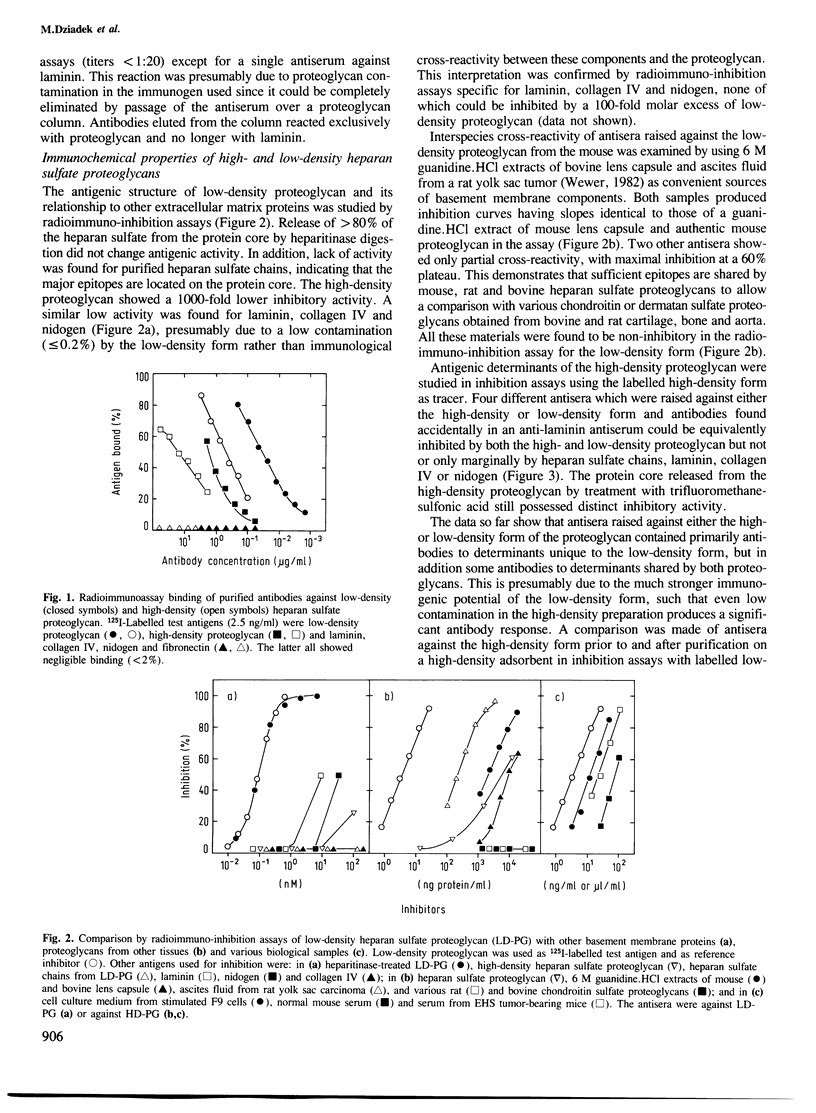
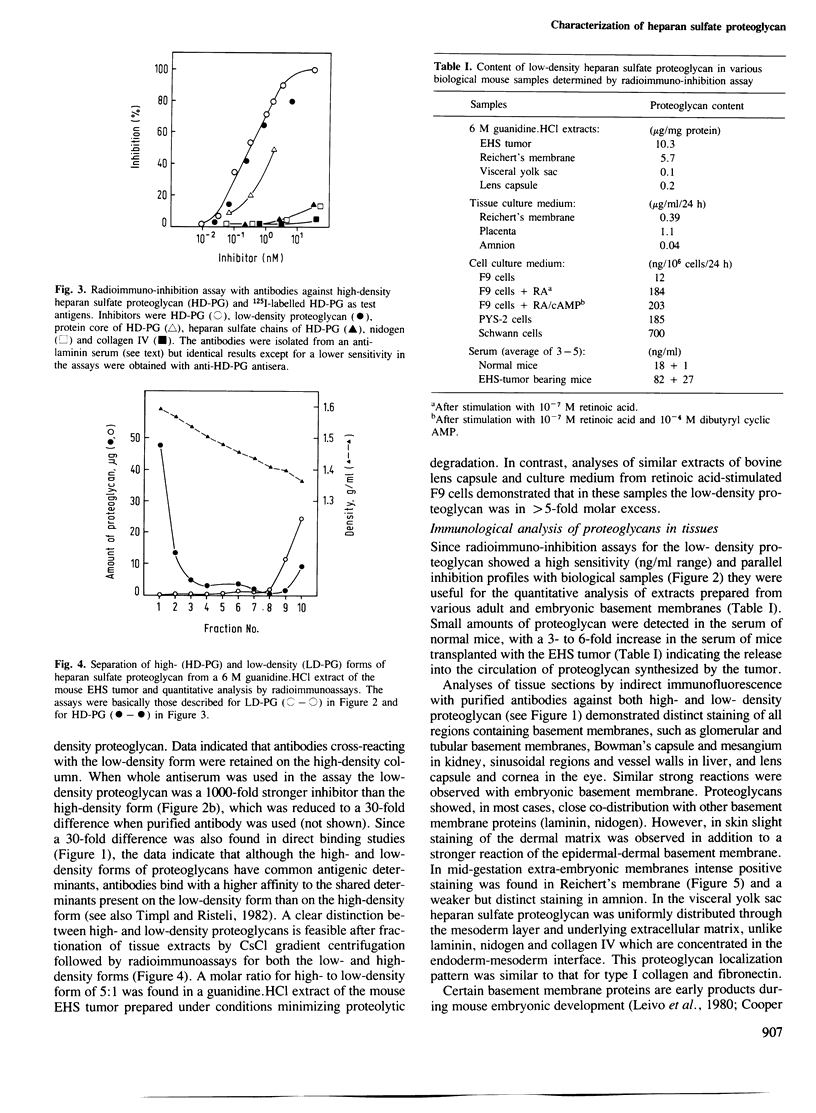
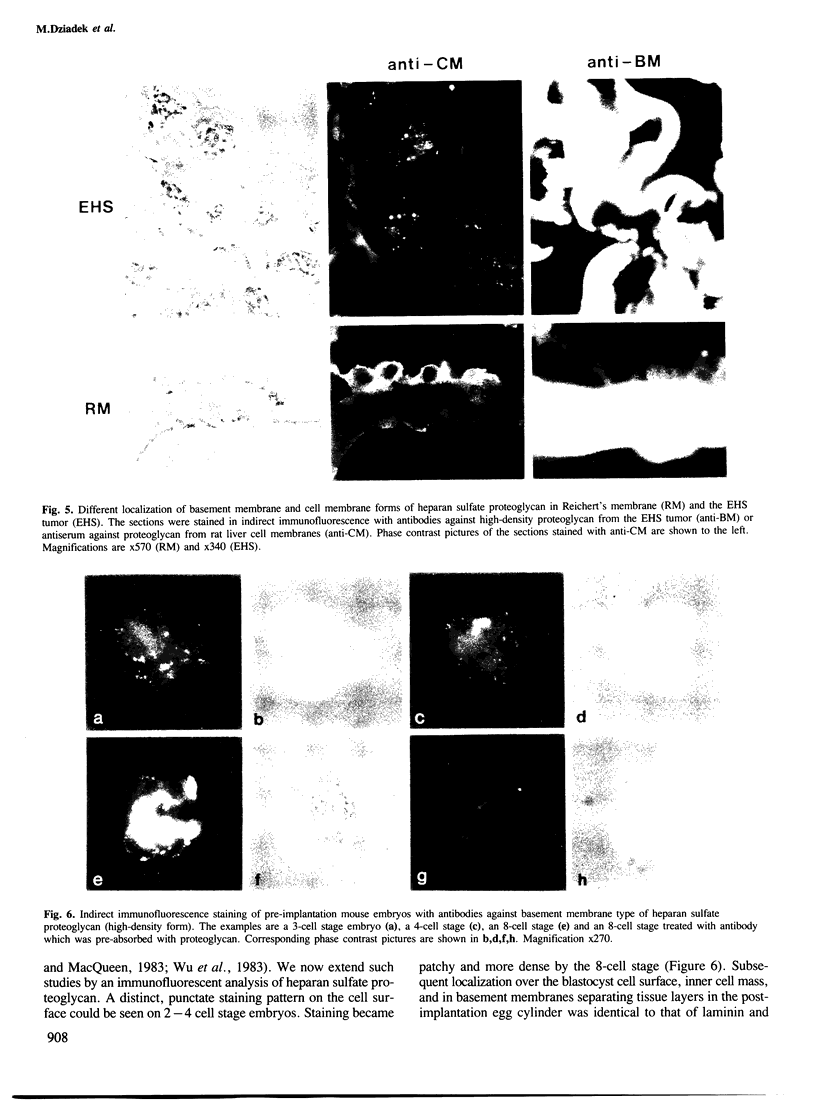
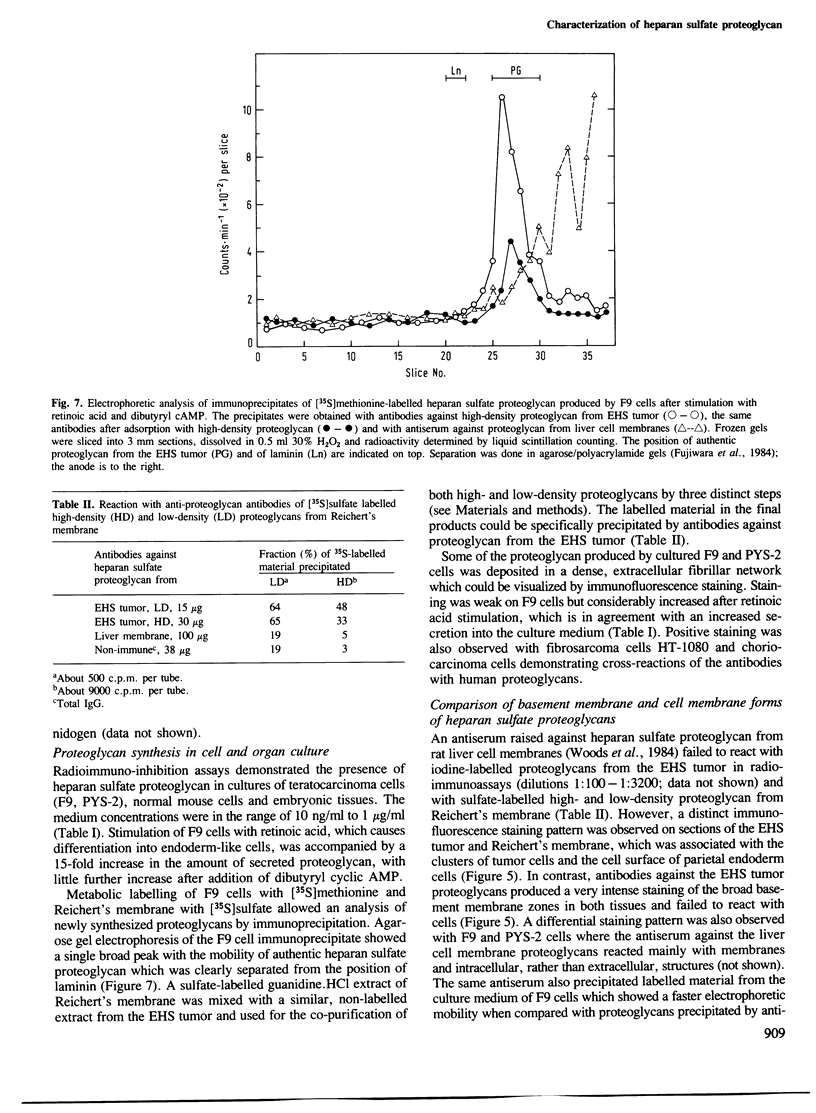
Abstract
Antibodies were raised against a small high-density and a large low-density form of heparan sulfate proteoglycan from a basement membrane-producing mouse tumor and were characterized by radioimmunoassays, immunoprecipitation and immunohistological methods. Antigenicity was due to the protein cores and included epitopes unique to the low density form as well as some shared by both proteoglycans. The antibodies did not cross-react with other basement membrane proteins or with chondroitin sulfate proteoglycans from interstitial connective tissues. The heparan sulfate proteoglycans occurred ubiquitously in embryonic and adult basement membranes and could be initially detected at the 2-4 cell stage of mouse embryonic development. Low levels were also found in serum. Biosynthetic studies demonstrated identical or similar proteoglycans in cultures of normal and carcinoembryonic cells and in organ cultures of fetal tissues. They could be distinguished from liver cell membrane heparan sulfate proteoglycan, indicating that the basement membrane types of proteoglycans represent a unique class of extracellular matrix proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan M. J., Oldberg A., Ruoslahti E., Brown K., Schwartz N. Immunological evidence for two distinct chondroitin sulfate proteoglycan core proteins: differential expression in cartilage matrix deficient mice. Dev Biol. 1983 Jul;98(1):139–147. doi: 10.1016/0012-1606(83)90342-1. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Colburn P. Antibodies to the heparan sulfate proteoglycans synthesized by endothelial cell cultures. Biochim Biophys Acta. 1983 Oct 4;760(1):1–12. doi: 10.1016/0304-4165(83)90118-6. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Cöster L., Malmström A., Fransson L. A. Proteoheparan sulfate from human skin fibroblasts. Isolation and structural characterization. J Biol Chem. 1983 Oct 10;258(19):11629–11635. [PubMed] [Google Scholar]
- Cooper A. R., MacQueen H. A. Subunits of laminin are differentially synthesized in mouse eggs and early embryos. Dev Biol. 1983 Apr;96(2):467–471. doi: 10.1016/0012-1606(83)90183-5. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Engel J., Schalch W. Antibody binding constants from Farr test and other radioimmunoassays. A theoretical and experimental analysis. Mol Immunol. 1980 May;17(5):675–680. doi: 10.1016/0161-5890(80)90166-2. [DOI] [PubMed] [Google Scholar]
- Fenger M., Wewer U., Albrechtsen R. Basement membrane heparan sulfate proteoglycan from the L2 rat yolk sac carcinoma. FEBS Lett. 1984 Jul 23;173(1):75–79. doi: 10.1016/0014-5793(84)81020-0. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Carlstedt I., Cöster L., Malmström A. Binding of transferrin to the core protein of fibroblast proteoheparan sulfate. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5657–5661. doi: 10.1073/pnas.81.18.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Extraction and purification of proteoglycans from mature bovine bone. Biochem J. 1984 Nov 15;224(1):47–58. doi: 10.1042/bj2240047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Wiedemann H., Timpl R., Lustig A., Engel J. Structure and interactions of heparan sulfate proteoglycans from a mouse tumor basement membrane. Eur J Biochem. 1984 Aug 15;143(1):145–157. doi: 10.1111/j.1432-1033.1984.tb08353.x. [DOI] [PubMed] [Google Scholar]
- Hampson I. N., Kumar S., Gallagher J. T. Heterogeneity of cell-associated and secretory heparan sulphate proteoglycans produced by cultured human neuroblastoma cells. Biochim Biophys Acta. 1984 Sep 28;801(2):306–313. doi: 10.1016/0304-4165(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Heathcote J. G., Orkin R. W. Biosynthesis of sulphated macromolecules by rabbit lens epithelium. I. Identification of the major macromolecules synthesized by lens epithelial cells in vitro. J Cell Biol. 1984 Sep;99(3):852–860. doi: 10.1083/jcb.99.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman K., Johansson S., Vartio T., Kjellén L., Vaheri A., Hök M. Structure of the pericellular matrix: association of heparan and chondroitin sulfates with fibronectin-procollagen fibers. Cell. 1982 Mar;28(3):663–671. doi: 10.1016/0092-8674(82)90221-5. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Taylor A., Cooper A. R. Murine parietal endoderm cells synthesise heparan sulphate and 170K and 145K sulphated glycoproteins as components of Reichert's membrane. Dev Biol. 1982 Mar;90(1):210–214. doi: 10.1016/0012-1606(82)90227-5. [DOI] [PubMed] [Google Scholar]
- Howe C. C., Solter D. Identification of noncollagenous basement membrane glycopolypeptides synthesized by mouse parietal entoderm and an entodermal cell line. Dev Biol. 1980 Jun 15;77(2):480–487. doi: 10.1016/0012-1606(80)90489-3. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V. Biosynthesis of heparan sulfate proteoglycan by human colon carcinoma cells and its localization at the cell surface. J Cell Biol. 1984 Aug;99(2):403–417. doi: 10.1083/jcb.99.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Hascall V. C., Farquhar M. G. Partial characterization of newly synthesized proteoglycans isolated from the glomerular basement membrane. J Cell Biol. 1981 Aug;90(2):527–532. doi: 10.1083/jcb.90.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Veis A., Kimura J. H., Jakubowski M. L. Characterization of heparan sulfate-proteoglycan of glomerular basement membranes. Proc Natl Acad Sci U S A. 1984 Feb;81(3):762–766. doi: 10.1073/pnas.81.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Pettersson I., Hök M. Cell-surface heparan sulfate: an intercalated membrane proteoglycan. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5371–5375. doi: 10.1073/pnas.78.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- Leivo I., Vaheri A., Timpl R., Wartiovaara J. Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol. 1980 Apr;76(1):100–114. doi: 10.1016/0012-1606(80)90365-6. [DOI] [PubMed] [Google Scholar]
- Lowe-Krentz L. J., Keller J. M. Multiple heparan sulfate proteoglycans synthesized by a basement membrane producing murine embryonal carcinoma cell line. Biochemistry. 1983 Sep 13;22(19):4412–4419. doi: 10.1021/bi00288a011. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Kleinman H. K., Terranova V. P., Ledbetter S., Hassell J. R. The regulation of basement membrane formation and cell-matrix interactions by defined supramolecular complexes. Ciba Found Symp. 1984;108:197–212. doi: 10.1002/9780470720899.ch13. [DOI] [PubMed] [Google Scholar]
- Morris J. E. Isolation of the major chondroitin sulfate/dermatan sulfate and heparan sulfate proteoglycans from embryonic chicken retina. Arch Biochem Biophys. 1984 Nov 15;235(1):127–140. doi: 10.1016/0003-9861(84)90261-3. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Yanagimachi R., Yanagimachi H. Ultrastructural localization of lectin-binding sites on the zonae pellucidae and plasma membranes of mammalian eggs. J Cell Biol. 1975 Aug;66(2):263–274. doi: 10.1083/jcb.66.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Kjellén L., Hök M. Cell-surface heparan sulfate. Isolation and characterization of a proteoglycan from rat liver membranes. J Biol Chem. 1979 Sep 10;254(17):8505–8510. [PubMed] [Google Scholar]
- Oldberg A., Schwartz C., Ruoslahti E. Isolation and partial characterization of a rat hepatoma heparan sulfate proteoglycan. Arch Biochem Biophys. 1982 Jul;216(2):400–406. doi: 10.1016/0003-9861(82)90228-4. [DOI] [PubMed] [Google Scholar]
- Oohira A., Wight T. N., Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983 Feb 10;258(3):2014–2021. [PubMed] [Google Scholar]
- Oohira A., Wight T. N., McPherson J., Bornstein P. Biochemical and ultrastructural studies of proteoheparan sulfates synthesized by PYS-2, a basement membrane-producing cell line. J Cell Biol. 1982 Feb;92(2):357–367. doi: 10.1083/jcb.92.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy N., Spiro R. G. Isolation and characterization of the heparan sulfate proteoglycan of the bovine glomerular basement membrane. J Biol Chem. 1984 Oct 25;259(20):12749–12755. [PubMed] [Google Scholar]
- Prehm P., Dessau W., Timpl R. Rates of synthesis of basement membrane proteins by differentiating teratocarcinoma stem cells and their modulation by hormones. Connect Tissue Res. 1982;10(3-4):275–285. doi: 10.3109/03008208209008053. [DOI] [PubMed] [Google Scholar]
- Radhakrishnamurthy B., Jeansonne N. E., Berenson G. S. Heterogeneity of heparan sulfate chains in a proteoglycan from bovine lung. Biochim Biophys Acta. 1984 Nov 28;802(2):314–320. doi: 10.1016/0304-4165(84)90177-6. [DOI] [PubMed] [Google Scholar]
- Risteli J., Draeger K. E., Regitz G., Neubauer H. P. Increase in circulating basement membrane antigens in diabetic rats and effects of insulin treatment. Diabetologia. 1982 Sep;23(3):266–269. doi: 10.1007/BF00252853. [DOI] [PubMed] [Google Scholar]
- Risteli J., Rohde H., Timpl R. Sensitive radioimmunoassays for 7 S collagen and laminin: application to serum and tissue studies of basement membranes. Anal Biochem. 1981 May 15;113(2):372–378. doi: 10.1016/0003-2697(81)90091-9. [DOI] [PubMed] [Google Scholar]
- Rohrbach D. H., Wagner C. W., Star V. L., Martin G. R., Brown K. S., Yoon J. W. Reduced synthesis of basement membrane heparan sulfate proteoglycan in streptozotocin-induced diabetic mice. J Biol Chem. 1983 Oct 10;258(19):11672–11677. [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Timpl R. Antibodies to collagens and procollagens. Methods Enzymol. 1982;82(Pt A):472–498. doi: 10.1016/0076-6879(82)82079-x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Dziadek M., Fujiwara S., Nowack H., Wick G. Nidogen: a new, self-aggregating basement membrane protein. Eur J Biochem. 1983 Dec 15;137(3):455–465. doi: 10.1111/j.1432-1033.1983.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Martin G. R., Bruckner P., Wick G., Wiedemann H. Nature of the collagenous protein in a tumor basement membrane. Eur J Biochem. 1978 Mar;84(1):43–52. doi: 10.1111/j.1432-1033.1978.tb12139.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Tyree B., Horigan E. A., Klippenstein D. L., Hassell J. R. Heterogeneity of heparan sulfate proteoglycans synthesized by PYS-2 cells. Arch Biochem Biophys. 1984 Jun;231(2):328–335. doi: 10.1016/0003-9861(84)90395-3. [DOI] [PubMed] [Google Scholar]
- Wewer U. Characterization of a rat yolk sac carcinoma cell line. Dev Biol. 1982 Oct;93(2):416–421. doi: 10.1016/0012-1606(82)90128-2. [DOI] [PubMed] [Google Scholar]
- Woodley D. T., Rao C. N., Hassell J. R., Liotta L. A., Martin G. R., Kleinman H. K. Interactions of basement membrane components. Biochim Biophys Acta. 1983 Dec 27;761(3):278–283. doi: 10.1016/0304-4165(83)90077-6. [DOI] [PubMed] [Google Scholar]
- Woods A., Hök M., Kjellén L., Smith C. G., Rees D. A. Relationship of heparan sulfate proteoglycans to the cytoskeleton and extracellular matrix of cultured fibroblasts. J Cell Biol. 1984 Nov;99(5):1743–1753. doi: 10.1083/jcb.99.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. C., Wan Y. J., Chung A. E., Damjanov I. Immunohistochemical localization of entactin and laminin in mouse embryos and fetuses. Dev Biol. 1983 Dec;100(2):496–505. doi: 10.1016/0012-1606(83)90242-7. [DOI] [PubMed] [Google Scholar]





