Abstract
In the gene therapy field, re-administration of adeno-associated virus (AAV) is an important topic because a decrease in therapeutic protein expression might occur over time. However, an efficient re-administration with the same AAV serotype is impossible due to serotype-specific, anti-AAV neutralizing antibodies (NABs) that are produced after initial AAV treatment. To address this issue, we explored the feasibility of using chimeric AAV serotype 5 (AAV5ch) and AAV1 for repeated liver-targeted gene delivery. To develop a relevant model, we immunized animals with a high dose of AAV5ch-human secreted embryonic alkaline phosphatase (hSEAP) that generates high levels of anti-AAV5ch NAB. Secondary liver transduction with the same dose of AAV1-human factor IX (hFIX) in the presence of high levels of anti-AAV5ch NAB proved to be successful because expression/activity of both reporter transgenes was observed. This is the first time that two different transgenes are shown to be produced by non-human primate (NHP) liver after sequential administration of clinically relevant doses of both AAV5ch and AAV1. The levels of transgene proteins achieved after delivery with AAV5ch and AAV1 illustrate the possibility of both serotypes for liver targeting. Furthermore, transgene DNA and RNA biodistribution patterns provided insight into the potential cause of decrease or loss of transgene protein expression over time in NHPs.
Keywords: AAV, gene therapy, re-administration of gene therapy
Although re-administration of AAV (adeno-associated virus)-based gene therapy with the same AAV serotype is impossible due to serotype-specific, anti-AAV neutralizing antibodies (NABs), Majowicz et al. (2017) report in Molecular Therapy a successful alternative for repeated AAV-based gene delivery when using sequentially two different AAV serotypes (AAV5ch followed by AAV1).
Introduction
Adeno-associated virus (AAV) vector-based gene therapy has been successfully employed in the treatment of genetic disorders in preclinical studies as well as in clinical trials. The clinical studies included hundreds of patients and indicate an excellent safety record for AAV-mediated gene therapy in humans.1, 2, 3 A major hurdle in achieving a successful AAV-based gene therapy is the presence of circulating neutralizing antibodies (NABs) directed against the AAV capsid proteins. NABs against specific AAV serotypes can be present in a patient’s blood prior to therapy because of naturally acquired infections with the wild-type AAV virus (pre-existing NABs) and are currently an exclusion criteria for participation in clinical trials that make use of AAV. Serotype-specific circulating NABs are formed after first administration of AAV vector in gene therapy. These NABs recognize AAV viral capsid proteins and block the secondary transduction with AAV of the same serotype.4, 5, 6, 7 Anti-AAV NABs after gene therapy treatment with AAV can reach high titers and have been reported to persist long after treatment. Although AAV-mediated gene therapy is expected to be long-lasting, it is anticipated that re-administration of the therapy may be necessary because of the potential decrease of transgene expression over time as a result of the natural turnover of transduced cells. Repeated AAV treatment might also be needed if the initial treatment does not result in a sufficient level of therapeutic protein expression or in the case of pediatric indications caused by fast liver growth leading to the loss of vector genomes and subsequent decline of transgene expression.8, 9 Therefore, strategies permitting a repeated gene delivery need to be developed.
Granted that serotypes with different antibody reactivity profiles and similar affinity for a particular target tissue are available, successive use of different AAV vector serotypes is a potential approach to escape the activity of NABs. We will refer to such a serotype-switching strategy as “cross-administration.” When considering cross-administration of two AAV serotypes to target a specific organ, not only the tissue tropism of those AAVs has to be considered, but also their serological prevalence in the patient population, as well as the level of NAB cross-reactivity between those serotypes. Serological studies in humans have shown a high prevalence of anti-AAV NABs with approximately 59% of the population being seropositive for AAV serotype 2 (AAV2), 50.5% for AAV1, 37% for AAV6, 33.5% for AAV9, 19% for AAV8, and 3.2% for AAV5.10 Furthermore, the degree of conservation in the amino acid sequences among AAVs is relatively high, with serotype 5 being the most divergent, and variable levels of cross-reactivity between anti-AAV antibodies have been shown for a wide range of serotypes.10, 11 From the therapeutic perspective, the cross-administration method has a significant advantage over the alternative approach of immune suppression,12, 13, 14, 15 which can lead to side effects and might not be applicable in all clinical settings depending on the patient’s treatment history and general health. In order to achieve cross-administration targeting the liver, we selected AAV1 and a chimeric AAV serotype 5 (AAV5ch) described and used previously16, 17 in which a portion of the viral protein (VP)1 polypeptide was replaced with the corresponding portion of type 2. Importantly, the VP1 substitution did not alter the outside structure of the capsid nor the tropism of AAV5, which behaved indistinguishably from AAV5 with wild-type VP1.16, 17 Our choice was based on their excellent safety profiles and their clinical application in humans.1, 17, 18 Also, AAV5 and AAV1 tropism for the liver have been reported to be similar in mice.2, 19 AAV5 and AAV1 immune epitopes have been partially characterized and reported as distinct.20 Whereas Grimm et al.2 reported cross-neutralization between AAV5 and AAV1 in mice, others reported only a weak cross-reactivity of anti-AAV5 and anti-AAV1 antibodies both in vitro21 and in vivo.6 The principle of cross-administration for liver targeting has previously been demonstrated in non-human primates (NHPs) that were administered intravenously (i.v.) an AAV5-based vector in the presence of anti-AAV8 NABs due to natural exposure (pre-existing NABs).22 In the present study, we used a model that reflects a clinical situation in which a second AAV-mediated gene delivery would be needed. Therefore, in order to explore the efficacy of AAV5ch and AAV1 cross-administration for liver targeting, we first injected mice and NHPs i.v. with a high dose of AAV5ch in order to raise high levels of anti-AAV5 NABs before re-administration with the same dose of AAV1.
Results
Successful Repeated AAV-Based Gene Delivery in Mice
Lack of Cross-Reactivity of Anti-AAV5ch and Anti-AAV1 Antibodies in Mice and Successful Repeated Hepatic Gene Delivery after Sequential Administration with AAV5ch and AAV1 Vectors
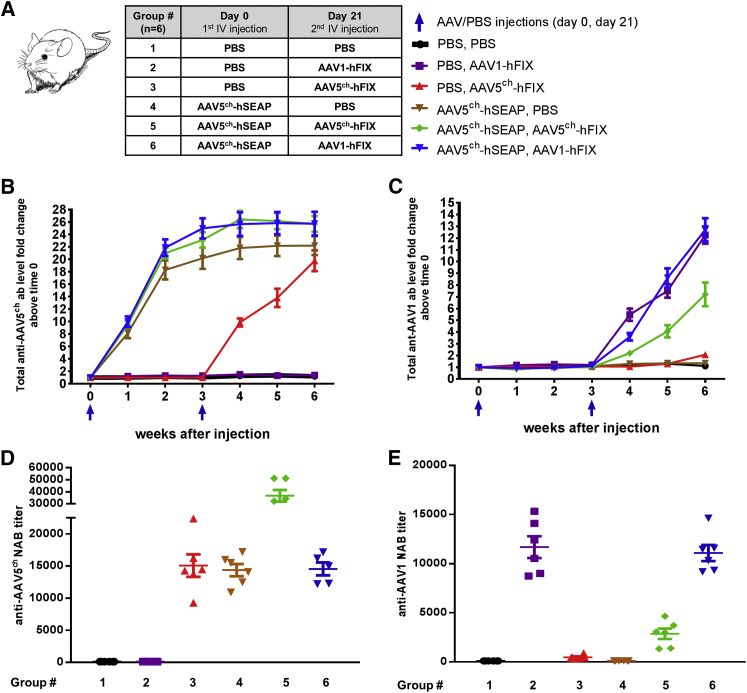
Mice were injected i.v. with AAV5ch-human secreted embryonic alkaline phosphatase (hSEAP) or PBS at week 0. A second i.v. administration with AAV1-human factor IX (hFIX) or AAV5ch-hFIX or PBS was performed in week 3 (Figure 1A). The injections were well tolerated by all of the animals. As expected, total anti-AAV5ch and anti-AAV1 antibodies were detected in the plasma of all of the mice that were injected with AAV5ch and AAV1 vectors, respectively (Figure 1B). Monitoring of the total antibodies showed no cross-recognition of AAV1 capsid by anti-AAV5ch antibodies or AAV5ch capsid by anti-AAV1 antibodies after primary administration with AAV1 or AAV5ch or in the case of cross-administration with AAV5ch followed by AAV1. When two sequential injections with AAV5ch-based vectors were given, recognition of AAV1 capsids by anti-AAV5ch antibodies was observed. This might be related to the induction of a humoral response against weaker epitopes, common for AAV5ch and AAV1, and recognized only after repeated exposure to high doses of AAV5ch (Figure 1C).
Figure 1.
Experimental Setup and Humoral Immune Response against AAV Vectors in Mice
(A–E) Mice receiving intravenous injections of PBS or AAV5ch-hSEAP at week 0 and second intravenous injection of PBS, AAV1-hFIX, or AAV5ch-hFIX at week 3 (A) develop total anti-AAV5ch antibodies (B) and total anti-AAV1 antibodies (C) in plasma over time, which correlates with anti-AAV5ch NAB (D) and anti-AAV1 NAB (E) levels at week 6. Data are presented as means ± SEM of all mice (n = 6).
In order to gain more insight into the humoral immune response against the AAV capsid, we performed NABs assays for both AAV5ch and AAV1 serotypes using plasma samples obtained at sacrifice (week 6). The anti-AAV5ch NAB titers measured were comparable in all of the mice injected once with AAV5ch-hSEAP or AAV5ch-hFIX; however, titers were higher in the group that was injected twice with AAV5ch (AAV5ch-hSEAP followed by AAV5ch-hFIX) (Figure 1D). Next, NABs against AAV1 capsid were measured in the plasma of mice that were injected with AAV1-hFIX. No NAB antibodies against AAV1 capsid were detected in the mice groups that were injected with AAV5ch-hSEAP alone, AAV5ch-hFIX alone, or PBS (Figure 1E). The results of our in vitro anti-AAV NABs assays demonstrate lack of cross-interference of anti-AAV5ch and anti-AAV1 NABs present in mice plasma, with in vitro transduction efficacy of AAV1 and AAV5ch, respectively, after mice received a single administration of each vector (Figures 1D and 1E).
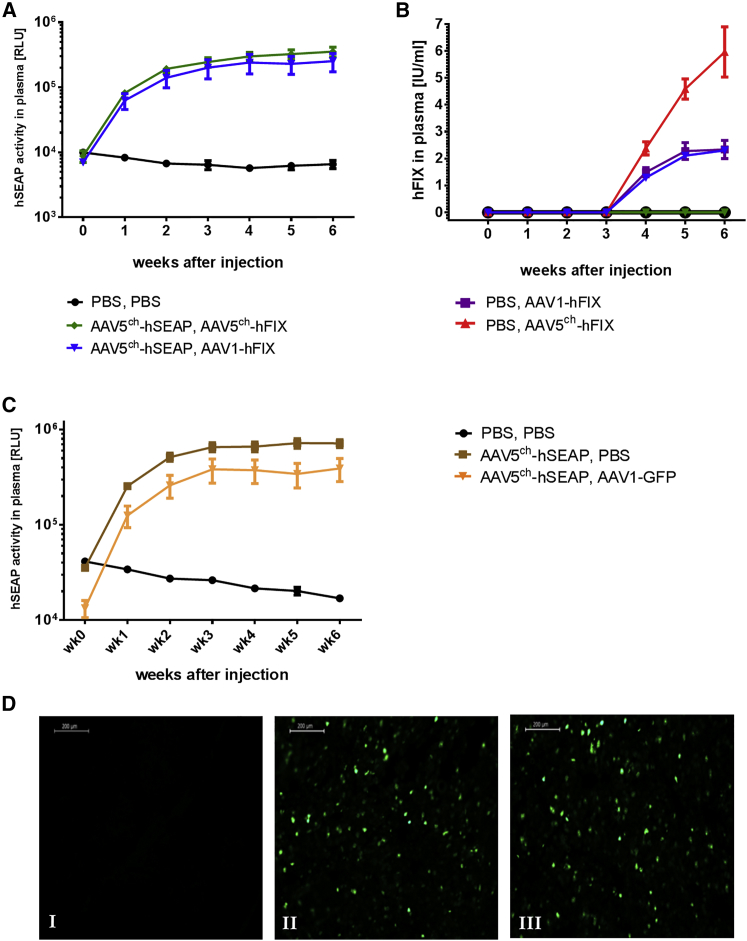
In order to determine the efficacy of re-administration with AAV1 after initial immunization with AAV5ch, we measured the activity or presence of hSEAP and hFIX protein in the plasma of the injected animals. As expected, hSEAP activity was detected in the plasma of all of the mice injected first with AAV5ch-hSEAP (Figures 2A and 2C). The mice that had received AAV1-hFIX as second injection in week 3 had hFIX protein in plasma from week 4 onward (Figure 2B). In contrast, the mice that received AAV5ch-hFIX as second injection following the first AAV5ch-hSEAP administration had no detectable level of hFIX in the plasma (Figure 2B). It should be noted that the amount of hFIX protein measured in plasma after injection with AAV1-hFIX was not influenced by the prior injection with AAV5ch-hSEAP because the same level of hFIX was measured in the control group that had received PBS as first injection and AAV1-hFIX in the second injection (Figure 2B). In general, the level of hFIX protein in plasma of mice injected with AAV5ch-hFIX was 2.5 times higher than in plasma of mice injected with AAV1-hFIX (Figure 2B). Additionally, successful cross-administration of AAV5ch and AAV1 vector was also achieved when combining other expression cassettes: AAV5ch-hSEAP followed by AAV1-EGFP (week 3) (Figures 2C and 2D).
Figure 2.
Transgene Activity or Presence in Mouse Plasma and Liver Tissue
(A–D) Mice intravenously cross-administered with AAV5ch followed by AAV1 present with stable reporter gene hSEAP activity (A and C) and hFIX presence (B) in mouse plasma over time and expression of GFP in representative mouse liver sections (D). Mice received intravenous injections of PBS or AAV5ch-hSEAP at week 0 and a second i.v. injection of PBS, AAV1-hFIX, or AAV5ch-hFIX at week 3 (n = 6/group) (A and B), or a first i.v. injection of PBS or AAV5ch-hSEAP at week 0 and a subsequent one of PBS, AAV1-GFP at week 3 (C and D). (D) PBS, PBS group (I); PBS, AAV1-GFP group (II); AAV5ch-hSEAP, AAV1-GFP group (III). RLU, relative luminescence units. Data in graphs (A)–(C) are presented as means ± SEM of all mice (n = 6).
Successful Repeated AAV-Based Gene Delivery in NHPs
Cross-Administration of AAV Serotypes AAV5ch and AAV1 Is Well Tolerated by NHPs
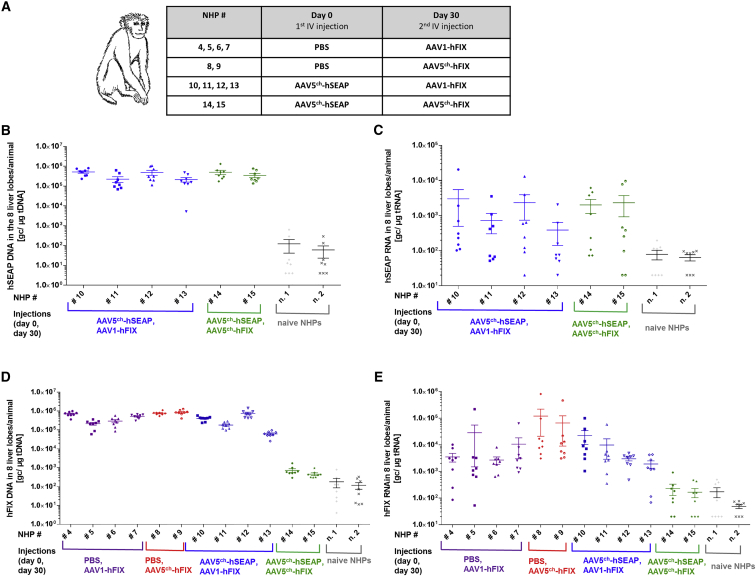
In order to test the cross-administration approach with the AAV serotypes 5ch and 1 in an animal model that is phylogenetically closer to humans, we conducted a study in NHPs (Macaca fascicularis). The animals were injected at day 0 with an AAV5ch-based vector (AAV5ch-hSEAP) or PBS, and at day 30 with either an AAV1- or AAV5ch-based vector (AAV1-hFIX or AAV5ch-hFIX) or PBS. Twelve NHPs were followed for 1 year after first AAV delivery. The two consecutive AAV injections at a dose of 3 × 1013 genome copies (gc)/kg each were well tolerated by all of the animals. No significant changes associated with experimental treatment were reported in routine hematology and biochemistry during the observation period (Figure S1). Additionally, regardless of the treatment group, no significant anatomical alteration or pathological abnormalities were observed at sacrifice. Further analysis of liver tissue (eight liver pieces per animal) was performed to assess the presence of immune inflammation markers CD1c (dendritic cells), CD3 (T cells), CD11b (APCs), CD16 (activated macrophages), CD20 (B cells), CD56 (NK cells), and CD14 (monocytes/macrophages), and no differences were observed between the experimental groups and the control PBS group (data not shown).
Successful Liver Transduction by AAV5ch and AAV1 after Sequential i.v. Delivery
In order to evaluate the transduction efficacy of AAV5ch-hSEAP, AAV5ch-hFIX, and AAV1-hFIX, we collected and processed tissue samples from eight liver regions of all animals at sacrifice (364 days after first injection). The AAV transduction efficacy in the liver was evaluated at the DNA and mRNA levels by qPCR and qRT-PCR with primer sets specific for hFIX and hSEAP, respectively. The data obtained are reported in Figure 3. Animals injected primarily with AAV5ch-hSEAP and re-administered with AAV1-hFIX presented both hSEAP and hFIX DNA and mRNA in the liver (NHPs 10–13). On the contrary and as expected, the animals initially injected with AAV5ch-hSEAP and re-administered with AAV5ch-hFIX had high levels of hSEAP DNA and mRNA in the liver, whereas DNA and RNA levels of hFIX were similar to PBS-injected animals, thus confirming that the secondary liver transduction with the same AAV serotype is unsuccessful.
Figure 3.
Experimental Setup and Presence of hSEAP and hFIX Vector DNA and mRNA in the Liver
(A) Monkeys were injected i.v. at day 0 with an AAV5ch-based vector (AAV5ch-hSEAP) or PBS and at day 30 with either an AAV1- or AAV5ch-based vector (AAV1-hFIX or AAV5ch-hFIX). (B–E) Reporter gene hSEAP DNA (B), mRNA (C), and hFIX DNA (D) and mRNA (E) in the monkey liver tissue at sacrifice. Mean ± SEM of eight different liver regions was plotted for every monkey. Monkeys were injected i.v. at day 0 with an AAV5ch-hSEAP (HNPs 10–15) or nothing (n. 1, n. 2) and at day 30 with AAV1-hFIX (HNPs 4–6 and 10–13) or AAV5ch-hFIX (HNPs 14 and 15) and sacrificed at day 364.
The levels of vector DNA as well as mRNA of hSEAP measured after primary administration with AAV5ch-hSEAP (3 × 1013 gc/kg, NHPs 10–15) were in the same range in all of the animals injected. The same observation was made for the levels of vector DNA and mRNA of hFIX in the liver of the animals injected only with AAV5ch-hFIX (3 × 1013 gc/kg, NHPs 8 and 9), as well as in the animals injected with AAV1-hFIX. Also, the same range of hFIX DNA and RNA was present in the livers of the animals that were injected only with AAV1-hFIX or initially injected with AAV5ch-hSEAP followed by AAV1-hFIX (3 × 1013 gc/kg, NHPs 4–7 and 10–13), showing that the initial injection with AAV5ch-hSEAP did not influence the result of secondary transduction with AAV1-hFX.
Interestingly, an uneven distribution of transgene mRNA was observed in the different liver pieces analyzed, whereas vector DNA presence was always found to be similar in all pieces of the liver (Figure 3).
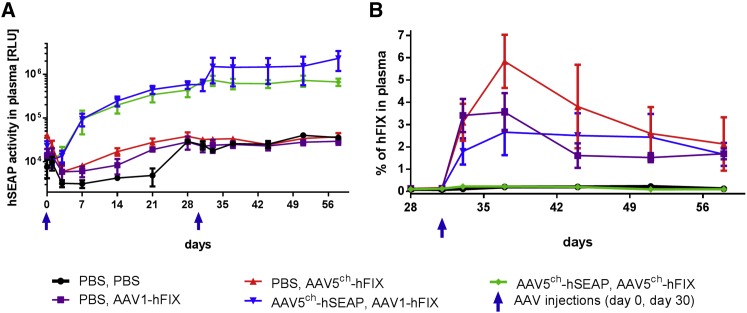
Presence and Activity of Transgene Products in the Plasma after Sequential i.v. AAV5ch- and AAV1-Mediated Gene Delivery in NHP
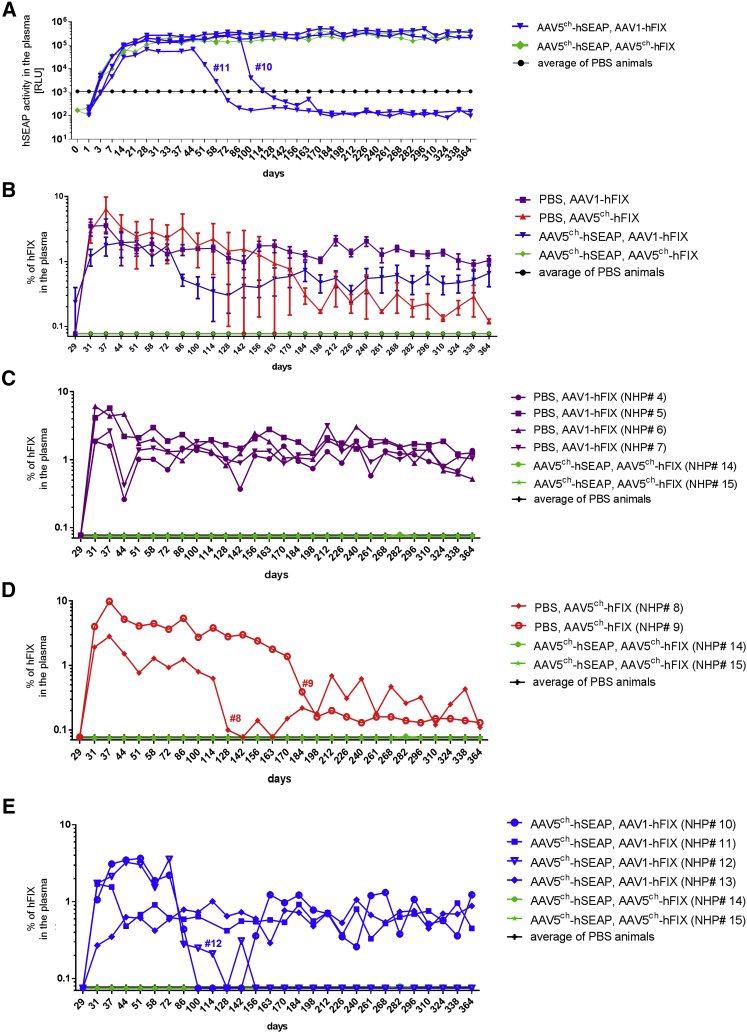
The efficacy of cross-administration with the combination of the AAV serotypes 5ch and 1 was also assessed by the analysis of the activity or presence of hSEAP and hFIX proteins in the plasma of the NHPs. Until 58 days after the initiation of the study, hSEAP activity was detected in the plasma of all animals that were injected with AAV5ch-hSEAP at day 0 of the experiment (Figure 4A). Cross-administration with AAV1-hFIX at day 30 of the experiment proved to be successful in NHPs because the cross-administered group (NHPs 10–13) showed not only hSEAP activity after primary AAV5ch-hSEAP administration (Figure 4A), but also the presence of hFIX protein in the plasma after secondary AAV1-hFIX injection (Figure 4B). In contrast and as expected, animals that were re-administered with the same serotype (AAV5ch-hFIX) did not have any hFIX present in the plasma (Figure 4B), confirming that secondary injection with the same serotype does not result in efficient transduction.
Figure 4.
Short-Term Transgene Activity or Presence in the Monkey Plasma
(A and B) Stable reporter gene hSEAP activity (A) and hFIX expression (B) in monkey plasma over time in animals cross-administered with AAV5ch-hSEAP followed by AAV1-hFX and in animals injected with AAV5ch-hFIX or AAV1-hFIX only. Monkeys were injected i.v. at day 0 with an AAV5ch-based vector (AAV5ch-hSEAP) or PBS and on day 30 with either an AAV1- or AAV5ch-based vector (AAV1-hFIX or AAV5ch-hFIX) or PBS. RLU, relative luminescence units. Data are presented as means ± SEM of all monkeys in each experimental group.
It should be noted that the levels of hFIX protein measured after injection with AAV1-hFIX were not influenced by prior injection with AAV5ch-hSEAP because similar levels of hFIX were measured in the control group that received PBS at first injection and AAV1-hFIX at second injection (Figure 4B).
Interestingly, the pattern of protein detection in the plasma was different for the two transgenes used in the study. hSEAP activity levels in plasma were found to increase until day 21 and then stabilize in all of the NHPs (NHPs 10–15) injected with AAV5ch-hSEAP (Figures 4A and 5A). On the other hand and similar to previously reported NHP studies,22, 23, 24, 25 the levels of hFIX peaked 1 week after injection with AAV5ch-hFIX or AAV1-hFIX before decreasing and stabilizing.
Figure 5.
Long-Term Transgene Activity or Presence in the Monkey Plasma
(A–E) Long-term reporter gene hSEAP activity (A), hFIX expression (grouped, B, means ± SEM), and hFIX expression in individual monkeys in PBS followed by AAV1-hFIX-injected group (C), PBS followed by AAV5ch-hFIX-injected group (D), and AAV5ch-hSEAP followed by AAV1-hFIX-injected group (E) measured in monkey plasma over time. Monkeys were injected i.v. at day 0 with an AAV5ch-based vector (AAV5ch-hSEAP) or PBS and on day 30 with either an AAV1- or AAV5ch-based vector (AAV1-hFIX or AAV5ch-hFIX) or PBS. RLU, relative luminescence units.
In order to determine the stability of hSEAP and hFIX transgene expression over time, we followed the animals beyond the first 58-day period until 1 year after the primary (AAV5ch-hSEAP) injection.
hSEAP activity levels in plasma remained constant in four out of six animals (NHPs 12–15) until sacrifice (Figure 5A). However, in two animals, a decrease and eventually a loss of the hSEAP activity in the plasma was observed from day 58 (NHP 11) and day 100 (NHP 10). Seven out of eight animals (NHPs 4–7, 10, 11, and 13) injected with AAV1-hFIX only or cross-administered with AAV5ch-hSEAP and AAV1-hFIX expressed levels of hFIX in the plasma at a mean of 0.96% ± 0.14% (mean ± SEM) on the last day of the experiment (Figures 5C and 5E). In one animal (NHP 12), circulating hFIX disappeared totally after 98 days (Figure 5E). The two animals injected once with AAV5ch-hFIX had a significant decrease of the protein in the plasma 98 days after AAV5ch-hFIX injection for NHP 8 and 168 days after AAV5ch-hFIX injection for NHP 9 (Figure 5D). The residual circulating levels of hFIX in plasma in NHPs 8 and 9 were at 0.12% ± 0.01% (mean ± SEM) on the last day of the experiment.
Appearance of a Transgene-Directed Immune Response in 5 out of 12 NHPs Injected with AAV Vectors
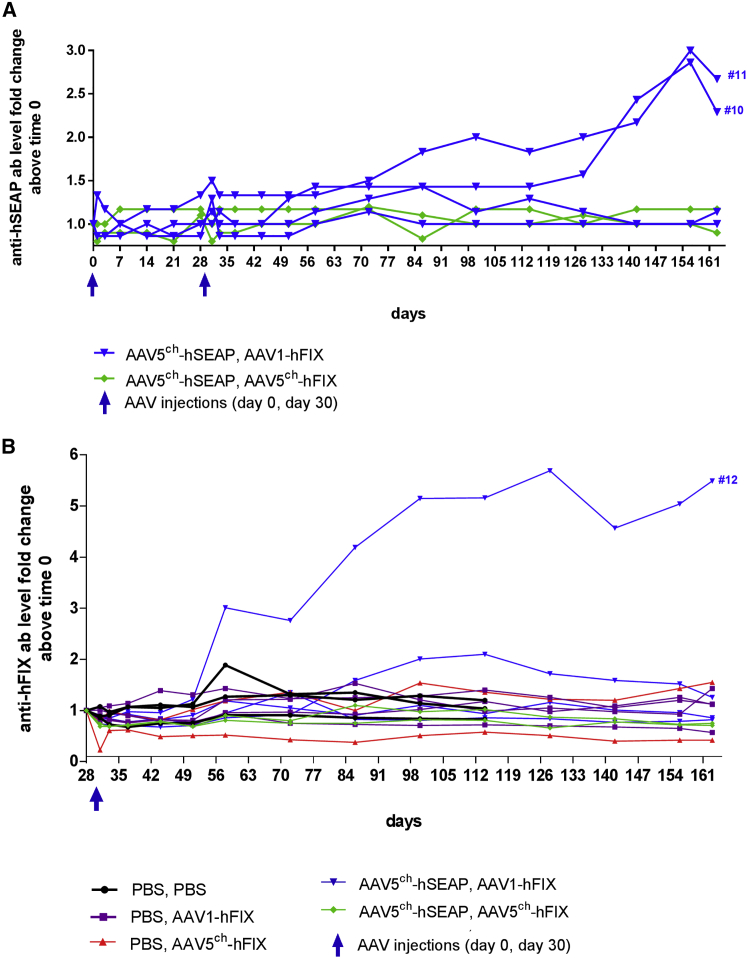
Both transgenes used in the study, hSEAP and hFIX, are encoding secreted proteins of human origin, and therefore the immune response in NHPs toward those proteins can be expected. In order to establish the origin of the decrease of hSEAP and hFIX observed at long-term follow-up in some of the animals, plasma samples were tested for the presence of antibodies against the transgene products. Both animals in which hSEAP activity was decreased (NHPs 10 and 11) presented elevated levels of antibodies against hSEAP in the plasma (Figure 6A) that correlated with the decrease of circulating hSEAP in the plasma. Antibodies against hFIX could be detected in the plasma of the animal with a complete loss of circulating hFIX protein (NHP 12) (Figure 6B). However, it should be noted that the strong association of the hFIX protein antigen-antibody complexes might have impaired the detection of anti-hFIX antibodies in other animals. Altogether the results indicate that the loss of hSEAP activity or hFIX presence in the plasma observed over time in some animals was due to a transgene-directed immune response and suggest that the levels of hSEAP and hFIX in the plasma might be underestimated because of the formation of antibody-antigen complexes that might hinder the detection of the transgene proteins. It should be noted that earlier described levels of vector DNA as well as mRNA measured in the liver after primary administration with AAV5ch-hSEAP were in the same range between all of the animals injected including the two animals (NHPs 11 and 10) that presented a decrease in the levels of hSEAP associated with the presence of circulating antibodies against hSEAP (Figures 3B and 3C). Also, the decrease or loss of hFIX in the plasma of three animals (NHPs 8, 9, and 12) was not associated with a decrease in the levels of vector DNA or mRNA copies (Figures 3D and 3E). Overall, these results suggest that disappearance of hSEAP and hFIX from the circulation was not due to destruction of transduced hepatocytes, because vector DNA and mRNA levels in the livers of animals that experienced transgene proteins presence/activity decrease or loss in plasma were in the same range as those observed in the animals that did not experience such decrease or loss.
Figure 6.
Humoral Immune Response against Transgene Products
(A and B) Fold change of anti-hSEAP (A) and anti-hFIX (B) IgG in monkey plasma over time. Monkeys were injected i.v. on day 0 with an AAV5ch-based vector (AAV5ch-hSEAP) or PBS and on day 30 with either an AAV1- or AAV5ch-based vector (AAV1-hFIX or AAV5ch-hFIX).
Vector DNA/mRNA Biodistribution between Organs
The biodistribution of vector genome DNA was further assessed in tissue samples from eight liver regions and 23 non-hepatic organs from all 12 NHPs that were injected with AAVs and 2 control NHPs that were injected only with PBS. The transgene copy number was determined in a qPCR assay for hSEAP and hFIX. In all NHPs injected with AAV5ch-hSEAP or AAV5ch-hFIX vector, the highest levels of DNA were detected in the liver and in the adrenal glands. Lower vector copy numbers were detected in some animals in spleen, heart, lungs, and kidney. Following AAV1-hFIX injection, most of the hFIX vector DNA was found in the liver and the inguinal lymph nodes. Lower amounts were also detected in mesenteric lymph nodes, spleen, adrenal glands, and heart (Figure S2). No signal was detected in any organ from animals infused with vehicle (PBS).
Additionally, the mRNA levels of hSEAP and hFIX were measured in the eight liver regions, adrenal gland, mesenteric and inguinal lymph nodes, spleen, and heart by qRT-PCR. As expected, both hSEAP and hFIX mRNA were present in the liver tissue. Interestingly, hFIX mRNA was also detected in the spleen of NHPs 9 and 12, whereas hSEAP mRNA was detected in the spleen of NHPs 10, 11, and 14 (Figure S3), adrenal glands of NHPs 12, 14, and 15, and inguinal lymph nodes of NHP 13 (data not shown).
Absence of Cross-Reactivity of Anti-AAV5ch and Anti-AAV1 Antibodies in NHP and Lack of T Cell Response against AAV Capsids
All of the NHPs entering the study were screened and selected for the absence of NABs against AAV5ch and AAV1. The animals were injected as described in Figure 3A. To evaluate the cross-reactivity of anti-AAV antibodies against serotypes 5ch and 1, we collected and analyzed plasma samples for the presence of anti-AAV5ch, anti-AAV1 neutralizing (NABs), and total antibodies.
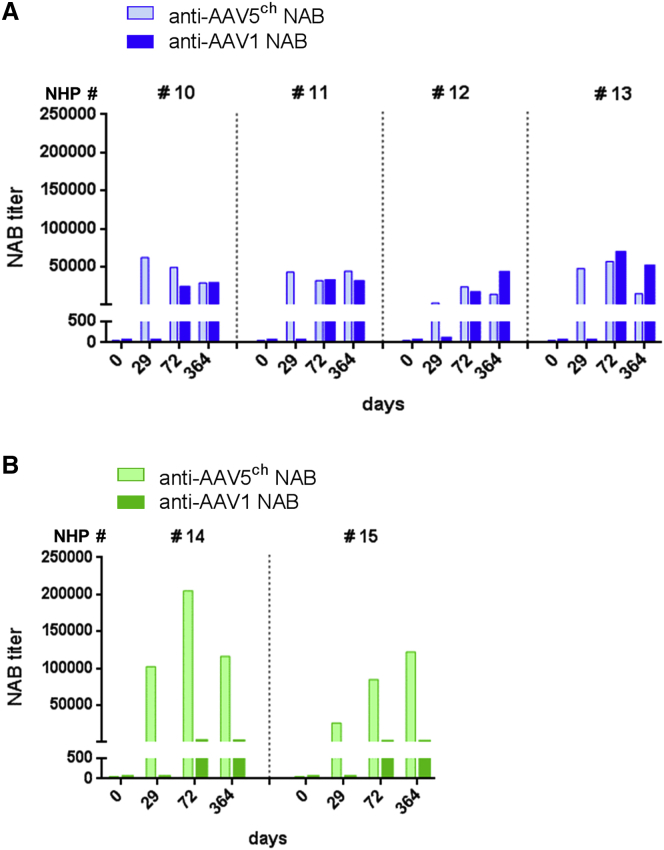
As expected, the animals cross-administered with AAV5ch and AAV1 (NHPs 10–13) (Figure 7A) developed circulating NABs against both AAV5ch and AAV1. Likewise, all of the NHPs administered twice with AAV5ch (NHPs 14 and 15) developed circulating NABs against AAV5ch (Figure 7B). Animals initially injected with AAV5ch followed by AAV1 did not significantly increase their anti-AAV5ch NAB level after injection with AAV1 (Figure 7A). As observed in mice, the NHPs administered twice with AAV5ch showed a significant increase in anti-AAV5ch NAB levels after the second administration (Figure 7B). In contrast, the primary injection with AAV5ch did not increase the magnitude of the anti-AAV1 antibody response after administration with AAV1 because the anti-AAV1 NAB levels obtained in animals from the cross-administered group were not higher than those from the animals that received only an AAV1 injection (data not shown). Similar to the results observed in mice, a low level of recognition of AAV1 capsid by anti-AAV5 antibodies was observed for the two animals injected twice with AAV5ch (NHPs 14 and 15) (Figure 7B).
Figure 7.
Neutralizing Humoral Immune Response against AAV Vectors in Monkeys
(A and B) Monkeys injected i.v. at day 0 with an AAV5ch-based vector (AAV5ch-hSEAP) or PBS and at day 30 with either an AAV1- or AAV5ch-based vector (AAV1-hFIX or AAV5ch-hFIX) develop anti-AAV5ch (A) and anti-AAV1 NABs (B).
Similar results to those obtained for NABs were observed for the total anti-AAV5ch and anti-AAV1 antibodies (Figure S4). Both neutralizing and total antibodies against AAV5ch and AAV1 generated after the injections with AAV5ch-hSEAP, AAV5ch-hFIX, and AAV1-hFIX were present in the plasma of all animals until the end of the experiment (day 364).
Cross-neutralization between AAV5ch and AAV1 was observed in vitro in one out of eight animals injected with AAV5ch (NHP 8) (Figure S4C). However, for this particular animal, binding and neutralization were observed in sera collected before administration with AAV5ch (Figure S4C) for both anti-AAV5ch IgG ELISA and anti-AAV5ch NAB assays. Furthermore, the transduction efficacy of AAV5ch vector in this animal was similar to the other NHPs (Figures 3D and 3E), which point out uncharacterized serum elements that might interfere with antibody-based in vitro assays as previously described by others.26
Overall, these results demonstrate the absence of cross-recognition of AAV1 capsid by anti-AAV5ch antibodies and AAV5ch capsid by anti-AAV1 antibodies.
To get more insight into the immune responses occurring after AAV delivery, we analyzed the cellular immune response against the viral capsid proteins (both whole capsid and pool of peptides covering the whole sequence). The interferon (IFN)-γ response was analyzed using peripheral blood mononuclear cells (PBMCs) extracted 28 days after the first administration and 28, 56, and 70 days after the second administration. The response of splenocytes obtained at necropsy was also analyzed. No IFN-γ response against the different capsids or peptides was observed in any of the samples (data not shown).
Discussion
The present study demonstrates a successful repeated hepatic gene delivery in both mice and NHPs by sequential use of the AAV serotypes 5ch and 1, and it also confirms the absence of anti-AAV NABs cross-reactivity in vivo between these serotypes. We report an efficacious expression of two different reporter genes after sequential delivery of AAV5ch and AAV1, respectively, which establishes that AAV1 could also be considered for liver-targeted gene therapy.
The cross-administration approach has been previously successfully used for liver targeting in NHPs. In that study AAV5 was administered i.v. to animals with circulating pre-existing anti-AAV8 NABs.22 However, the pre-existing anti-AAV NAB titers that are present in the circulation due to natural infection with wild-type AAVs are significantly lower than the levels that are raised after a gene therapy treatment with AAV. In our current report, we describe a study model that reflects a clinical situation in which a second administration of gene delivery with the AAV vector would be needed. Therefore, we injected animals first with a high dose of AAV5ch-hSEAP in order to raise high levels of anti-AAV5ch NAB, before re-administrating them with the same dose of AAV1-hFIX. We demonstrate the feasibility of secondary gene delivery with an AAV1 serotype in the presence of high titer of anti-AAV5ch NAB as activity of hSEAP delivered by AAV5ch and expression of hFIX delivered by AAV1 were observed.
Cross-neutralization between AAV5ch and AAV1 was observed in vitro in one out of eight animals initially injected with AAV5ch. However, for this animal, seroconversion occurred over time before any administration of AAV5ch. Considering that the liver transduction efficacy of AAV5ch vector in this animal was similar to the other NHPs (Figures 3D and 3E), those results point to uncharacterized serum elements that might interfere with in vitro antibody-based assays. Our results support the hypothesis that the interaction of AAV vector with certain serum proteins can impact AAV transduction efficiency, by binding to the AAV surface and influencing AAV transduction.26
From a safety point of view, the sequential administration of AAV5ch and AAV1 was well tolerated. All the parameters such as weight gain, temperature, activity, skin and mucosa color, serum biochemistry, and hematological analysis were in the normal range for all four animals that had been cross-administered with AAV5ch-hSEAP followed by AAV1-hFIX. Only a few notable observations were reported during the follow-up period. In one out of four animals injected with AAV5ch-hFIX (NHP 9) a transient elevation of CPK was observed, which was considered unrelated to the AAV vector itself, because it was an isolated event. Additionally, a transient elevation of aspartate aminotransferase (AST) concomitant with a non-significant ALT elevation was observed after the second AAV injection in the two NHPs that received the same AAV5ch serotype twice (NHPs 14 and 15). Although ketamine anesthesia, which was used in our study, has been reported to elevate the activity of AST in serum,24, 27 it cannot be excluded that the repeated AAV5ch injection might have caused the AST elevation. Furthermore, no cellular immune responses were observed against AAV1 and AAV5ch capsid proteins, and no elevation of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-2, and IL-4 at 24 or 72 hr after injections of AAVs was observed in any of the animals. Histology and immunohistochemistry analysis of the liver, spleen, and lymph nodes did not reveal significant anatomical alteration or pathological abnormalities.
All NHPs cross-administered with AAV5ch-hSEAP followed by AAV1-hFIX showed hSEAP and hFIX protein expression in plasma through 8 weeks of the experiment. Interestingly, in a long-term follow-up, a decrease of transgene activity or presence in plasma was observed in 5 out of the 12 NHPs in the study, associated with specific immune responses directed against hSEAP or hFIX (Figure 6).
Importantly, in the monkeys that experienced decrease or loss of hSEAP or hFIX activity or presence in the plasma, there was no loss of DNA or mRNA of hSEAP or hFIX in the liver tissue at sacrifice. The levels of DNA and mRNA of hSEAP and hFIX in the liver of all animals were in the same range, indicating that the disappearance of hSEAP and hFIX from the circulation was not due to immune-mediated destruction of transduced hepatocytes. Remarkably, and consistent with a previous report showing activity of human alpha 1-antitrypsin (hAAT) promoter in splenic cells,28 four out of five of the monkeys experiencing a decrease or loss of transgene expression in the plasma presented significant levels of mRNA of the same transgene in the spleen. Considering that splenic tissue contains a large number of antigen-presenting cells responsible for the initiation of immune responses,28, 29 this observation suggests a relationship between the presence of transgene expression in the spleen and the occurrence of immune response against the transgene products.
The decrease or loss of transgene proteins in plasma of NHPs associated with an immune response against the transgene protein was previously reported by other groups.22, 25, 30, 31, 32, 33 Together, these findings raise the question whether NHPs are an appropriate animal model for long-term pre-clinical studies when a human protein is expressed, because the transgene protein levels might be at times underestimated because of the transgene-directed immune response. However, the immune responses against the transgene proteins do not occur systematically in all of the animals, and the generation of immune responses against transgene products has been suggested in clinical trials.34, 35 Therefore, the immune mechanisms occurring in NHPs might be relevant as a model to develop strategies to prevent, avoid, or abolish such immune responses.
Interestingly, in cynomolgus macaques, whereas vector DNA presence was found to be equivalent in the different parts of the liver tissue, the expression pattern of transgene mRNA was found to be diverse. This observation might be related to the transcriptional capabilities and promoter activity profile in different cell types that are being transduced. The underlying cellular mechanisms for such a phenomenon require further investigation.
In summary, even though our study reveals some limitations of NHP use as a model for a clinical translation, we demonstrate that sequential AAV delivery of serotypes AAV5ch and AAV1 is a safe and successful method in both mice and NHPs for re-administration of gene delivery when targeting the liver tissue. This approach could be an option for patients who have pre-existing immunity toward AAV5ch or who have high titers of anti-AAV5 NAB after initial AAV5ch treatment but need to be re-administered with the AAV therapy in case of decrease or loss of transgene activity.
Materials and Methods
Ethics Statement
Mouse experiments were approved by the local animal welfare committee (University of Amsterdam). The NHP study was performed following guidelines from the institutional ethical commission. The experimental design employing NHPs was approved by the Ethical Committee for Animal Testing of the University of Navarra and by the Department of Health at the Government of Navarra (CEEA 092/13).
AAV Production
The AAV vector batches (AAV5ch-hSEAP, AAV5ch-hFIX, AAV1-EGFP, and AAV1-hFIX) were produced in insect cells according to a technology adapted from R.M. Kotin and colleagues.36 All of the transgenes were under the control of liver-specific promoters. hAAT promoter was used in the human SEAP construct, whereas human FIX and EGFP were expressed from the synthetic liver promoter 1 (LP1), which consists of liver-specific elements from the human apolipoprotein E/C-I gene locus control region and the hAAT promoter. AAV5 vectors used in the above described studies are hybrid vectors and they contain inverted terminal repeats (ITRs) and VP1 derived from AAV2, whereas VP2 and VP3 come from AAV5 vector. The AAV5ch vectors used in the above described studies are chimeric AAV5 in which the serotype 5 VP1-unique portion was replaced with the equivalent one of AAV serotype 2.16 All AAV vector batches were purified with an AVB Sepharose column using the ÄKTA explorer system (GE Healthcare). After purification, the concentration of AAV vector gc (gc/mL) was determined by TaqMan qPCR amplification.
Animal Experiments
Male C57BL/6 mice (8–10 weeks) were obtained from Harlan and maintained in specific pathogen-free conditions at the animal facility. In the first experiment, mice (n = 6/group) were injected i.v. with AAV5ch-hSEAP or PBS at week 0. The second i.v. administration with AAV1-hFIX, AAV1-EGFP, or PBS was performed at week 3 (Figure 1). In the second experiment, mice (n = 6/group) were injected i.v. with AAV5ch-hSEAP or PBS at week 0. The second i.v. administration with AAV1-hFIX, AAV5ch-hFIX, or PBS was performed at week 3 (Figure 2). The dose of all of the AAV batches that were injected was 1.46 × 1013 gc/kg, and the injection volume was 10 μL/g. In both experiments, blood was collected weekly by submandibular vein puncture into tubes containing sodium citrate. Plasma was isolated after centrifugation at 2,500 × g for 20 min at 4°C and frozen at −80°C until further analysis. All mice were sacrificed after 7 weeks. Liver tissues were collected, processed, and snap frozen in liquid nitrogen with or without pre-fixation in picric acid and stored at −80°C until further analysis.
Female Macaca fascicularis (cynomolgus monkeys) at age 3–5 years were obtained from R.C. Hartelust. Monkeys were injected with PBS or AAV5ch-hSEAP at day 0 and with PBS, AAV1-hFIX, or AAV5ch-hFIX at day 30. The dose of all of the AAV batches that were injected was 3 × 1013 gc/kg. For the injection procedure, animals were anesthetized by intramuscular injection of 10 mg/kg ketamine (Imalgene 100 mg/mL; Merial). When animals showed any signal of waking up from anesthesia, they received an extra half-dose. Vector administration was performed by an i.v. bolus injection of viral suspension in the saphenous vein. Plasma and whole blood were collected at day 0 (basal, previous to the first administration), 24 hr, 72 hr, day 7, and then every week during the first month after vector injection. After the second administration (day 30), the samples were collected at days 31, 33, and 37 and every week thereafter for 1 month after vector injection. From the third month and until the sacrifice, the samples were collected twice a month. The heparinized blood samples were collected in tubes (brown-top BD Vacutainer SST II Advance). The samples were mixed by inversion and left in vertical position at least 1 hr at room temperature. The plasma was collected and stored at −20°C freezer. Blood cells were purified for ELISPOT analysis once per month. The NHPs were sacrificed 364 days after administration by i.v. injection of T61 (Intervet) in the saphenous vein. Tissue and organ collection at sacrifice included eight different liver regions (I–VIII), cerebrum, cerebellum, salivary glands, spinal cord, thymus, spleen, inguinal nodes, mesenteric nodes, ovaries, uterus, oviducts, uterine cervix, vagina, lung, heart, skeletal muscle, kidney, adrenal, pancreas, stomach, small intestine, large intestine, and application site. Heart, spleen, liver, adrenals, kidney, and ovaries were weighed before processing.
Assessment of Transgene Expression
Human FIX expression was measured in murine plasma using the FIX ELISA kit (VisuaLize FIX Antigen Kit; Affinity Biologicals). Human FIX expression was measured in monkey plasma by a sandwich ELISA developed in-house. In brief, plasma samples were 100-fold diluted and loaded for capture into ELISA wells coated with the hFIX-specific monoclonal antibody AHIX-5041 (Hematologic Technologies). Detection was performed using a peroxidase FIX-specific mouse monoclonal antibody followed by colorimetric reaction. To provide a calibration curve, we analyzed a dilution series of calibrated human reference plasma in parallel to the samples. Results were reported as the percentage of hFIX relative to normal human levels, i.e., with 100% corresponding to the normal human population average. hSEAP activity was measured in mouse and monkey plasma with the use of chemiluminescent “HSEAP Reporter Gene Assay” (Roche). GFP expression was assessed by post mortem fluorescent microscopy of mouse liver tissue sections. Mouse livers were fixed in picric acid upon harvesting at sacrifice.
Assessment of Anti-AAV5ch and Anti-AAV1 Antibody Levels
Levels of anti-AAV5ch and anti-AAV1 antibodies in mouse plasma were measured by an anti-AAV5ch and anti-AAV1-specific ELISA. In short, 96-well flat-bottom plates (MaxiSorp; Thermo Scientific) were coated with AAV5ch or AAV1 capsid incubated with mouse plasma, and specific antibodies were detected with 1:1,000 rabbit anti-mouse Ig-horseradish peroxidase (HRP; DAKO). Levels of anti-AAV5ch and anti-AAV1 antibodies in NHP plasma were measured by specific ELISA as described in mice, but to detect monkey antibodies protein A/HRP (Amersham).
NAB Assay against AAV5ch and AAV1 Capsid
HEK293T cells were seeded in black 96-well culture plates (Costar) coated with 0.25% poly-L-lysine (Sigma-Aldrich) at a density of 0.5 × 105 cells/well in 100 μL of DMEM with 10% fetal bovine serum (FBS) and antibiotics (1% penicillin/streptomycin; GIBCO). Cells were incubated overnight at 37°C. Medium was then removed and the following mix was added: AAV1-cytomegalovirus promoter- luciferase transgene (CMV-luc) (for anti-AAV1) or AAV5ch-CMV-luc (for anti-AAV5) incubated with heat-inactivated plasma sample at a total volume of 100 μL of DMEM supplemented with antibiotics. The mix was kept for 1 hr at 4°C before being added to cells. Medium of the HEK293T cells was removed by aspiration and the mix added to cells and incubated for 20 hr at 37°C. Serial dilutions of each plasma sample were prepared in triplicate. As a positive control, cells without any plasma addition were analyzed, while as a negative control, cells without any plasma or virus were used. The cells were washed with PBS and lysed by addition of Glo Lysis Buffer (Promega); then the ONE-Glo Luciferase Assay (Promega) protocol was followed and luciferase expression was measured with the use of GloMax Discover (Promega). The anti-AAV NAB titer (half maximal inhibitory concentration [IC50]) was calculated with the use of LabKey Software. Plasma samples were considered to have neutralizing activity if the lowest plasma dilution inhibited vector transduction by at least 50%, as described before.37, 38
ELISPOT
The IFN-γ ELISPOT assay was performed with frozen isolated PBMCs (28, 58, 86, and 100 days after injection) or splenocytes (harvested at sacrifice) plated following the manufacturer’s instructions (BD Biosciences). In short, cells were seeded in duplicate at 2 × 105 per well and incubated at 37°C for 22 hr. The negative control wells were incubated in RPMI medium. As positive control, PBMCs were stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin at a final concentration of 0.05/0.5 μg/mL. Cells were stimulated with AAV1 and AAV5ch capsids and 24-peptide pools including a total of 143 peptides covering the complete AAV5ch capsid sequence. Furthermore, splenocytes were incubated with a pool of peptides described by Hui et al.,39 but from AAV5ch and AAV1 capsid sequences. Spots were counted automatically using a CTL ImmunoSpot Analyzer and processed with CTL ImmunoSpot Software. The number of spots was correlated to the amount of cells that had been activated in the presence of each corresponding stimulus.
Biochemical Analysis
Serum biochemistry was assessed by a Good Laboratory Practices (GLP)-certified laboratory (Toxicology Lab CIFA University of Navarra). The biochemical parameters were analyzed using the autoanalyzer Hitachi 911. The parameters analyzed were creatine phosphokinase (CPK), creatinine (Crea), total protein (TP), urea, alkaline phosphatase (ALP), AST, alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT).
DNA/RNA Liver Biodistribution
The extraction of total DNA was performed using the QIAamp DNA Mini Kit (QIAGEN). Total RNA was extracted by an initial step of mechanical homogenization in TRIzol (Invitrogen). Subsequently, a microgram of total RNA was treated with TURBO DNA-free Kit (Ambion) according to the manufacturer’s protocol to obtain the corresponding cDNA. Vector DNA/cDNA was quantified by means of real-time qPCR, using a specific qPCR for the detection of the transgenes hFIX and hSEAP. Gene-specific primers for the hFIX promoter (sense 5′-CAAGTATGGCATCTACACCAAAGTCT-3′ and antisense 5′-GCAATAGCATCACAAATTTCACAAA-3′) and for hSEAP (sense 5′-CCTGTTTGCTCCTCCGAT-3′ and antisense 5′-GGGTTCTCCTCCTCAACT-3′) were used for each reaction. Amplification was performed using iQ SYBR Green Supermix in an iQ5 real-time PCR detection system (Bio-Rad). The amplification protocol started with 10 min at 95°C, followed by 40 cycles of amplification (95°C for 15 s; 60°C for 60 s; 72°C for 25 s). A melting curve was generated by raising the incubation temperature from 65°C to 95°C to confirm amplification specificity. Results are expressed as gene copies per microgram total DNA or total RNA.
Author Contributions
A.M., study design; mouse experiment execution, analytics, and analysis; NHP study plasma sample analysis; manuscript writing; D.S., NHP study execution, NHP biodistribution analysis, NHP ELISPOT assay on PBMCs, manuscript review, and material and method contribution; N.Z., NHP study analytics; E.R.-G., NHP study analytics; G.G.-A., NHP study supervision, manuscript review and editing; H.P., study design review, manuscript review and editing; V.F., design of studies and supervision, manuscript writing.
Acknowledgments
The work described in the manuscript was funded by uniQure. The authors would like to thank Karin Kwikkers for help performing antibody assays and Richard van Logtenstein for assistance with mouse study execution.
Footnotes
Supplemental Information includes four figures and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.05.003.
Supplemental Information
References
- 1.Gaudet D., Méthot J., Déry S., Brisson D., Essiembre C., Tremblay G., Tremblay K., de Wal J., Twisk J., van den Bulk N. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20:361–369. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimm D., Zhou S., Nakai H., Thomas C.E., Storm T.A., Fuess S., Matsushita T., Allen J., Surosky R., Lochrie M. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–2419. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- 3.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halbert C.L., Rutledge E.A., Allen J.M., Russell D.W., Miller A.D. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halbert C.L., Standaert T.A., Aitken M.L., Alexander I.E., Russell D.W., Miller A.D. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J. Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivière C., Danos O., Douar A.M. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- 7.Xiao W., Chirmule N., Berta S.C., McCullough B., Gao G., Wilson J.M. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs F., Gordts S.C., Muthuramu I., De Geest B. The liver as a target organ for gene therapy: state of the art, challenges, and future perspectives. Pharmaceuticals (Basel) 2012;5:1372–1392. doi: 10.3390/ph5121372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kok C.Y., Cunningham S.C., Carpenter K.H., Dane A.P., Siew S.M., Logan G.J., Kuchel P.W., Alexander I.E. Adeno-associated virus-mediated rescue of neonatal lethality in argininosuccinate synthetase-deficient mice. Mol. Ther. 2013;21:1823–1831. doi: 10.1038/mt.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 11.Gao G.P., Alvira M.R., Wang L., Calcedo R., Johnston J., Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntosh J.H., Cochrane M., Cobbold S., Waldmann H., Nathwani S.A., Davidoff A.M., Nathwani A.C. Successful attenuation of humoral immunity to viral capsid and transgenic protein following AAV-mediated gene transfer with a non-depleting CD4 antibody and cyclosporine. Gene Ther. 2012;19:78–85. doi: 10.1038/gt.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mingozzi F., Chen Y., Edmonson S.C., Zhou S., Thurlings R.M., Tak P.P., High K.A., Vervoordeldonk M.J. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther. 2013;20:417–424. doi: 10.1038/gt.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mingozzi F., Chen Y., Murphy S.L., Edmonson S.C., Tai A., Price S.D., Metzger M.E., Zhou S., Wright J.F., Donahue R.E. Pharmacological modulation of humoral immunity in a nonhuman primate model of AAV gene transfer for hemophilia B. Mol. Ther. 2012;20:1410–1416. doi: 10.1038/mt.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unzu C., Hervás-Stubbs S., Sampedro A., Mauleón I., Mancheño U., Alfaro C., de Salamanca R.E., Benito A., Beattie S.G., Petry H. Transient and intensive pharmacological immunosuppression fails to improve AAV-based liver gene transfer in non-human primates. J. Transl. Med. 2012;10:122. doi: 10.1186/1479-5876-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urabe M., Nakakura T., Xin K.Q., Obara Y., Mizukami H., Kume A., Kotin R.M., Ozawa K. Scalable generation of high-titer recombinant adeno-associated virus type 5 in insect cells. J. Virol. 2006;80:1874–1885. doi: 10.1128/JVI.80.4.1874-1885.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Avola D., López-Franco E., Sangro B., Pañeda A., Grossios N., Gil-Farina I., Benito A., Twisk J., Paz M., Ruiz J. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J. Hepatol. 2016;65:776–783. doi: 10.1016/j.jhep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Mendell J.R., Sahenk Z., Malik V., Gomez A.M., Flanigan K.M., Lowes L.P., Alfano L.N., Berry K., Meadows E., Lewis S. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol. Ther. 2015;23:192–201. doi: 10.1038/mt.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pañeda A., Vanrell L., Mauleon I., Crettaz J.S., Berraondo P., Timmermans E.J., Beattie S.G., Twisk J., van Deventer S., Prieto J. Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum. Gene Ther. 2009;20:908–917. doi: 10.1089/hum.2009.031. [DOI] [PubMed] [Google Scholar]
- 20.Tseng Y.S., Gurda B.L., Chipman P., McKenna R., Afione S., Chiorini J.A., Muzyczka N., Olson N.H., Baker T.S., Kleinschmidt J., Agbandje-McKenna M. Adeno-associated virus serotype 1 (AAV1)- and AAV5-antibody complex structures reveal evolutionary commonalities in parvovirus antigenic reactivity. J. Virol. 2015;89:1794–1808. doi: 10.1128/JVI.02710-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harbison C.E., Weichert W.S., Gurda B.L., Chiorini J.A., Agbandje-McKenna M., Parrish C.R. Examining the cross-reactivity and neutralization mechanisms of a panel of mAbs against adeno-associated virus serotypes 1 and 5. J. Gen. Virol. 2012;93:347–355. doi: 10.1099/vir.0.035113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathwani A.C., Gray J.T., McIntosh J., Ng C.Y., Zhou J., Spence Y., Cochrane M., Gray E., Tuddenham E.G., Davidoff A.M. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H., Couto L.B., Patarroyo-White S., Liu T., Nagy D., Vargas J.A., Zhou S., Scallan C.D., Sommer J., Vijay S. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim C.Y., Lee H.S., Han S.C., Heo J.D., Kwon M.S., Ha C.S., Han S.S. Hematological and serum biochemical values in cynomolgus monkeys anesthetized with ketamine hydrochloride. J. Med. Primatol. 2005;34:96–100. doi: 10.1111/j.1600-0684.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- 25.Mingozzi F., Hasbrouck N.C., Basner-Tschakarjan E., Edmonson S.A., Hui D.J., Sabatino D.E., Zhou S., Wright J.F., Jiang H., Pierce G.F. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M., Crosby A., Hastie E., Samulski J.J., McPhee S., Joshua G., Samulski R.J., Li C. Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther. 2015;22:984–992. doi: 10.1038/gt.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pañeda A., Lopez-Franco E., Kaeppel C., Unzu C., Gil-Royo A.G., D’Avola D., Beattie S.G., Olagüe C., Ferrero R., Sampedro A. Safety and liver transduction efficacy of rAAV5-cohPBGD in nonhuman primates: a potential therapy for acute intermittent porphyria. Hum. Gene Ther. 2013;24:1007–1017. doi: 10.1089/hum.2013.166. [DOI] [PubMed] [Google Scholar]
- 28.Park F., Ohashi K., Kay M.A. The effect of age on hepatic gene transfer with self-inactivating lentiviral vectors in vivo. Mol. Ther. 2003;8:314–323. doi: 10.1016/s1525-0016(03)00169-2. [DOI] [PubMed] [Google Scholar]
- 29.Sack B.K., Herzog R.W. Evading the immune response upon in vivo gene therapy with viral vectors. Curr. Opin. Mol. Ther. 2009;11:493–503. [PMC free article] [PubMed] [Google Scholar]
- 30.Davidoff A.M., Gray J.T., Ng C.Y., Zhang Y., Zhou J., Spence Y., Bakar Y., Nathwani A.C. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol. Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Nathwani A.C., Davidoff A.M., Hanawa H., Hu Y., Hoffer F.A., Nikanorov A., Slaughter C., Ng C.Y., Zhou J., Lozier J.N. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- 32.Nathwani A.C., Gray J.T., Ng C.Y., Zhou J., Spence Y., Waddington S.N., Tuddenham E.G., Kemball-Cook G., McIntosh J., Boon-Spijker M. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathwani A.C., Rosales C., McIntosh J., Rastegarlari G., Nathwani D., Raj D., Nawathe S., Waddington S.N., Bronson R., Jackson S. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol. Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendell J.R., Campbell K., Rodino-Klapac L., Sahenk Z., Shilling C., Lewis S., Bowles D., Gray S., Li C., Galloway G. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flotte T.R., Trapnell B.C., Humphries M., Carey B., Calcedo R., Rouhani F., Campbell-Thompson M., Yachnis A.T., Sandhaus R.A., McElvaney N.G. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urabe M., Ding C., Kotin R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 37.Nelson E.K., Piehler B., Eckels J., Rauch A., Bellew M., Hussey P., Ramsay S., Nathe C., Lum K., Krouse K. LabKey Server: an open source platform for scientific data integration, analysis and collaboration. BMC Bioinformatics. 2011;12:71. doi: 10.1186/1471-2105-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piehler B., Nelson E.K., Eckels J., Ramsay S., Lum K., Wood B., Greene K.M., Gao H., Seaman M.S., Montefiori D.C., Igra M. LabKey Server NAb: a tool for analyzing, visualizing and sharing results from neutralizing antibody assays. BMC Immunol. 2011;12:33. doi: 10.1186/1471-2172-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hui D.J., Edmonson S.C., Podsakoff G.M., Pien G.C., Ivanciu L., Camire R.M., Ertl H., Mingozzi F., High K.A., Basner-Tschakarjan E. AAV capsid CD8+ T-cell epitopes are highly conserved across AAV serotypes. Mol. Ther. Methods Clin. Dev. 2015;2:15029. doi: 10.1038/mtm.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.