Abstract
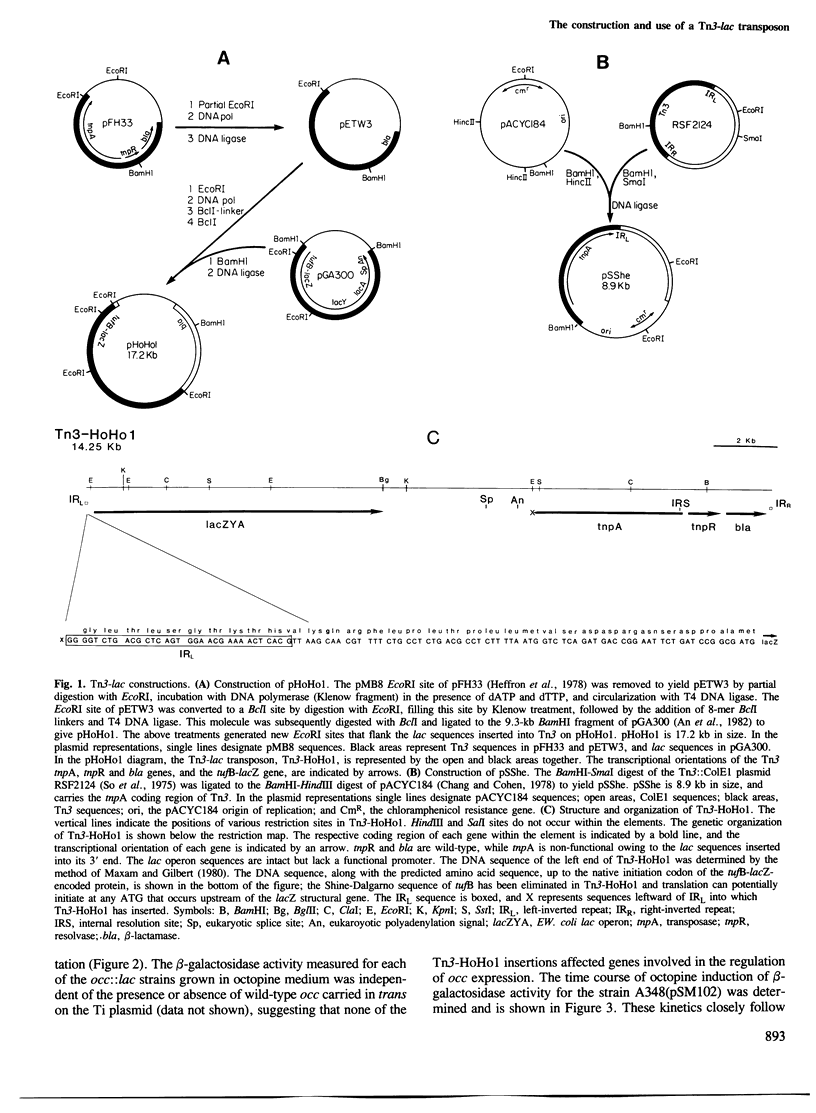
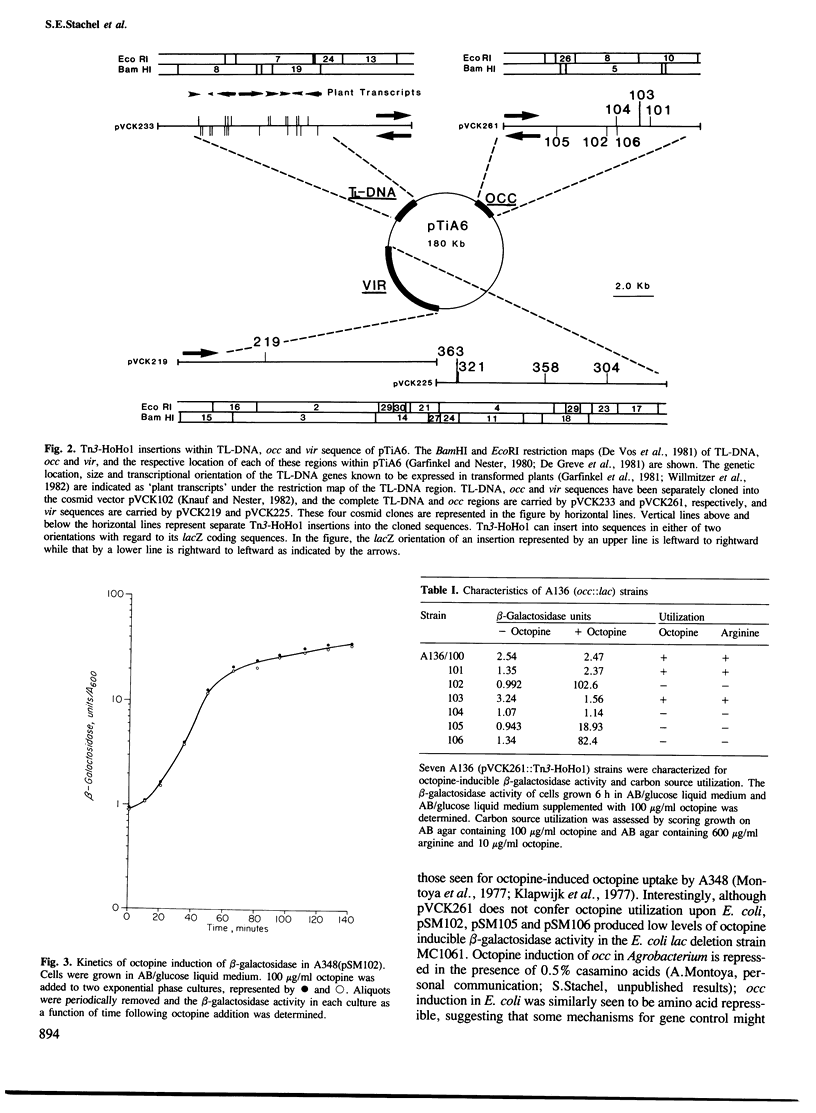
The construction and use of a Tn3-lac transposon, Tn3-HoHo1, is described. Tn3-HoHo1 can serve as a transposon mutagen and provides a new and useful system for the random generation of both transcriptional and translational lacZ gene fusions. In these fusions the production of beta-galactosidase, the lacZ gene product, is placed under the control of the gene into which Tn3-HoHo1 has inserted. The expression of the gene can thus be analyzed by monitoring beta-galactosidase activity. Tn3-HoHo1 carries a non-functional transposase gene; consequently, it can transpose only if transposase activity is supplied in trans, and is stable in the absence of this activity. A system for the insertion of Tn3-HoHo1 into sequences specifically contained within plasmids is described. The applicability of Tn3-HoHo1 was demonstrated studying three functional regions of the Agrobacterium tumefaciens A6 Ti plasmid. These regions code for octopine catabolism, virulence and plant tumor phenotype. The regulated expression of genes contained within each of these regions was analyzed in Agrobacterium employing Tn3-HoHo1 generated lac fusions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Hidaka K., Siminovitch L. Expression of bacterial beta-galactosidase in animal cells. Mol Cell Biol. 1982 Dec;2(12):1628–1632. doi: 10.1128/mcb.2.12.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Caplan A., Herrera-Estrella L., Inzé D., Van Haute E., Van Montagu M., Schell J., Zambryski P. Introduction of genetic material into plant cells. Science. 1983 Nov 18;222(4625):815–821. doi: 10.1126/science.222.4625.815. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. Overproduction of the Tn3 transposition protein and its role in DNA transposition. Cell. 1982 Feb;28(2):345–354. doi: 10.1016/0092-8674(82)90352-x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J. In vivo formation of gene fusions encoding hybrid beta-galactosidase proteins in one step with a transposable Mu-lac transducing phage. Proc Natl Acad Sci U S A. 1984 Jan;81(2):535–539. doi: 10.1073/pnas.81.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., Currier T. C., Farrand S. K., Bendich A. J., Gordon M. P., Nester E. W. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A., Lee B. K. Association of the lethal yellow (Ay) coat color mutation with an ecotropic murine leukemia virus genome. Proc Natl Acad Sci U S A. 1983 Jan;80(1):247–249. doi: 10.1073/pnas.80.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Greve H., Decraemer H., Seurinck J., Van Montagu M., Schell J. The functional organization of the octopine Agrobacterium tumefaciens plasmid pTiB6s3. Plasmid. 1981 Sep;6(2):235–248. doi: 10.1016/0147-619x(81)90069-x. [DOI] [PubMed] [Google Scholar]
- De Vos G., De Beuckeleer M., Van Montagu M., Schell J. Restriction endonuclease mapping of the octopine tumor-inducing plasmid pTiAch5 of Agrobacterium tumefaciens. Plasmid. 1981 Sep;6(2):249–253. doi: 10.1016/0147-619x(81)90070-6. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R., Eady R. R., Espin G., Hill S., Iaccarino M., Kahn D., Merrick M. Analysis of regulation of Klebsiella pneumoniae nitrogen fixation (nif) gene cluster with gene fusions. Nature. 1980 Jul 10;286(5769):128–132. doi: 10.1038/286128a0. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin N. C. Genetic fusions for operon analysis. Annu Rev Genet. 1978;12:193–221. doi: 10.1146/annurev.ge.12.120178.001205. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Gelvin S. B., Gordon M. P., Nester E. W., Aronson A. I. Transcription of the Agrobacterium Ti plasmid in the bacterium and in crown gall tumors. Plasmid. 1981 Jul;6(1):17–29. doi: 10.1016/0147-619x(81)90051-2. [DOI] [PubMed] [Google Scholar]
- Gielen J., De Beuckeleer M., Seurinck J., Deboeck F., De Greve H., Lemmers M., Van Montagu M., Schell J. The complete nucleotide sequence of the TL-DNA of the Agrobacterium tumefaciens plasmid pTiAch5. EMBO J. 1984 Apr;3(4):835–846. doi: 10.1002/j.1460-2075.1984.tb01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Heffron F., Bedinger P., Champoux J. J., Falkow S. Deletions affecting the transposition of an antibiotic resistance gene. Proc Natl Acad Sci U S A. 1977 Feb;74(2):702–706. doi: 10.1073/pnas.74.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Hille J., van Kan J., Schilperoort R. trans-Acting virulence functions of the octopine Ti plasmid from Agrobacterium tumefaciens. J Bacteriol. 1984 May;158(2):754–756. doi: 10.1128/jb.158.2.754-756.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J., Hofker M., den Dulk-Ras H., Schilperoort R. A. A comparison of virulence determinants in an octopine Ti plasmid, a nopaline Ti plasmid, and an Ri plasmid by complementation analysis of Agrobacterium tumefaciens mutants. Plasmid. 1984 May;11(3):195–205. doi: 10.1016/0147-619x(84)90026-x. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Brent R., Ptashne M., Walker G. C. Regulation of damage-inducible genes in Escherichia coli. J Mol Biol. 1982 Sep 25;160(3):445–457. doi: 10.1016/0022-2836(82)90307-2. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk P. M., Schilperoort R. A. Negative control of octopine degradation and transfer genes of octopine Ti plasmids in Agrobacterium tumefaciens. J Bacteriol. 1979 Aug;139(2):424–431. doi: 10.1128/jb.139.2.424-431.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee H. J., White F. F., Iyer V. N., Gordon M. P., Nester E. W. Mutational analysis of the virulence region of an Agrobacterium tumefaciens Ti plasmid. J Bacteriol. 1983 Feb;153(2):878–883. doi: 10.1128/jb.153.2.878-883.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., An G., Friesen J. D., Fill N. P. Location of the tufB promoter of E. coli: cotranscription of tufB with four transfer RNA genes. Cell. 1981 Jul;25(1):251–258. doi: 10.1016/0092-8674(81)90250-6. [DOI] [PubMed] [Google Scholar]
- Lis J. T., Simon J. A., Sutton C. A. New heat shock puffs and beta-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell. 1983 Dec;35(2 Pt 1):403–410. doi: 10.1016/0092-8674(83)90173-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G., Klapwijk P. M., Poulis J. A., Schilperoort R. A. Characterization of Tn904 insertions in octopine Ti plasmid mutants of Agrobacterium tumefaciens. J Bacteriol. 1980 Oct;144(1):82–91. doi: 10.1128/jb.144.1.82-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Schröder G., Waffenschmidt S., Weiler E. W., Schröder J. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem. 1984 Jan 16;138(2):387–391. doi: 10.1111/j.1432-1033.1984.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J., Beckwith J. R. Labeling of proteins with beta-galactosidase by gene fusion. Identification of a cytoplasmic membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1980 Jan 10;255(1):168–174. [PubMed] [Google Scholar]
- So M., Gill R., Falkow S. The generation of a ColE1-Apr cloning vehicle which allows detection of inserted DNA. Mol Gen Genet. 1975 Dec 30;142(3):239–249. doi: 10.1007/BF00425649. [DOI] [PubMed] [Google Scholar]
- Stern M. J., Higgins C. F., Ames G. F. Isolation and characterization of lac fusions to two nitrogen-regulated promoters. Mol Gen Genet. 1984;195(1-2):219–227. doi: 10.1007/BF00332750. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Wieder S., McSharry R. Use of bacteriophage transposon Mu d1 to determine the orientation for three proC-linked phosphate-starvation-inducible (psi) genes in Escherichia coli K-12. J Bacteriol. 1981 Apr;146(1):93–101. doi: 10.1128/jb.146.1.93-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. F., Klee H. J., Nester E. W. In vivo packaging of cosmids in transposon-mediated mutagenesis. J Bacteriol. 1983 Feb;153(2):1075–1078. doi: 10.1128/jb.153.2.1075-1078.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Simons G., Schell J. The TL-DNA in octopine crown-gall tumours codes for seven well-defined polyadenylated transcripts. EMBO J. 1982;1(1):139–146. doi: 10.1002/j.1460-2075.1982.tb01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullems G. J., Molendijk L., Ooms G., Schilperoort R. A. Differential expression of crown gall tumor markers in transformants obtained after in vitro Agrobacterium tumefaciens-induced transformation of cell wall regenerating protoplasts derived from Nicotiana tabacum. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4344–4348. doi: 10.1073/pnas.78.7.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]