiver operating characteristic analysis for HF events
Receiver operating characteristic analyses were performed to determine optimal cut‐off values for MMP‐9, TIMP‐1, IL‐6, BNP, NA, TNF‐alpha, and the ejection fraction to detect changes in HF events. The area under the curve of MMP‐9 levels was 0.63. BNP had the highest area under the curve value among the analysed variables. For prediction of HF events, the optimal cut‐off value of MMP‐9 was 23.2 ng/mL, with a sensitivity of 64.7% and a specificity of 56.6%. Results of ROC analysis of other variables are shown in Table 2.
Table 2.
Receiver operating curve analysis for HF events
| MMP‐9, ng/mL | TIMP‐1, ng/mL | BNP, pg/mL | NA, ng/mL | TNF‐alpha, pg/mL | IL‐6, pg/mL | EF, % | |
|---|---|---|---|---|---|---|---|
| AUC | 0.63 | 0.62 | 0.75 | 0.64 | 0.60 | 0.62 | 0.76 |
| Cut‐off | 23.2 | 171.5 | 210.0 | 0.38 | 0.73 | 7.5 | 47.4 |
| Sensitivity (%) | 64.7 | 44.4 | 71.4 | 46.7 | 31.0 | 37.1 | 71.9 |
| Specificity (%) | 56.6 | 78.6 | 75.0 | 82.2 | 91.0 | 81.2 | 69.2 |
AUC, area under the curve; BNP, brain natriuretic peptide; HF, heart failure; MMP, matrix metalloproteinase; NA, noradrenaline; TIMP, tissue inhibitor of MMP; TNF, tumor necrosis factor.
Hazard ratios for HF events when incorporating MMP‐9 and brain natriuretic peptide levels
In the setting of both of MMP‐9 and BNP levels, high MMP‐9 levels (>23.2 ng/mL) and high BNP levels (>210 pg/mL) showed the highest HR for HF events (HR, 6.46; 95% CI 2.40–17.43; P < 0.001). Moreover, even in the lower BNP group (≤210 pg/mL), the HR for high MMP‐9 values (>23.2 ng/mL) was 3.63 (95% CI, 1.20–11.02, P = 0.023) for HF events compared with low BNP values (≤210 pg/mL) and low MMP‐9 values (≤23.2 ng/mL) (Figure 2).
Figure 2.
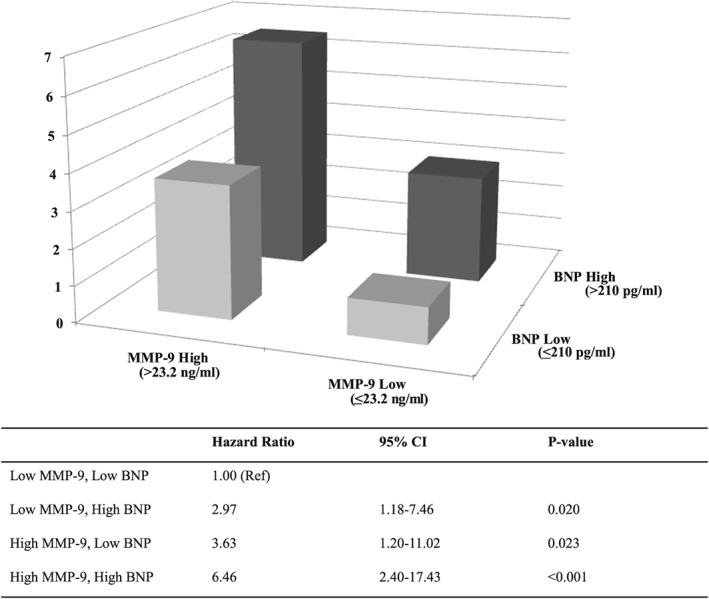
Hazard ratios for heart failure (HF) events based on combinations of MMP‐9 and BNP levels. Low MMP‐9, ≤23.2 ng/mL; high MMP‐9, >23.2 ng/mL; low BNP, ≤210 pg/mL; high BNP, >210 pg/mL. BNP, brain natriuretic peptide; CI, confidence interval; MMP, matrix metalloproteinase; Ref, reference.
Kaplan–Meier analysis for HF events
Kaplan–Meier analysis demonstrated a higher probability of HF events in patients with high MMP‐9 values (>23.2 ng/mL; P = 0.005). Consideration of both MMP‐9 and BNP levels further improved the risk stratification. For patients with low BNP levels (≤210 pg/mL), the risk of HF events was higher for those with higher MMP‐9 levels (>23.2 ng/mL; log rank P = 0.016) (Figure 3).
Figure 3.
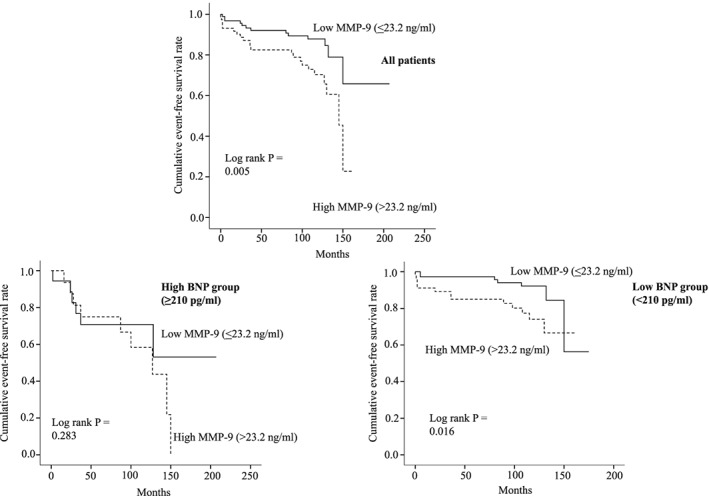
Kaplan–Meier curves for HF events. Upper panel: All patients were stratified by MMP‐9. MMP‐9 ≤23.2 ng/mL (solid line) vs. MMP‐9 >23.2 ng/mL (broken line). Lower left panel: Patients in the high BNP group (BNP >210 pg/mL) were stratified into a low MMP‐9 group (≤23.2 ng/mL) (solid line) and a high MMP‐9 group (>23.2 ng/mL) (broken line). Lower right panel: Patients in the low BNP group (≤210 pg/mL) were stratified into a low MMP‐9 group (≤23.2 ng/mL) (solid line) and a high MMP‐9 group (>23.2 ng/mL) (broken line). BNP, brain natriuretic peptide; HF events, heart failure events; MMP, matrix metalloproteinase.
Cox proportional hazard analysis for HF events
Table 3 shows results of univariate and multivariate Cox proportional hazard model analysis for detecting HF events. In the univariate analysis, MMP‐9, IL‐6, ejection fraction, and BNP were predictive for HF events. The multivariate Cox proportional hazard model showed that high levels of MMP‐9 were an independent predictor for HF events (HR, 3.73; 95% CI, 1.03–13.46; P = 0.043).
Table 3.
Cox proportional hazard analysis for HF events
| Univariate HR (95% CI) | P value | Multivariate HR (95% CI) | P value | |
|---|---|---|---|---|
| Age (years) | 1.00 (0.97–1.03) | 0.78 | Not selected | |
| Sex (male) | 0.90 (0.45–1.80) | 0.77 | Not selected | |
| MMP‐9 (>23.2 ng/mL) | 2.57 (1.29–5.12) | 0.007 | 3.73 (1.03–13.46) | 0.043 |
| TIMP‐1 (>171.5 ng/mL) | 2.02 (0.97–4.20) | 0.05 | Not selected | |
| Taking a CCB | 0.48 (0.23–1.01) | 0.05 | Not selected | |
| BNP (>210 pg/mL) | 2.83 (1.43–5.62) | 0.025 | Not selected | |
| NA (>0.38 ng/mL) | 2.44 (0.87–6.81) | 0.08 | Not selected | |
| IL‐6 (>7.5 pg/mL) | 2.47 (1.25–4.86) | 0.009 | Not selected | |
| TNF‐alpha (>0.73 pg/mL) | 1.21 (0.56–2.58) | 0.62 | Not selected | |
| EF (>47.4%) | 3.50 (1.59–7.67) | 0.002 | Not selected |
BNP, brain natriuretic peptide; CCB, calcium channel blocker; CI, confidence interval; HF, heart failure; HR, hazard ratio; MMP, matrix metalloproteinase; NA, noradrenaline; TIMP, tissue inhibitor of MMP; TNF, tumor necrosis factor.
Characteristics of patients categorized by MMP‐9 level
The demographics and clinical characteristics were compared between patients who had an MMP‐9 level >23.2 ng/mL and those with a level ≤23.2 ng/mL. No significant differences were found between these groups except for MMP family members (see Supporting Information, Table S2 ).
Additive information of MMP‐9 to brain natriuretic peptide
Incorporating MMP‐9 into BNP yielded a significant category‐free NRI of 0.291 (95% CI 0.015 to 0.567) and IDI of 0.055 (95% CI 0.018 to 0.093), these findings were statistically significant.
Discussion
The main findings of the present study are: first, in patients with chronic HF, MMP‐9, TIMP‐1, and the MMP‐9/TIMP‐1 ratio were correlated with disease severity as determined by the NYHA functional class. Second, MMP‐9 values were correlated with inflammatory cytokines and neurohormonal factors in patients with chronic HF. Third, even in patients with low BNP levels, high MMP‐9 levels were a strong predictor of HF events in a long‐term follow‐up of a median of 109 months. Fourth, reclassification metrics such as NRI and IDI were statistically improved on incorporation of the MMP‐9 level, the additive clinical usefulness of MMP‐9 to BNP was shown.
We demonstrated the additive prognostic value of considering both MMP‐9 and BNP levels. Several potential reasons may explain our observations. BNP‐guided therapy does not always improve clinical outcomes as previously reported.9, 10, 11 The reason for this lack of significant improvement may be that BNP levels only change upon ventricular wall stretching. Thus, worsening of HF must occur before BNP levels rise. Elevated MMP‐9 levels may help identify patients at risk before an increase occurs in ventricular pressure overload, which reflects ongoing ventricular remodeling. The value of BNP levels for guiding therapy in addition to clinical symptom‐based treatment seems to be limited,9, 10, 11, 21 despite the undisputed diagnostic and prognostic importance of these values.6, 7, 8 The benefits of predicting HF events may be offset by non‐HF events. Although BNP measurement can help detect worsening HF, the current standard HF therapy is not sufficient to prevent subsequent HF events. Because deterioration of heart function must occur before BNP levels rise, elevated levels of another biomarker before an increase in cardiac pressure occurs may help identify patients at risk for HF events. At such an early phase, medical interventions can prevent a poor outcome.
BNP is a cardiac loading marker that responds to ventricular and myocardial stretching and wall stress, whereas MMP is regarded as a marker of fibrosis and is less responsive to loading. Our study demonstrated that in HFPEF patients, levels of BNP, and the MMP‐9/TIMP‐1 ratio were lower compared with those in HFREF patients. An imbalance in the MMP/TIMP ratio and a robust increase in BNP levels reflect advanced ventricular remodeling, dilatation, and wall stretching. MMP and TIMP levels were similar in HFREF and HFPEF patients and may represent ongoing myocardial injury and extracellular matrix remodeling before an increase in BNP and a decreased ejection fraction are seen. HFPEF is characterized by matrix apposition and myocardial stiffening. Thus, a matrix and fibrosis marker such as MMP may also be an important prognostic marker in HFPEF. We focused on MMP‐9, TIMP‐1, and the MMP‐9/TIMP‐1 ratio as candidate markers for predicting HF events in this study. No significant change was observed in the MMP‐9/TIMP‐1 ratio in patients with and without HF events. TIMPs have been proposed as potential biomarkers of HF development. However, in our study, the combination of MMP/TIMP levels could not predict HF events during the follow‐up period. These observations imply that only measuring MMP‐9 and not TIMP‐1 may be sufficient to assess the risk of future HF events.
MMP molecules are up‐regulated in non‐structural remodeling situations, such as lethal arrhythmia.22, 23 The HR for HF events was highest in conditions of high BNP and high MMP‐9 levels. These conditions appear to reflect the status of ongoing inflammation and catecholamine spillover in addition to ventricular wall stretching. Thus, the combination of both high BNP and high MMP‐9 levels was associated with a poor outcome in HF patients. Notably, high MMP‐9 levels even in the presence of low BNP levels still represented a high clinical risk for HF events (HR 3.63). This situation represents ongoing ventricular remodeling via inflammation and catecholamine spillover before a ventricular pressure overload occurs. Indeed, we observed significant correlations between MMP‐9 and factors such as IL‐6, NA, and TNF‐alpha levels, whereas no correlation was found between MMP‐9 and BNP levels. We therefore consider that elevated MMP‐9 levels are a prognostic factor for a worsening clinical course, regardless of BNP levels.
The addition of MMP‐9‐guided therapy to BNP‐guided therapy leads to additional risk stratification, which allows further detection of patients at risk of HF events and allows more aggressive drug and/or mechanical intervention to avoid HF events. Compared with BNP monitoring, MMP‐9 monitoring was associated with significantly better prediction of worsening HF events. Cox hazard analysis demonstrated that MMP‐9 was still prognostic in the setting of low BNP. In this condition, although extracellular matrix remodeling and turnover were accelerated, ventricular wall stress was not increased enough to stimulate a robust increase in BNP. Hence, MMP‐9 monitoring enables identification of patients with a risk of worsening HF events at an earlier time compared with BNP monitoring. In this study, we could not clarify the determinant of differences in plasma MMP‐9 levels. However, several interventions attenuate MMP activity24, 25, 26, 27, 28, 29, 30; thus, assessing MMP levels is useful for identifying patients at risk for HF events and could be used to monitor patients who may require modification of their therapeutic options. Increased MMP‐9 may identify a subset of HF patients who would benefit from intensive medical therapy. Risk stratification may be best achieved by using models that incorporate multiple biomarkers, including inflammatory cytokines and natriuretic peptides. Associations between adverse left ventricular remodeling and worse prognosis, and MMPs have been investigated in various HF conditions and subject's background.31, 32, 33 Our study showed MMP‐9 levels are a strong predictor of HF events in patients with chronic HF. Our findings go line with recent findings on the impact of MMP‐9 in patient with HF, while there are inconsistent findings regarding TIMP‐1 levels. This discordance may reflect the changes of MMP species expressions in various stages and conditions of HF in each study. For cardiac pressure overload, there are increased levels of TIMPs in the early stages of HF associated with extracellular matrix accumulation. In the late phase, such as left ventricular dilatation, there is an up‐regulation of MMPs leading to extracellular degradation.1, 34, 35 Up‐regulations of both MMP‐9 ad TIMP‐1 in patients with poor prognosis seems to reflect varieties of ventricular remodeling stages at the time of assessment.
Our study has several limitations. This study consisted of observational research in patients already diagnosed with HF, and hence did not establish a cause‐effect relationship of interventional therapy. We also did not examine individuals without HF. ROC analyses showed relatively low areas under the curves for all variables. ROC analyses are suitable for events that are currently happening, rather than predicting events for long‐term follow‐up periods. Another limitation is that MMP molecules are up‐regulated in non‐HF events such as unstable angina, myocardial infarction, and cerebral infarction. Thus, specificity and the area under the curve were relatively low. To predict major adverse cerebro‐cardiovascular events, MMP‐9, and BNP were still strong predictors (data not shown). The proportion of patients receiving beta‐blockers was low at the time of blood collection. In the era of enrollment, calcium channel antagonists were widely used to treat hypertension and angina pectoris, particularly in Japan36 where coronary artery spasm is more prevalent than in the West.37 Beta‐blockers are thought to aggravate coronary spasm.38 After enrollment, patients received tailored medical therapy by the in‐charge cardiologists. Tailored HF therapy may have affected the incidence of HF events. However, in‐charge cardiologists and patients were blinded to the results of biomarkers, except for BNP and ejection fraction values. Thus, we consider that baseline MMP‐9 values are still predictive regardless of tailored therapy. In HF conditions, the majority of BNP is produced from ventricular myocardium. BNP is synthesized as preprohormones and cleaved to prohormones, proBNP1–108, that are proteolytically cleaved to bioactive form, BNP1‐32. 39, 40 Conventional assay for BNP, which was available in our study, are not specific because antibody against BNP1–32 has cross‐activity with proBNP1–108. Our measurement of BNP recognized both proBNP1–108 and BNP1–32. Therefore, novel, specific assay for proBNP1–108 may establish additional predictive ability for progressive HF events. The incidence of cardiac events was substantially lower than that reported in western countries.36, 41 Racial or ethnic differences between Japanese and western populations may mediate a difference in the response to HF therapy. However, the possibility that the low event rate may be due to the selection of low‐risk patients cannot be excluded. Over‐activation of MMPs, or an imbalance of active MMPs and their inhibitor TIMPs, is considered to be a key factor in the remodeling of extracellular matrix found in HF conditions. It is therefore of interest to determine not only the amount of enzyme expressed, but also what proportion subsequently becomes activated or has the ability to become active. However, ELISA system, which was used in our study, detects proMMP‐9 and their complex form with TIMP‐1.18 Thus, our study may not reflect the actual ratio of MMP‐9: TIMP‐1. This may lead to underestimate the competence of predictive ability of MMP‐9/TIMP‐1 ratio.
In conclusion, MMP‐9 levels and the level of inflammatory cytokines were correlated with the severity of chronic HF. MMP‐9 levels are a strong predictor of HF events and allow further risk stratification of patients, suggesting that disparity between MMP‐9 and TIMP‐1 levels may contribute to HF events and mortality.
Conflict of interest
None declared.
Source of funding
This work was partially supported by a Research Grant from the University of Fukui.
Supporting information
Table S1. Correlations with MMP‐9 levels.
Table S2 Patient characteristics by MMP‐9 value.
Figure S1. Bar graphs illustrating MMP‐9, TIMP‐1, the MMP‐9/TIMP‐1 ratio, and BNP levels in HFREF and HFPEF patients.
Acknowledgements
The authors thank Hiromi Nishimura, Yumie Yasusaki, Motoko Oku, Mari Kurata, and Yoshiko Kurose for providing excellent technical assistance. The first author would also like to express his gratitude to his wife for her moral support and constant encouragement.
Morishita, T. , Uzui, H. , Mitsuke, Y. , Amaya, N. , Kaseno, K. , Ishida, K. , Fukuoka, Y. , Ikeda, H. , Tama, N. , Yamazaki, T. , Lee, J.‐D. , and Tada, H. (2017) Association between matrix metalloproteinase‐9 and worsening heart failure events in patients with chronic heart failure. ESC Heart Failure, 4: 321–330. doi: 10.1002/ehf2.12137.
References
- 1. Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)‐9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 2013; 139: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 2006; 113: 2089–2096. [DOI] [PubMed] [Google Scholar]
- 3. Kelly D, Cockerill G, Ng LL, Thompson M, Khan S, Samani NJ, Squire IB. Plasma matrix metalloproteinase‐9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. Eur Heart J 2007; 28: 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation 1998; 98: 1728–1734. [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, Cardiology TFftDaToAaCHFotESo , Guidelines ECfP . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 6. Tsutamoto T, Wada A, Maeda K, Hisanaga T, Mabuchi N, Hayashi M, Ohnishi M, Sawaki M, Fujii M, Horie H, Sugimoto Y, Kinoshita M. Plasma brain natriuretic peptide level as a biochemical marker of morbidity and mortality in patients with asymptomatic or minimally symptomatic left ventricular dysfunction. Comparison with plasma angiotensin II and endothelin‐1. Eur Heart J 1999; 20: 1799–1807. [DOI] [PubMed] [Google Scholar]
- 7. Tang WH, Girod JP, Lee MJ, Starling RC, Young JB, Van Lente F, Francis GS. Plasma B‐type natriuretic peptide levels in ambulatory patients with established chronic symptomatic systolic heart failure. Circulation 2003; 108: 2964–2966. [DOI] [PubMed] [Google Scholar]
- 8. Eurlings LW, van Pol PE, Kok WE, van Wijk S, Lodewijks‐van der Bolt C, Balk AH, Lok DJ, Crijns HJ, van Kraaij DJ, de Jonge N, Meeder JG, Prins M, Pinto YM. Management of chronic heart failure guided by individual N‐terminal pro‐B‐type natriuretic peptide targets: results of the PRIMA (Can PRo‐brain‐natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol 2010; 56: 2090–2100. [DOI] [PubMed] [Google Scholar]
- 9. Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, Vuillomenet A, Jeker U, Dubach P, Beer H, Yoon SI, Suter T, Osterhues HH, Schieber MM, Hilti P, Schindler R, Brunner‐La Rocca HP, Investigators T‐C. BNP‐guided vs symptom‐guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME‐CHF) randomized trial. JAMA 2009; 301: 383–392. [DOI] [PubMed] [Google Scholar]
- 10. Sanders‐van Wijk S, Maeder MT, Nietlispach F, Rickli H, Estlinbaum W, Erne P, Rickenbacher P, Peter M, Pfisterer MP, Brunner‐La Rocca HP, Investigators T‐C. Long‐term results of intensified, N‐terminal‐pro‐B‐type natriuretic peptide‐guided versus symptom‐guided treatment in elderly patients with heart failure: five‐year follow‐up from TIME‐CHF. Circ Heart Fail 2014; 7: 131–139. [DOI] [PubMed] [Google Scholar]
- 11. Savarese G, Trimarco B, Dellegrottaglie S, Prastaro M, Gambardella F, Rengo G, Leosco D, Perrone‐Filardi P. Natriuretic peptide‐guided therapy in chronic heart failure: a meta‐analysis of 2,686 patients in 12 randomized trials. PLoS One 2013; 8: e58287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello‐Boerrigter LC, Chen HH, Burnett JC. Brain natriuretic peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res 2002; 91: 1127–1134. [DOI] [PubMed] [Google Scholar]
- 13. Oral H, Sivasubramanian N, Dyke DB, Mehta RH, Grossman PM, Briesmiester K, Fay WP, Pagani FD, Bolling SF, Mann DL, Starling MR. Myocardial proinflammatory cytokine expression and left ventricular remodeling in patients with chronic mitral regurgitation. Circulation 2003; 107: 831–837. [DOI] [PubMed] [Google Scholar]
- 14. Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R, Liu PP. Tumor necrosis factor‐alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation 2007; 115: 1398–1407. [DOI] [PubMed] [Google Scholar]
- 15. Siwik DA, Chang DL, Colucci WS. Interleukin‐1beta and tumor necrosis factor‐alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res 2000; 86: 1259–1265. [DOI] [PubMed] [Google Scholar]
- 16. Coker ML, Jolly JR, Joffs C, Etoh T, Holder JR, Bond BR, Spinale FG. Matrix metalloproteinase expression and activity in isolated myocytes after neurohormonal stimulation. Am J Physiol Heart Circ Physiol 2001; 281: H543–H551. [DOI] [PubMed] [Google Scholar]
- 17. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 18. Fujimoto N, Hosokawa N, Iwata K, Shinya T, Okada Y, Hayakawa T. A one‐step sandwich enzyme immunoassay for inactive precursor and complexed forms of human matrix metalloproteinase 9 (92 kDa gelatinase/type IV collagenase, gelatinase B) using monoclonal antibodies. Clin Chim Acta 1994; 231: 79–88. [DOI] [PubMed] [Google Scholar]
- 19. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984; 311: 819–823. [DOI] [PubMed] [Google Scholar]
- 20. Yamazaki T, Lee JD, Shimizu H, Uzui H, Ueda T. Circulating matrix metalloproteinase‐2 is elevated in patients with congestive heart failure. Eur J Heart Fail 2004; 6: 41–45. [DOI] [PubMed] [Google Scholar]
- 21. Maeder MT, Rickenbacher P, Rickli H, Abbühl H, Gutmann M, Erne P, Vuilliomenet A, Peter M, Pfisterer M, Brunner‐La Rocca HP, Investigators T‐C. N‐terminal pro brain natriuretic peptide‐guided management in patients with heart failure and preserved ejection fraction: findings from the Trial of Intensified versus standard medical therapy in elderly patients with congestive heart failure (TIME‐CHF). Eur J Heart Fail 2013; 15: 1148–1156. [DOI] [PubMed] [Google Scholar]
- 22. Flevari P, Theodorakis G, Leftheriotis D, Kroupis C, Kolokathis F, Dima K, Anastasiou‐Nana M, Kremastinos D. Serum markers of deranged myocardial collagen turnover: their relation to malignant ventricular arrhythmias in cardioverter‐defibrillator recipients with heart failure. Am Heart J 2012; 164: 530–537. [DOI] [PubMed] [Google Scholar]
- 23. Kanoupakis EM, Manios EG, Kallergis EM, Mavrakis HE, Goudis CA, Saloustros IG, Milathianaki ME, Chlouverakis GI, Vardas PE. Serum markers of collagen turnover predict future shocks in implantable cardioverter‐defibrillator recipients with dilated cardiomyopathy on optimal treatment. J Am Coll Cardiol 2010; 55: 2753–2759. [DOI] [PubMed] [Google Scholar]
- 24. Li YY, Feng Y, McTiernan CF, Pei W, Moravec CS, Wang P, Rosenblum W, Kormos RL, Feldman AM. Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation 2001; 104: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 25. Nakaya R, Uzui H, Shimizu H, Nakano A, Mitsuke Y, Yamazaki T, Ueda T, Lee JD. Pravastatin suppresses the increase in matrix metalloproteinase‐2 levels after acute myocardial infarction. Int J Cardiol 2005; 105: 67–73. [DOI] [PubMed] [Google Scholar]
- 26. Chi JF, Uzui H, Guo HY, Ueda T, Lee JD. Effects of eplerenone on the activation of matrix metalloproteinase‐2 stimulated by high glucose and interleukin‐1β in human cardiac fibroblasts. Genet Mol Res 2014; 13: 4845–4855. [DOI] [PubMed] [Google Scholar]
- 27. Guo XG, Uzui H, Mizuguchi T, Ueda T, Chen JZ, Lee JD. Imidaprilat inhibits matrix metalloproteinase‐2 activity in human cardiac fibroblasts induced by interleukin‐1beta via NO‐dependent pathway. Int J Cardiol 2008; 126: 414–420. [DOI] [PubMed] [Google Scholar]
- 28. Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res 2001; 89: 201–210. [DOI] [PubMed] [Google Scholar]
- 29. Hessel MH, Bleeker GB, Bax JJ, Henneman MM, den Adel B, Klok M, Schalij MJ, Atsma DE, van der Laarse A. Reverse ventricular remodelling after cardiac resynchronization therapy is associated with a reduction in serum tenascin‐C and plasma matrix metalloproteinase‐9 levels. Eur J Heart Fail 2007; 9: 1058–1063. [DOI] [PubMed] [Google Scholar]
- 30. Uzui H, Nakano A, Mitsuke Y, Geshi T, Sakata J, Sarazawa K, Morishita T, Satou T, Ishida K, Lee J‐D. Acarbose treatments improve arterial stiffness in patients with type 2 diabetes mellitus. Journal of Diabetes Investigation 2011; 2: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franz M, Berndt A, Neri D, Galler K, Grün K, Porrmann C, Reinbothe F, Mall G, Schlattmann P, Renner A, Figulla HR, Jung C, Küthe F. Matrix metalloproteinase‐9, tissue inhibitor of metalloproteinase‐1, B+ tenascin‐C and ED‐A+ fibronectin in dilated cardiomyopathy: potential impact on disease progression and patients' prognosis. Int J Cardiol 2013; 168: 5344–5351. [DOI] [PubMed] [Google Scholar]
- 32. Buralli S, Dini FL, Ballo P, Conti U, Fontanive P, Duranti E, Metelli MR, Marzilli M, Taddei S. Circulating matrix metalloproteinase‐3 and metalloproteinase‐9 and tissue Doppler measures of diastolic dysfunction to risk stratify patients with systolic heart failure. Am J Cardiol 2010; 105: 853–856. [DOI] [PubMed] [Google Scholar]
- 33. Dini FL, Buralli S, Bajraktari G, Elezi S, Duranti E, Metelli MR, Carpi A, Taddei S. Plasma matrix metalloproteinase‐9 better predicts outcome than N‐terminal protype‐B natriuretic peptide in patients with systolic heart failure and a high prevalence of coronary artery disease. Biomed Pharmacother 2010; 64: 339–342. [DOI] [PubMed] [Google Scholar]
- 34. Kitaoka H, Kubo T, Okawa M, Hayato K, Yamasaki N, Matsumura Y, Doi YL. Impact of metalloproteinases on left ventricular remodeling and heart failure events in patients with hypertrophic cardiomyopathy. Circ J 2010; 74: 1191–1196. [DOI] [PubMed] [Google Scholar]
- 35. Nishikawa N, Yamamoto K, Sakata Y, Mano T, Yoshida J, Miwa T, Takeda H, Hori M, Masuyama T. Differential activation of matrix metalloproteinases in heart failure with and without ventricular dilatation. Cardiovasc Res 2003; 57: 766–774. [DOI] [PubMed] [Google Scholar]
- 36. Yasue H, Ogawa H, Tanaka H, Miyazaki S, Hattori R, Saito M, Ishikawa K, Masuda Y, Yamaguchi T, Motomiya T, Tamura Y. Effects of aspirin and trapidil on cardiovascular events after acute myocardial infarction. Japanese Antiplatelets Myocardial Infarction Study (JAMIS) Investigators. Am J Cardiol 1999; 83: 1308–1313. [DOI] [PubMed] [Google Scholar]
- 37. Pristipino C, Beltrame JF, Finocchiaro ML, Hattori R, Fujita M, Mongiardo R, Cianflone D, Sanna T, Sasayama S, Maseri A. Major racial differences in coronary constrictor response between japanese and caucasians with recent myocardial infarction. Circulation 2000; 101: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 38. Yasue H, Omote S, Takizawa A, Nagao M, Miwa K, Tanaka S. Exertional angina pectoris caused by coronary arterial spasm: effects of various drugs. Am J Cardiol 1979; 43: 647–652. [DOI] [PubMed] [Google Scholar]
- 39. Huntley BK, Sandberg SM, Heublein DM, Sangaralingham SJ, Burnett JC, Ichiki T. Pro‐B‐type natriuretic peptide‐1‐108 processing and degradation in human heart failure. Circ Heart Fail 2015; 8: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Costello‐Boerrigter LC, Lapp H, Boerrigter G, Lerman A, Bufe A, Macheret F, Heublein DM, Larue C, Burnett JC. Secretion of prohormone of B‐type natriuretic peptide, proBNP1‐108, is increased in heart failure. JACC Heart Fail 2013; 1: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left‐ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001; 357: 1385–1390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlations with MMP‐9 levels.
Table S2 Patient characteristics by MMP‐9 value.
Figure S1. Bar graphs illustrating MMP‐9, TIMP‐1, the MMP‐9/TIMP‐1 ratio, and BNP levels in HFREF and HFPEF patients.


