Abstract
The underrepresentation of ethnically diverse populations in cancer clinical trials results in the inequitable distribution of the risks and benefits of this research. Using a case study approach, we apply a conceptual framework of factors associated with the participation of diverse population groups in cancer clinical trials developed by Dr. Jean Ford and colleagues to increase understanding of the specific strategies, and barriers and promoters addressed by these strategies, that resulted in marked success in accrual of racially and ethnically diverse populations in cancer clinical research. Results indicate that the studies presented were able to successfully engage minority participants due to the creation and implementation of multi-level, multifaceted strategies that included: culturally and linguistically appropriate outreach, education, and research studies that were accessible in local communities; infrastructure to support engagement of key stakeholders, clinicians, and organizations serving minority communities; testimonials by ethnically diverse cancer survivors; availability of medical interpretation services; and providing infrastructure that facilitated the engagement in clinical research of clinicians who care for minority patient populations. These strategic efforts were effective in addressing limited awareness of trials, lack of opportunities to participate, and acceptance of engagement in cancer clinical trials. Careful attention to the context and population characteristics in which cancer clinical trials are conducted will be necessary to address disparities in research participation and cancer outcomes. These studies illustrate that progress on minority accrual into clinical research requires intentional efforts to overcome barriers at all three stages of the accrual process: awareness, opportunity and acceptance of participation.
Keywords: recruitment, diverse populations, cancer clinical trials, conceptual framework
INTRODUCTION
Cancer is a major public health problem, with racial and ethnic minorities bearing a disproportionate burden of cancer morbidity and mortality relative to other groups. Despite their disproportionate burden of cancer, racial and ethnic minorities are less likely to participate in cancer clinical trials (Ford JG, 2008; Ford et al., 2012; Ford ME, 2013; Langford A, 2010; Seifer, Michaels, & Collins, 2010). The underrepresentation of ethnically diverse populations in cancer clinical trials results in the inequitable distribution of the risks and benefits of this research (Hartz et al., 2011). The National Institute of Health (NIH) Revitalization Act stipulates that investigators make every effort to achieve participation rates among racial and ethnic minorities that mirror their representation in the U.S. population or in the geographic regions where the trials take place (Pinsky et al., 2008). However, in 2013, only 2% of National Cancer Institute (NCI) sponsored clinical trials focused on racial/ethnic minorities, a proportion substantially lower than their percentage of the U.S. population (36.3%) (Chen, Lara, Dang, Paterniti & Kelly, 2014).
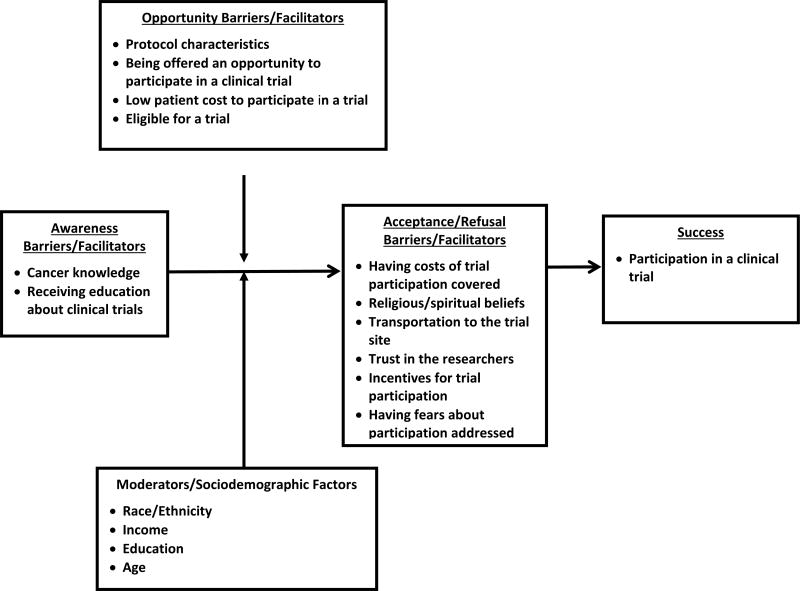
Using a conceptual framework of factors that can affect the degree of success in recruiting and retaining ethnically diverse populations in research can facilitate and inform the development of strategies to address disparities in clinical research participation. Jean Ford and colleagues (Ford et al., 2008) developed such a framework. Their model describes two categories of factors that can affect accrual during distinct and consecutive phases of the recruitment process: 1) awareness barriers/promoters and 2) acceptance or refusal barriers/promoters. In addition, the model specifies two general types of moderators that can affect the utility of various recruitment strategies used throughout the phases (Figure 1): sociodemographic factors and opportunity barriers/promoters (Ford et al., 2008). In this paper, we utilize the framework as a valuable heuristic for understanding the types of factors that affected recruitment in three studies that included ethnically diverse populations across different population groups and contexts. Therefore, the elements of the Jean Ford et al. (Ford et al., 2008) framework are presented from the perspective of clinical trial participation barriers.
Figure 1.
Conceptual Framework
METHODS
We employ a case study approach to illustrate how the Ford framework can be used to increase our understanding of factors that impede or promote research participation among diverse populations and develop recruitment plans that take into account these factors. First, we describe briefly each stage of the Ford model and its implications for equitable enrollment of racial and ethnic minorities in clinical research. Then, we present three case studies in which we apply the model to understand the effectiveness of specific strategies, to identify potential challenges and successful strategies for engaging ethnically diverse populations in cancer clinical trials based on our experiences and the framework.
Clinical Trial Participation Barriers Identified in the Conceptual Framework
In the Jean Ford et al. (Ford et al., 2008) conceptual framework, awareness barriers refer factors contributing to lack of awareness that trials are open and accruing. Opportunity barriers refer to lack of a personal invitation to participate in a trial, as well as lack of trial access. Acceptance barriers refer to a lack of strategies to address factors that could lead individuals to refuse clinical trial participation, even when offered. These factors include perceptions of the health-care settings where trials are conducted, cancer risk perceptions, and health beliefs.
Awareness Barriers
Based on the model, awareness barriers can include limited information about cancer and lack of awareness or knowledge of clinical trials (Green et al., 2015; Haynes-Maslow et al., 2014; Leiter, Diefenbach, Doucette, Oh, & Galsky, 2015). These factors could be major contributors to lower participation rates in cancer clinical trials among racially and ethnically diverse populations. Lack of awareness of clinical trials is common among diverse populations with low educational attainment, including African Americans and Asians, and is associated with negative perceptions of trial participation (Advani et al., 2003; Ford et al., 2013; Lara et al., 2005). Awareness barriers to clinical trial participation include lack of culturally appropriate information regarding the importance of research on cancer treatments or behavioral interventions and limited cancer knowledge.
Opportunity Barriers
In the Ford conceptual framework (Ford et al., 2008), a major factor influencing study participation is having an opportunity to participate in a cancer clinical trial. Thus, providing ethnic minorities with access to cancer clinical trials is a promising approach to address disparities in participation. Factors that promote opportunity to participate in cancer clinical trials include physicians’ knowledge and presentation of opportunities for participating in clinical trials to patients. However, studies have shown that physicians may be unaware that their ethnic minority patients may require additional information related to the importance of participation in clinical trials (Ford et al., 2013; Langford et al., 2010). Whether physicians inform their patients of an open clinical trial depends on several factors, including their knowledge of the trial, beliefs about the trial, and beliefs about whether their patients can successfully enroll and complete the trial (Fouad, Reed, & Martin, 2011).
In addition to physician referrals, the setting in which a clinical trial takes place affects the opportunity to participate. For example, patients’ geographic distance to the clinical trial location may serve as a serious impediment to participation, especially among low income and ethnically diverse patients who tend to be more likely to experience transportation issues (Brown, Fouad, Basen-Engquist, & Tortolero-Luna, 2000; Wallace & Bartlett, 2013; Williams et al., 2011). Distance from a physician's practice to the nearest clinical trial site was inversely associated with physician referral to and patient enrollment in a trial (Kaplan et al., 2013) suggesting that geographic distance is an important determinant of clinical trial participation.
Acceptance Barriers
Acceptance barriers are addressed when patients are educated about cancer clinical trials and given a meaningful opportunity to participate. Under these conditions, it is anticipated that disparities in cancer clinical trial participation rates could be greatly diminished. In fact, a meta-analysis of 20 health research studies reporting on the enrollment decisions of over 70,000 individuals found that when access to studies was provided, participation rates of ethnic minorities and whites did not differ significantly, and in clinical intervention studies, rates were actually higher for Latinos than whites or African Americans (Wendler et al., 2006). Perceptions of the health care settings where the trial takes place can influence the decision to accept an invitation to participate (Ford et al., 2013; Fouad et al., 2011). For example, if a health care setting historically provided a segregated system of health care prior to integration, lingering negative perceptions of it may exist in local communities of color. Also, if a health care system is viewed by community members as not being responsive to the needs or concerns of local diverse communities, this perception could negatively affect community members’ views of the clinical staff and/or investigators in the system who conducts clinical trials. Other factors include the cultural and linguistic competence of trials. A study of multi-level factors associated with participation among ethnically diverse women in 230 breast cancer clinical trials found that the provision of study information in English-only and the lack of community outreach were common and significant barriers to the enrollment of ethnic minorities (Livaudais-Toman, Burke, Napoles, & Kaplan, 2014).
Other factors that influence acceptance of participation in cancer clinical trials include risk perceptions and health beliefs. According to the Health Belief Model, increased perceived risk of a potential diagnosis (or perception of recurrence risk) is associated with behavior change to reduce that risk (Glanz, Lewis, & Rimer, 1997; Janz & Becker, 1984; Lipkus, Iden, Terrenoire, & Feaganes, 1999). Thus, providing ethnic minorities with information on their higher risk of poorer quality cancer care and outcomes relative to whites could serve to promote acceptance of participation in cancer clinical trials. Alternatively, however, this information could also play a role in reducing trust in the researchers conducting the trials.
Moderators of Participation in Clinical Trials: Sociodemographic Characteristics
Sociodemographic factors such as age, income, and education also play a role; individuals who participate in cancer research are more likely to be better educated and to have higher income levels than non-participants (Bernard-Davila et al., 2015; Bussey-Jones et al., 2010; Kehl et al., 2014; Unger, Gralow, Albain, Ramsey, & Hershman, 2016). Furthermore, individuals with these characteristics have a higher likelihood of being offered opportunities to participate in cancer research compared with individuals who are less well educated and who have lower income levels (Bernard-Davila et al., 2015; Kehl et al., 2014; Unger et al., 2016). Hence, individuals of a higher socioeconomic status may be more aware of cancer research, have more opportunities to participate, and face fewer economic and logistical barriers to participation than less educated and lower income individuals.
RESULTS
Case Studies of the Application of the Conceptual Framework
Next, we present case studies in which we applied the conceptual framework to understand the factors that influenced successful enrollment of ethnic minorities in three clinical trials. For each study, we present the study design and accrual results, application of the conceptual framework, and a summary of major highlights.
Case Study 1: The Selenium and Vitamin E Cancer Prevention Trial (SELECT)
Study Design and Results
SELECT was a phase III study assessing the impact of selenium and vitamin E, alone and in combination, on the incidence of prostate cancer. SELECT was designed as a double blind, placebo-controlled, two-by-two factorial trial with planned accrual of 32,400 men. The accrual period was designed to last 5 years with a follow-up period of 7 – 12 years depending on when the participant entered the trial. Men were recruited from over 400 SELECT study sites in the United States, Canada, and Puerto Rico. The minority recruitment goals for SELECT were 20% African Americans, 3% Latinos, and 1% Asians. Black men were over-sampled because of their higher prostate cancer incidence and mortality rates.
Fifteen percent of participants accrued to the SELECT Trial were African American (versus the targeted goal of 20%), representing the highest percentage of blacks ever recruited to a cancer prevention trial. Because of the successful overall recruitment strategies employed, SELECT closed accrual more than two years early, with a total of 35,333 men recruited, exceeding the targeted goal of 32,400. Latinos comprised 6% of the sample versus a goal of 3%, and Asian American accrual was on target at 1%. Minority recruitment to SELECT is described in detail by (Cook et al., 2005).
Application of the Conceptual Framework
Awareness Barriers/Promoters
Patient level promoters included the development of African American sensitive videos and patient education materials, and targeted community outreach activities. For example, “For Men Only” Retreats, men’s health education sessions across the country that included clinical trial and SELECT information conducted in collaboration with the National Black leadership Initiative on Cancer III (NBLIC III) were important in spreading the work about the trial. The “Wellness on Wheels” initiative was a similar education program conducted on trains that were frequented by African American men. Two faith-based programs, “Taking it to the Top” and “SELECT Sunday,” also had local SELECT site participation. SELECT sites partnered with churches and mosques where pastors delivered the prostate cancer and SELECT message from the pulpit. Prostate cancer survivors were often present and served as advocates for the SELECT trial, giving testimonials about the study. We also presented SELECT at national and local fraternity meetings that targeted African American men. Extensive outreach was negotiated for no cost radio media tours, a television program, and SELECT information on various websites to enhance awareness of the trial. Provider and system level promoters include presenting SELECT at national physician meetings, including the National Medical Association, Southwest Oncology Group (SWOG), and local physician meetings.
Opportunity Barriers/Promoters
Patient level opportunity promoters included allowing men to participate with co-morbid conditions, as long as they were controlled, and reducing the minimum age criterion for African American men from 55 to 50 due to the equivalent risk of prostate cancer to white men in that age range. Relaxing eligibility criteria related to comorbidity and age are critical for minority populations because they are more likely to have other chronic illnesses and may be more susceptible at earlier ages (Mathioudakis et al., 2016; Prasad, Helder, Brown, & Schaff, 2016; Yoo, De, Wilkins, Smith, & Blumenthal, 2016). We also provided small site grants each year to assist with retention items, parking and refreshments. Many of the required SELECT pre-enrollment participant educational sessions were conducted after working hours.
At the provider and system levels, we encouraged physicians to enroll men in the trial with controlled co-morbid conditions. We provided accrual updates by sites to physicians and staff at each semi-annual SWOG meeting in hopes of increasing accrual. We conducted three separate one-day workshops that included discussing recruitment issues/needs, developing mentoring relationships, and sharing recruitment strategies. One workshop included panel discussions with local community leaders and SELECT investigators. Another workshop involved pairing underperforming sites with sites performing well serving as mentors. Workshop proceedings were disseminated to all SELECT staff at the semi-annual SWOG/SELECT meetings.
Acceptance Barriers/Promoters
SELECT promoted acceptance at the patient level by collaborating with trusted national media personalities and obtaining endorsements by members of well-known community organizations, pastors, and local prostate cancer survivors for many local outreach programs. To promote acceptance at the provider/system levels, SELECT formed the SELECT Minority and Medically Underserved Subcommittee (MMUS) that partnered with the National Black Leadership Initiative on Cancer (NBLIC III) and the American Cancer Society. MMUS had a diverse membership with longstanding relationships in minority communities and minority recruitment expertise. MMUS also monitored overall and African American recruitment with monthly accrual reports listed by site. Further analyses, recommendations, and occasional mentoring were provided to specific sites based on these reports. SELECT awarded Minority Recruitment Enhancement Grants (MREGs) were awarded via a competitive review process to sites that demonstrated the ability to recruit substantial numbers of minorities (Cook et al., 2010). Fifteen sites were awarded grants for one or two years. Sites were required to provide monthly reports detailing implemented recruitment strategies with a simple success rating per strategy. MREGs often supported additional recruitment and adherence materials and local site staff.
Summary
Most of the SELECT recruitment initiatives addressed minority accrual barriers at all three phases of the recruitment process: awareness, opportunity, and acceptance. Strategies that worked well included adjusting eligibility criteria, radio media tours, hiring additional staff, mass mailings, and supporting participants with parking reimbursements, transportation, etc. What rarely or didn’t work included the television program (SELECT was not mentioned), health fairs, churches, barbershops, laundromats, and networking with clinics and community leaders.
Case Study 2: Statewide Cancer Clinical Trial Educational Intervention in South Carolina
Study Design and Results
The “Improving Perceptions of Cancer Clinical Trials” Study was designed to address the hypothesis that increasing knowledge about cancer clinical trials leads to more positive perceptions of trials among study participants. Therefore, the study intervention, a cancer clinical trial education program, was conducted with predominantly African American communities in South Carolina. These communities experience large cancer disparities, limited access to quality cancer care, and constitute a large population of African Americans who could potentially gain much from participating in cancer clinical trials. The clinical trial education intervention was part of a larger 4-hour evidence-based cancer education program that included a 3-hour general cancer information component, a 30-minute prostate cancer knowledge component, and a 30-minute cancer clinical trials component. The intervention was designed to be highly interactive and “hands on” rather than merely didactic. Participants engaged in role playing and small group activities as they practiced sharing the information that they learned with others.
The intervention took place at ten sites in 11 counties representing several different geographic regions of South Carolina. These eleven counties (and sites) include Berkeley (Varnertown Indian Community), Georgetown (Georgetown), Charleston (Charleston and Johns Island), Greenville (Greenville), Orangeburg (both Orangeburg sites), Richland (Columbia), Bamberg (Denmark), Florence (Florence), Darlington (Darlington), Hampton (Yemassee), and Williamsburg (Kingstree). The study included a convenience sample of participants residing in counties with large racial disparities in cancer mortality.
A pretest/posttest design was used. The survey instrument assessed general sociodemographic information (e.g., age, race, income). Perceptions of randomized trials were evaluated using the 7-item Attitudes to Randomized Trial Questionnaire (ARTQ) (Fallowfield et al., 1998). The instrument was interviewer-administered and each question was read aloud to the participants to address health literacy issues. Explanations were provided when participants indicated that statements were unclear to them. The ARTQ includes three domains: positive or negative perceptions of medical research in general, willingness to personally participate in research, and willingness to personally participate in research involving randomization. Previous validation in mainly European populations showed that ARTQ scores predicted trial enrollment with 80.4% accuracy (Fallowfield et al., 1998).
Of 315 study participants, most were African American (81.4%) and female (84.8%). Slightly more than 60% of participants had at least a college degree. With the exception of one of seven items on the ARTQ, the majority of participants who had less favorable perceptions of cancer clinical trials before the intervention changed their perceptions to more positive ones from pretest to posttest (p < .01). The exception, Item 3, read “usually the only scientific way to compare one treatment with another is for the choice between the two to be made randomly, rather like tossing a coin.” When asked about their responses to this item, participants typically stated that their preference was to be assigned to the intervention group, rather than be randomized.
Application of the Conceptual Framework
Awareness Barriers/Promoters
The intervention focused on awareness promoters and sought to address these in a comprehensive manner. The intervention was designed to promote awareness and ultimately acceptance by helping individuals understand what a cancer clinical trial is, its purpose, and their rights as study participants. All of these factors were addressed in the intervention. It is important to note that given the responses to Item 3 of the ARTQ, additional attention could be paid to the discussion of randomization procedures during future cancer clinical trial education interventions.
Opportunity Barriers/Promoters
Although the study did not directly target the creation of opportunities for engagement in research, participants were armed with the knowledge and skills to request information from clinicians and other sources regarding the availability of clinical trials.
Acceptance Barriers/Promoters
Pre-post intervention results supported that participants’ willingness to personally participate in research improved significantly as a result of the educational program. Overall, participants’ perceptions of cancer clinical trials were more favorable after the intervention, suggesting that such programs can mitigate barriers to their acceptance.
Summary
Study results demonstrate that the intervention positively influenced trial perceptions and willingness to participate in a clinical trial. The intervention was based on the premise that lack of knowledge about trials can lead to negative perceptions of them, which can have a negative impact on trial participation. Recruitment difficulties often arise from misconceptions of what trials are, the randomization process, and participants’ rights in the trial. For example, in the present study, the investigators learned that some participants thought that if they were in a cancer clinical trial, they might be given a placebo rather than the treatment that is being tested. The investigators explained that at minimum, participants would receive the current standard of care if they were assigned to the usual care group, and that under no circumstances would patients with cancer receive a placebo, or no treatment at all. This helped to allay their fears. The intervention evolved from Adult Learning Theory (SD, 2003) and Diffusion of Innovation Theory (EM, 2003) in which trained participants then train others and learning is fostered through trusted social networks. The trained study participants have conducted 104 additional cancer clinical trial education sessions in their communities, reaching 3,292 people.
To enhance awareness education in future cancer clinical trials, the ARTQ could be administered to potential trial participants to identify those who could benefit from participating in the intervention. Alternatively, the administration of the ARTQ and the intervention could be incorporated as standard recruitment procedures.
Case Study 3: Strategies for Recruitment of Minority Patients to Clinical Trials in an Academic Cancer Center in a Minority-majority State
Study Design and Results
Effective recruitment of patients to cancer preventative and therapeutic trials in hospitals and clinics that serve large ethnic minority populations has the potential to decrease cancer disparities. This case study reviews strategies employed by a Minority-Serving Academic Cancer Center (MSACC) to increase recruitment of minority patients to clinical trials. The MSACC was funded in 2000 as one of 13 centers in the National Cancer Institute (NCI)-funded Minority-Based Community Clinical Oncology Program (MB-CCOP). MB-CCOPs were academic centers in which a minimum of 40% of new cancer cases occurred in minority populations. The minority-majority state with the highest percentage of Latinos and American Indians in the nation was the catchment area for the MSACC and cancer clinical trials. The findings reported here are based on the analysis of qualitative interviews with research administrators and staff in this MSACC and publically available research documents.
The Partnership increased the number of NCI Cooperative Group trials, as well as investigator initiated and industry trials available in the state. Following two funding cycles of five years each, the organization developed a network of about 100 physicians, which included all practicing community-based oncologists in the major Metropolitan area and most community-based oncologists in the state. In its first year of operation, the Partnership reported enrolling 76 patients in clinical trials. In the first five years, 521 patients were accrued to trials; of these, 319 (61.2%) were ethnic minorities (180 Hispanic, 36 American Indian, and 103 mixed race, other minority, or undeclared). In the following years, they were able to increase accrual drastically and have enrolled nearly 3,000 patients in the first decade of operation. Rates of minority patient recruitment remained strong in the following years.
Application of the Conceptual Framework
Awareness Barriers/Promoters
This Partnership’s mission statement included the provision of educational opportunities to raise awareness of clinical trials. Awareness-related strategies included outreach to the general community, conducted by an outreach person contracted by the non-profit organization Partnership. In addition, two community educators hired by the MSACC targeted Native American and Spanish-speaking communities in the State with information about cancer; however, the amount of attention paid to increasing awareness to cancer clinical trials was limited.
Strategies that were utilized to promote awareness among providers regarding cancer clinical trials consisted of the provision of information about research protocols, research support, and research nurses and staff involved in the administration of cancer clinical trials. Prior to the MSACC and Partnership creation, this type of research support was not available for community physicians who partnered with the MSACC. In interviews, physicians who reported a higher number of patients accrued attributed their engagement to their beliefs in the importance of cancer clinical trials to patients and to science and their training prior to being hired by the MSACC.
Opportunity Barriers/Promoters
The MBACC recognized the need to change research infrastructure in order to remove barriers to patient accrual to clinical trials. The MB-CCOP funding allowed the MSACC to recruit new oncology clinical specialists and to develop a new expanded cancer clinical trial infrastructure at the Academic Center and its affiliate sites. The MSACC leadership proactively identified regulatory and administrative barriers to accrual at the MSACC. Practitioners in non-academic clinical settings in the state were not able to accrue patients to clinical trials citing lack of research staff and knowledge about such trials as barriers. One of the main strategies employed by the MBACC for recruitment of patients to clinical trials included the creation of a non-profit organization whose mission was to create a statewide partnership to increase access to clinical trials. The organization, referred to here as the “Partnership,” streamlined administrative and regulatory hurdles of accrual, including provision of research staff and Institutional Review Board (IRB) support. As a non-profit independent legal entity, the Partnership was able to respond efficiently to changes in the regulatory, administrative, and clinical environments.
Opportunity barriers to participation in clinical trials were significant. Patients who were interested in participating in clinical trials had to travel out of state, and the financial, familial, and emotional resources needed for the travel prohibited most patients from participating, particularly those from marginalized communities. Analysis of the interviews revealed that the MBACC and the Partnership leaders focused predominantly on opportunity-related promoters in their strategies to increase recruitment of minority patients to cancer clinical trials. One of the major promoters to patient accrual cited by MBACC and Partnership leaders included the opportunity for patients to access trials in their own communities. Increasing the number of trials available for patients and physicians was achieved by opening trials throughout the state, providing access at different sites, and increasing the number of physicians conducting cancer clinical trials. In addition, these leaders also felt that they were able to increase the number of available CCTs in the state by streamlining the review process by having one Protocol Review and Monitoring Committee that evaluated quality of proposed trials and a centralized IRB that facilitated approval of protocols for all Partnership’s sites. Strategies to increase opportunities to participate focused at the provider level, assuming that this strategy when applied in a diverse catchment area would increase opportunities for multiethnic patients to enroll.
At the organizational level, medical translation services at the time of recruitment, mostly in Spanish, served to expand opportunities for the participation of ethnically diverse patients.
Acceptance Barriers/Promoters
The MSACC and Partnership relied predominantly on physicians to communicate information and promote acceptance of cancer clinical trials to patients. No communication strategies directly targeted patients with respect to clinical trials accrual. Physicians at the MSACC received support from research staff, including research nurses who participated in screening patients for eligibility and at times also recruited patients.
Summary
The case study above demonstrates the importance of increasing opportunity for CCTs participation for minority patients by reducing administrative and clinical barriers to CCTs in minority-serving institutions. Additionally, it underscores the gap in understanding and bridging awareness-related needs among minority patients and the potential for incentivizing academic centers to implement interventions to support CCT-related awareness among minority patients.
DISCUSSION
Applying the Ford model to the three case studies demonstrates how implementation of strategic promoters to overcome key barriers can increase awareness of clinical research, provide meaningful opportunities to participate, and facilitate informed decisions to participate, thereby contributing to the equitable representation in clinical research of racially and ethnically diverse populations. Key promoters of recruitment and retention of diverse populations included multi-level approaches that targeted patients, physicians, organizations, systems, communities, and an entire state. The use of linguistically and culturally appropriate information and delivery channels was pivotal in increasing awareness. Community-based outreach strategies that engaged key stakeholders from the targeted communities ensured that opportunities to participate and acceptance of research were maximized. Testimonials by ethnically diverse cancer survivors served to enhance acceptance of participation trials. Availability of medical interpretation services was essential in certain geographic areas. And, providing for infrastructure that facilitated the engagement in clinical research of clinicians who care for minority patient populations proved effective.
These studies illustrate that progress on minority accrual into clinical research requires intentional efforts to overcome barriers at all three stages of the accrual process: awareness, opportunity and acceptance of participation. The case studies highlight the need to take the research to the communities. These studies were able to achieve recruitment and retention targets by implementing systematic approaches that took the research to the minority communities. Such strategies included the placement of educational programs within ethnically diverse communities, building social networks with minority organizations and community providers, and providing research infrastructure and resources within these communities to support engagement in cancer research.
These case studies provide support for the validity of the Ford model. Awareness and acceptance promoters were present when patients were educated about cancer clinical trials and given a meaningful opportunity to participate. These case studies indicate that education that is tailored and taken to communities via outreach can improve attitudes and increase acceptance of clinical research. The Ford model can be elaborated further by addressing sensitizing conditions. For example, preparatory knowledge of the community can enhance recruitment efforts. Formative research with key stakeholders to identify preferred learning styles, social networks, and communication channels can result in more efficient and effective use of recruitment resources.
While a great number of studies have identified barriers to recruitment among racially and ethnically diverse populations, this study serves to illustrate strategies that were applied successfully to enhance participation rates among these populations. However, this success hinged on a culturally-informed investment of resources that required key stakeholder input via the establishment of genuine partnerships with the targeted populations and their providers. Most researchers underestimate the time, attention and resources required to engage minority populations in research (National Academics of Sciences, 2016). With the rapid advancement of precision medicine approaches to cancer treatments, issues of genomic literacy, patient distrust and effective clinician-patient communication, as well as equitable access to state-of-the-science treatments, will become even more pronounced. If we continue to dedicate insufficient attention and resources to the engagement of racially and ethnically diverse populations in cancer clinical trials, this could serve to exacerbate disparities in cancer outcomes. Inclusion of these populations in numbers that are proportionate to the populations of persons affected by specific types of cancers is scientifically and ethically sound.
Acknowledgments
This research was supported by the following funding sources: NIH/NCI Grant Number P20 CA157071; NIH/NCI Grant Number P30 CA138313; NIH/NIMHD Grant Number R01 MD005892; NIH/NCI Grant Number 3U10CA086780-10S1; ACS IRG Grant Number 92-024; NIH/NCI Grant Number 1U54CA153511; and NIH/NIA Grant Number 1 P30 AG15272.
References
- Advani AS, Atkeson B, Brown CL, Peterson BL, Fish L, Johnson JL, Gautier M. Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer. 2003;97(6):1499–1506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- Bernard-Davila B, Aycinena AC, Richardson J, Gaffney AO, Koch P, Contento I, Greenlee H. Barriers and facilitators to recruitment to a culturally-based dietary intervention among urban Hispanic breast cancer survivors. J Racial Ethn Health Disparities. 2015;2(2):244–255. doi: 10.1007/s40615-014-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Ann Epidemiol. 2000;10(8):S13–21. doi: 10.1016/s1047-2797(00)00197-6. [DOI] [PubMed] [Google Scholar]
- Bussey-Jones J, Garrett J, Henderson G, Moloney M, Blumenthal C, Corbie-Smith G. The role of race and trust in tissue/blood donation for genetic research. Genet Med. 2010;12(2):116–121. doi: 10.1097/GIM.0b013e3181cd6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, L P, Dang JHT, Paterniti DA, Paterniti DA, Kelly K. Twenty years post-NIH revitalization act: enhancing minority participation in clinical trials (EMPaCT): laying groundwork for improving minority clinical trial accrual. Cancer. 2014;120(7):1091–1096. doi: 10.1002/cncr.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook ED, Arnold KB, Hermos JA, McCaskill-Stevens W, Moody-Thomas S, Probstfield JL, Minasian LM. Impact of supplemental site grants to increase African American accrual for the Selenium and Vitamin E Cancer Prevention Trial. Clin Trials. 2010;7(1):90–99. doi: 10.1177/1740774509357227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook ED, Moody-Thomas S, Anderson KB, Campbell R, Hamilton SJ, Harrington JM, Probstfield JL. Minority recruitment to the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Clin Trials. 2005;2(5):436–442. doi: 10.1191/1740774505cn111oa. [DOI] [PubMed] [Google Scholar]
- EM R. Diffusion of innovations. 5. New York: Free Press; 2003. [Google Scholar]
- Fallowfield LJ, Jenkins V, Brennan C, Sawtell M, Moynihan C, Souhami RL. Attitudes of patients to randomised clinical trials of cancer therapy. Eur J Cancer. 1998;34(10):1554–1559. doi: 10.1016/s0959-8049(98)00193-2. [DOI] [PubMed] [Google Scholar]
- Ford JG, H M, Lai GY, et al. Barriers to recruiting underrepresented population to cancer clinical trials: a systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- Ford M, Wahlquist A, Blake R, Green C, Streets J, Fuller E, Garrett-Mayer E. Assessing an intervention to improve clinical trial perceptions among predominately African-American communities in South Carolina. Prog Community Health Partnersh. 2012;6(3):249–263. doi: 10.1353/cpr.2012.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ME, S L, Pickelsimer E, et al. Unequal burden of disease, unequal participation in clinical trials: solutions from African American and Latino community members. Health Soc Work. 2013;38:29–38. doi: 10.1093/hsw/hlt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad M, D R, Martin Michelle. Successful models for minority recruitment. Retrieved from http://www.empactconsortium.com/material/qa-with-dr-jean-ford/#sthash.8lgLTakq.dpuf.
- Glanz Lewis F, R B. Health behavior and health education: theory, research, and practice. San Francisco: Jossey-Bass; 1997. [Google Scholar]
- Green MA, Michaels M, Blakeney N, Odulana AA, Isler MR, Richmond A, Corbie-Smith G. Evaluating a community-partnered cancer clinical trials pilot intervention with African American communities. J Cancer Educ. 2015;30(1):158–166. doi: 10.1007/s13187-014-0764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz SM, Johnson EO, Saccone NL, Hatsukami D, Breslau N, Bierut LJ. Inclusion of African Americans in genetic studies: what is the barrier? Am J Epidemiol. 2011;174(3):336–344. doi: 10.1093/aje/kwr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes-Maslow L, Godley P, Dimartino L, White B, Odom J, Richmond A, Carpenter W. African American women's perceptions of cancer clinical trials. Cancer Med. 2014;3(5):1430–1439. doi: 10.1002/cam4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11(1):1–47. doi: 10.1177/109019818401100101. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6392204. [DOI] [PubMed] [Google Scholar]
- Kaplan CP, Napoles AM, Dohan D, Shelley Hwang E, Melisko M, Nickleach D, Haas J. Clinical trial discussion, referral, and recruitment: physician, patient, and system factors. Cancer Causes Control. 2013;24(5):979–988. doi: 10.1007/s10552-013-0173-5. [DOI] [PubMed] [Google Scholar]
- Kehl KL, Arora NK, Schrag D, Ayanian JZ, Clauser SB, Klabunde CN, Keating NL. Discussions about clinical trials among patients with newly diagnosed lung and colorectal cancer. J Natl Cancer Inst. 2014;106(10) doi: 10.1093/jnci/dju216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford A, R K, Lawrence AN. Clinical trial awareness among racial/ethnic minorities in HINTS 2007: sociodemographic, attitudinal and knowledge correlates. Journal of Health Communication: International Perspectives. 2010;15:92–101. doi: 10.1080/10810730.2010.525296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara PN, Jr, Paterniti DA, Chiechi C, Turrell C, Morain C, Horan N, Chen MS., Jr Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282–9289. doi: 10.1200/JCO.2005.02.6245. [DOI] [PubMed] [Google Scholar]
- Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: Changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215–223. doi: 10.1177/1740774515571917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkus IM, Iden D, Terrenoire J, Feaganes JR. Relationships among breast cancer concern, risk perceptions, and interest in genetic testing for breast cancer susceptibility among African-American women with and without a family history of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8(6):533–539. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10385144. [PubMed] [Google Scholar]
- Livaudais-Toman J, Burke NJ, Napoles A, Kaplan CP. Health Literate Organizations: Are Clinical Trial Sites Equipped to Recruit Minority and Limited Health Literacy Patients? J Health Dispar Res Pract. 2014;7(4):1–13. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26295011. [PMC free article] [PubMed] [Google Scholar]
- Mathioudakis NN, Giles M, Yeh HC, Haywood C, Jr, Greer RC, Golden SH. Racial differences in acute kidney injury of hospitalized adults with diabetes. J Diabetes Complications. 2016;30(6):1129–1136. doi: 10.1016/j.jdiacomp.2016.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academics of Sciences, E., and Medicine. Applying an implementation science approach to genomic medicine. Workshop summary. Washington, DC: 2016. [PubMed] [Google Scholar]
- Pinsky PF, Ford M, Gamito E, Higgins D, Jenkins V, Lamerato L, Gohagan JK. Enrollment of racial and ethnic minorities in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Med Assoc. 2008;100(3):291–298. doi: 10.1016/s0027-9684(15)31241-4. [DOI] [PubMed] [Google Scholar]
- Prasad A, Helder MR, Brown DA, Schaff HV. Understanding Differences in Administrative and Audited Patient Data in Cardiac Surgery: Comparison of the University HealthSystem Consortium and Society of Thoracic Surgeons Databases. J Am Coll Surg. 2016 doi: 10.1016/j.jamcollsurg.2016.06.393. [DOI] [PubMed] [Google Scholar]
- SD B. Pedagogy and androagogy. In: DiStefano KRA, Silverman R, Taira S, editors. Encyclopedia of distributed learning. Thousand Oaks, CA: Sage; 2003. [Google Scholar]
- Seifer SD, Michaels M, Collins S. Applying community-based participatory research principles and approaches in clinical trials: forging a new model for cancer clinical research. Prog Community Health Partnersh. 2010;4(1):37–46. doi: 10.1353/cpr.0.0103. [DOI] [PubMed] [Google Scholar]
- Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient Income Level and Cancer Clinical Trial Participation: A Prospective Survey Study. JAMA Oncol. 2016;2(1):137–139. doi: 10.1001/jamaoncol.2015.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Bartlett R. Recruitment and retention of African American and Hispanic girls and women in research. Public Health Nurs. 2013;30(2):159–166. doi: 10.1111/phn.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3(2):e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams IC, Utz SW, Jones R, Hinton I, Steeves R, Alexander G. Recruitment of Rural African Americans for Research Projects: Lessons Learned. South Online J Nurs Res. 2011;11(1):8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24791157. [PMC free article] [PubMed] [Google Scholar]
- Yoo W, De S, Wilkins T, Smith SA, Blumenthal D. Age, Race and Regional Disparities in Colorectal Cancer Incidence Rates in Georgia between 2000 and 2012. Ann Public Health Res. 2016;3(2) Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27042701. [PMC free article] [PubMed] [Google Scholar]