Abstract
Purpose:
Laparoscopic intracorporeal colorectal anastomosis with double stapling technique is difficult because of unsuitable cutting angle in narrow pelvic cavity. For reasons of tilted and long linear staple line of rectal stump, circular anastomotic plane can make multiple intersections. The present study was designed to assess whether multiple intersections after double stapling technique is the risk factor of anastomotic complication in laparoscopic colorectal surgery.
Materials and Methods:
In total, 128 consecutive left colon and rectal cancer patients who underwent laparoscopic rectal resection with double stapling technique were enrolled in this study. In all cases, operator tried to reduce intersections by inversion and invagination techniques. They were subdivided into 3 groups: 58 patients with no intersection of staple lines (group A), 62 patients with 1 point of intersection (group B) and 8 patients with 2 points of intersection (group C). Intraoperative air leakage, incomplete cut ring, postoperative bleeding, anastomotic stenosis, and leakage were compared between the 3 groups.
Results:
Clinical anastomotic leakage was identified in 1 (group C) of 128 patients (0.7%). Overall anastomotic leakage rate was 0% (0/58) in group A, 0% (0/62) in group B, and 12.5% (1/8) in group C (P=0.001). In univariate analysis, intersections of staple lines were associated with anastomotic complications. There were no statistically significant differences between the 3 groups in multivariate analysis.
Conclusions:
The number of intersections of staple lines is associated with anastomotic leakage, and the inversion technique is a useful method for avoiding anastomotic leakage. Using an appropriate technique by skilled operator, double stapling technique for laparoscopic anterior resection is safe and feasible.
Key Words: double stapling technique, anastomotic leakage, colorectal surgery
In 1980, Knight and Griffen1 published the first report of a modification of the stapling technique for low rectal anastomosis using both linear and circular stapler. The advantages of this method include the fact that no purse-string suture is placed deep in the pelvis, and the distal segment is not opened, which avoids spillage from the rectal stump and size disparity between the 2 ends of the bowel.2 However, there is some concern about the safety of the double stapling technique: the number of linear stapler firings during rectal division is associated with anastomotic leakage,3–6 lateral intersecting staple lines (dog-ears) are a weak point in the anastomosis,7 and intersections of staple lines after the double stapling technique tend to be associated with anastomotic leakage.7–9 Laparoscopic intracorporeal colorectal anastomosis with double stapling technique is difficult because of the unsuitable cutting angle associated with using a linear stapler in the narrow pelvic cavity. Consequently, we sometimes have used multiple stapler firings during division of the rectum. Because of the long and tilted linear staple line placed on the rectal stump, a circular anastomotic plane can create multiple intersections of staple lines and dog-ears. Anastomotic leakage is a major problem in patients with colorectal cancer who have undergo laparoscopic surgery. Despite technical improvements in laparoscopic surgery, recent studies have reported that the anastomotic leakage rate remains at 6.3% to 13.7%.5,10–14 Unlike open surgery, there are few studies describing the anastomotic leakage rate and risk factors associated with laparoscopic colorectal surgery using a double stapling technique.3,4,15,16 The present study was designed to assess whether intersection of staple lines after use of the double stapling technique are a risk factor for anastomotic leakage. In addition, we evaluated the association between the number of intersections and clinical parameters, including instrument factors, in laparoscopic colorectal surgery.
MATERIALS AND METHODS
Patient Selection
We retrospectively analyzed 166 consecutive cases of laparoscopic surgery for sigmoid colon and rectal cancer between June 2014 and August 2015. All patients were seen by a single skilled operator who had performed more than 1500 laparoscopic colorectal surgery. We excluded 38 patients for extracorporeal side-to-side anastomosis by 2 linear staplers (n=16), extracorporeal end to end hand sewn anastomosis (n=3), abdominoperineal resection (n=3), Hartmann operation (n=1), protective ileostomy (n=3), and open surgery (n=12). We finally enrolled 128 patients with sigmoid colon and rectal cancer, who underwent laparoscopic colorectal resection with the double stapling technique (Fig. 1). Seven patients had preoperative chemoradiotherapy. For upper rectal cancer (>10 cm from anal verge), the mesorectum was excised 5 cm below the tumor, whereas total mesorectal excision was performed for middle and lower rectal cancer (≤10 cm from anal verge). The distance from the anal verge to the lower edge of the tumor was measured by digital rectal examination and colonoscopy. Twelve patients underwent open surgery with the double stapling technique for sigmoid colon and rectal cancer during the same period (laparoscopic surgery ratio: 93%). No patients were converted to open surgery (open conversion rate: 0%). In all cases, the operator tried to remove lateral intersecting staple lines (dog-ears) and the intersections of staple lines by the inversion and invagination technique. In this technique, after introducing a circular endostapler through the anus and opening the integrated trocar of the endostapler on the linear staple line, either one or both edges of the linear staple line were folded or pierced by integrated trocar to hide the staple line inside the rim of the circular stapler head. Patients were subdivided into 3 groups (Fig. 2): 58 patients had no intersection of staple lines (group A), 62 patients had 1 point of intersection (group B), and 8 patients had 2 points of intersection (group C). We retrospectively collected data on patient-related factors (age, sex, body mass index, American Society of Anesthesiologists’ score, previous laparotomy, and preoperative chemoradiotherapy), tumor-related factors (tumor location, distance of tumor from the anal verge, and American Joint Committee on Cancer TNM stage 7th edition), and surgery-related factors (type of operation, operation time, estimated blood loss, reinforcement suture at the points of intersections, distance of anastomosis from the anal verge, number of cartridges of the linear stapler used for rectal division, diameter of circular stapler, and anastomotic leakage).
FIGURE 1.
Exclusion criteria of patients.
FIGURE 2.
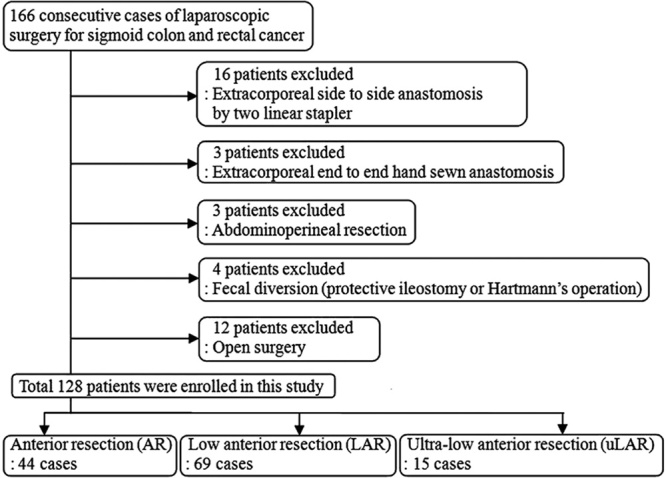
A, Group A had no intersection of staple lines (arrows). B, Group B had 1 point of intersection (arrow). C, Group C had 2 points of intersection, either 1 at left side (arrow).
Surgical Technique
The surgical procedure was standardized, as described previously.17 We routinely performed high ligation of the inferior mesenteric artery. The splenic flexure was mobilized totally or partially, depending on the bowel length. After mobilization of the left colon, we performed tumor-specific mesorectal excision. The distal rectum was divided intracorporeally with a linear articulated endostapler (Endo GIA Ultra Universal, COVIDIEN, Mansfield, MA) loaded with a 45 or 60 mm purple cartridge (Endo GIA Reloads with Tri-Staple Technology, COVIDIEN) through the 12 mm trocar with no precompression. The circular endostapler (DST Series EEA Stapler, COVIDIEN) was introduced through the anus (Fig. 3). The circumferential border of the rectal remnant was freed completely of perirectal fat for 1.0 to 1.5 cm of the staple line by electrocautery (ENDOPATH Electrosurgery PROBE PLUS II System, ETHICON, Cincinnati, OH), and then the inversion and invagination technique was performed. In this technique, the integrated trocar of the circular endostapler was opened on the linear staple line, which was free of fat; then, either one or both edges of the linear staple line were folded or pierced by the integrated trocar to hide the staple line inside the rim of the circular stapler head (Figs. 4, 6A). If the rectal remnant could not be mobilized distally enough to invaginate both edges of linear staple lines, left edge was pierced by integrated trocar and right edge was abandoned avoiding 2 intersections of staple lines and for ease of reinforcement suture (Figs. 5, 6B). After articulating the anvil with the integrated trocar of the endostapler and firing with no precompression, 2 distal and proximal “donuts” were immediately inspected and their integrity was verified. A pneumatic test was routinely carried out by transanal instillation of air. If there were intersections of staple lines, an intracorporeal reinforcement suture with 3-0 silk was placed at the intersection point. The level of anastomosis from the anal verge was measured by digital rectal examination.
FIGURE 3.
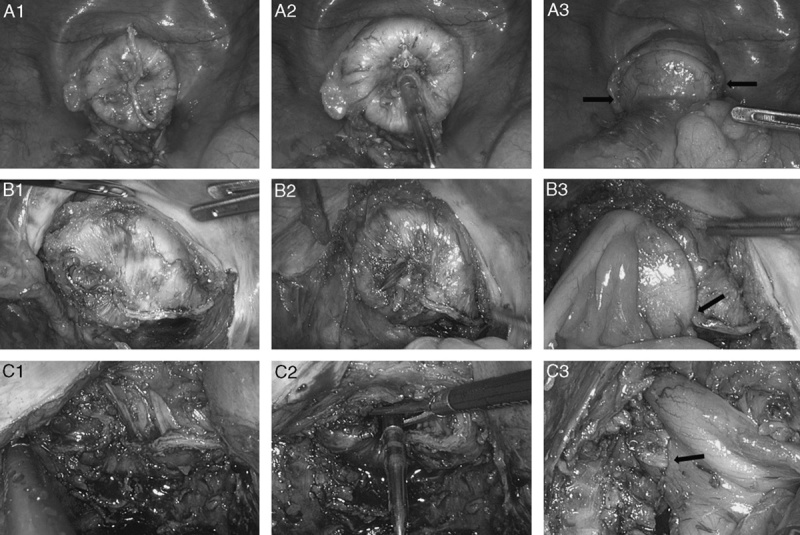
The circular endostapler was introduced through the anus. “Dog-ear” (arrows) and either one or both edges of linear staple line (arrowheads) were located out of the rim of circular stapler head.
FIGURE 4.
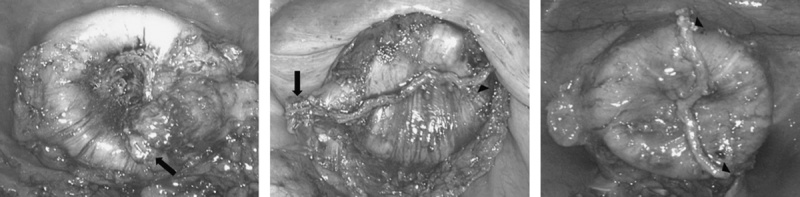
A, Either one or both edges of linear staple line were folded or pierced by the integrated trocar of circular endostapler. B, Linear staple line hid inside the rim of the circular stapler head.
FIGURE 6.
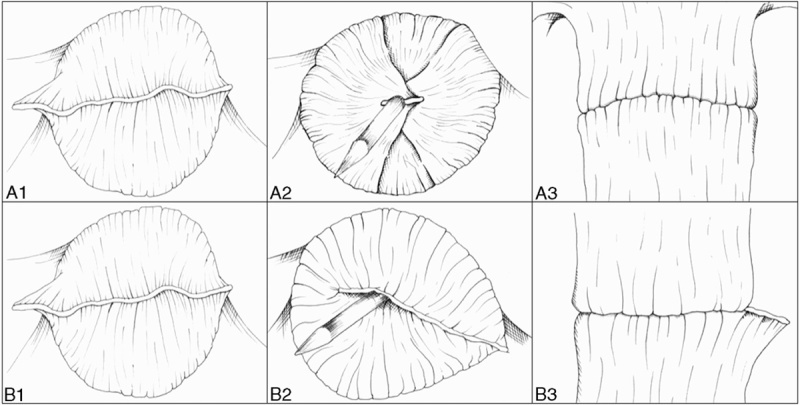
A, Inversion or invagination technique was performed perfectly. B, Right edge was abandoned avoiding 2 intersections of staple lines.
FIGURE 5.
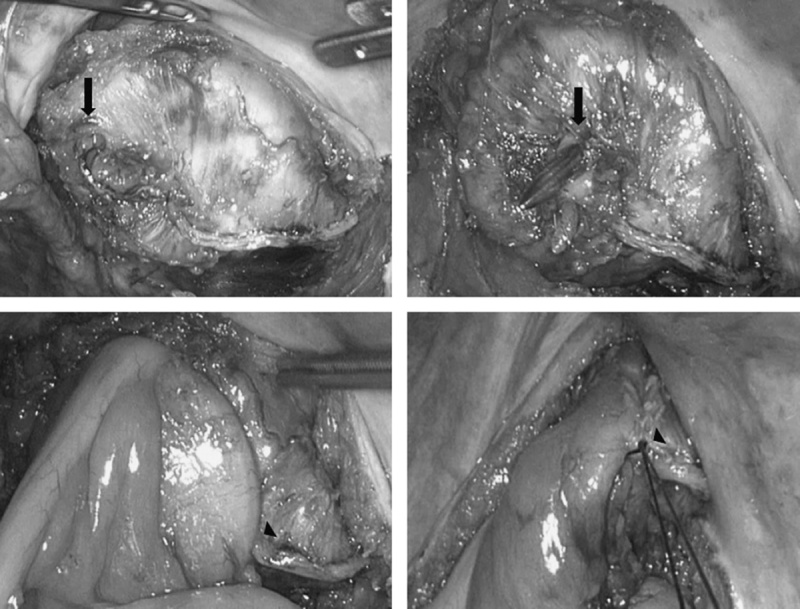
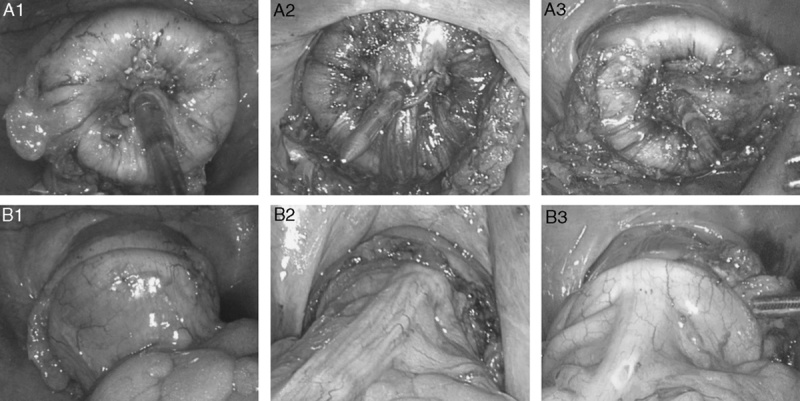
If the rectal remnant could not be mobilized distally enough to invaginate both edges of linear staple lines, left edge (arrows) was pierced by integrated trocar of circular endostapler and right edge (arrowheads) was abandoned avoiding 2 intersections of staple lines and for ease of reinforcement suture.
Clinical Anastomotic Leakage
Anastomotic leakage was investigated in the presence of clinical leakage signs (discharge of pus or feces from the pelvic drain and signs of peritonitis including abdominal pain, tenderness, fever, or leukocytosis). Once leakage signs were suspected, a computerized tomography (CT) scan was performed to check anastomotic leakage. Diagnosis required positive findings, such as an abscess at the level of the anastomosis and fluid or air bubbles surrounding the anastomosis on CT scan. Asymptomatic anastomotic leakage was not considered because routine contrast enemas were not performed in our institution. We included only symptomatic anastomotic leakage (anastomotic leakage requiring active therapeutic interventions or an operation) in this study.
Statistical Analysis
Statistical analysis was performed using SPSS software, version 18.0 for Windows (SPSS Inc.). Continuous variables (age, body mass index, tumor location, distance of tumor from the anal verge, operation time, estimated blood loss, and distance of anastomosis from the anal verge) were dichotomized. The χ2 test and Fisher extract test were used for categorical variables. Multivariate analysis was conducted with logistic regression analysis to detect risk factors for anastomotic leakage, and factors with a P-value of <0.05 were included in the model.
RESULTS
Patient and Tumor Characteristics
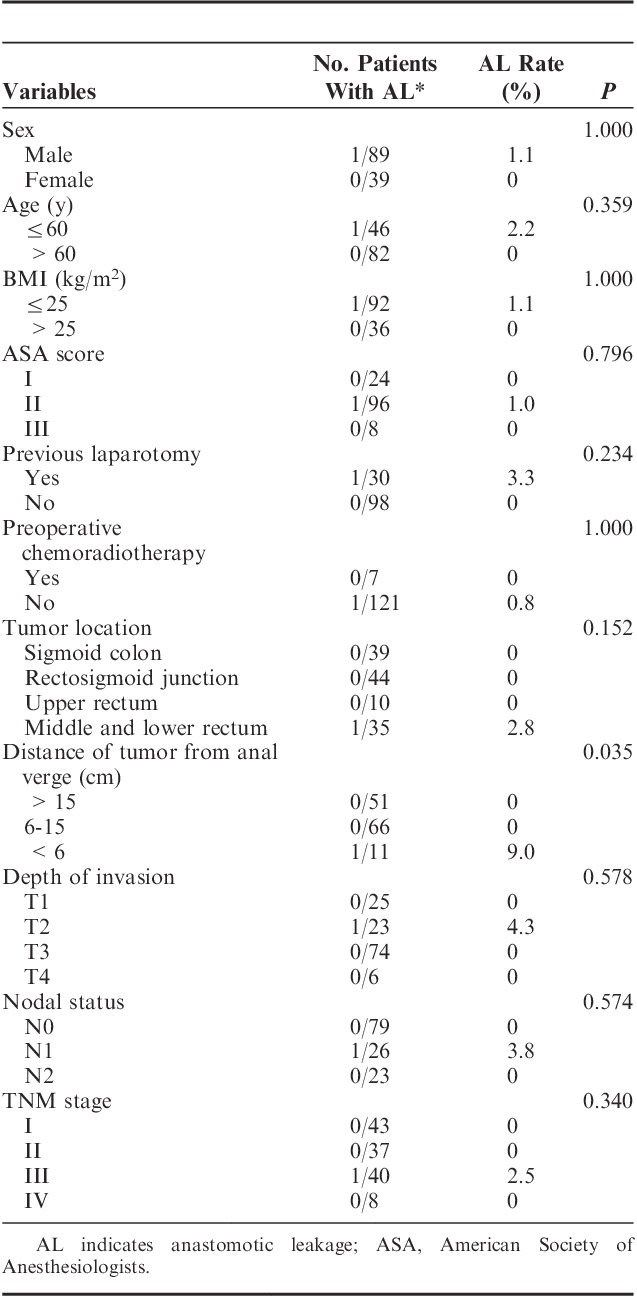
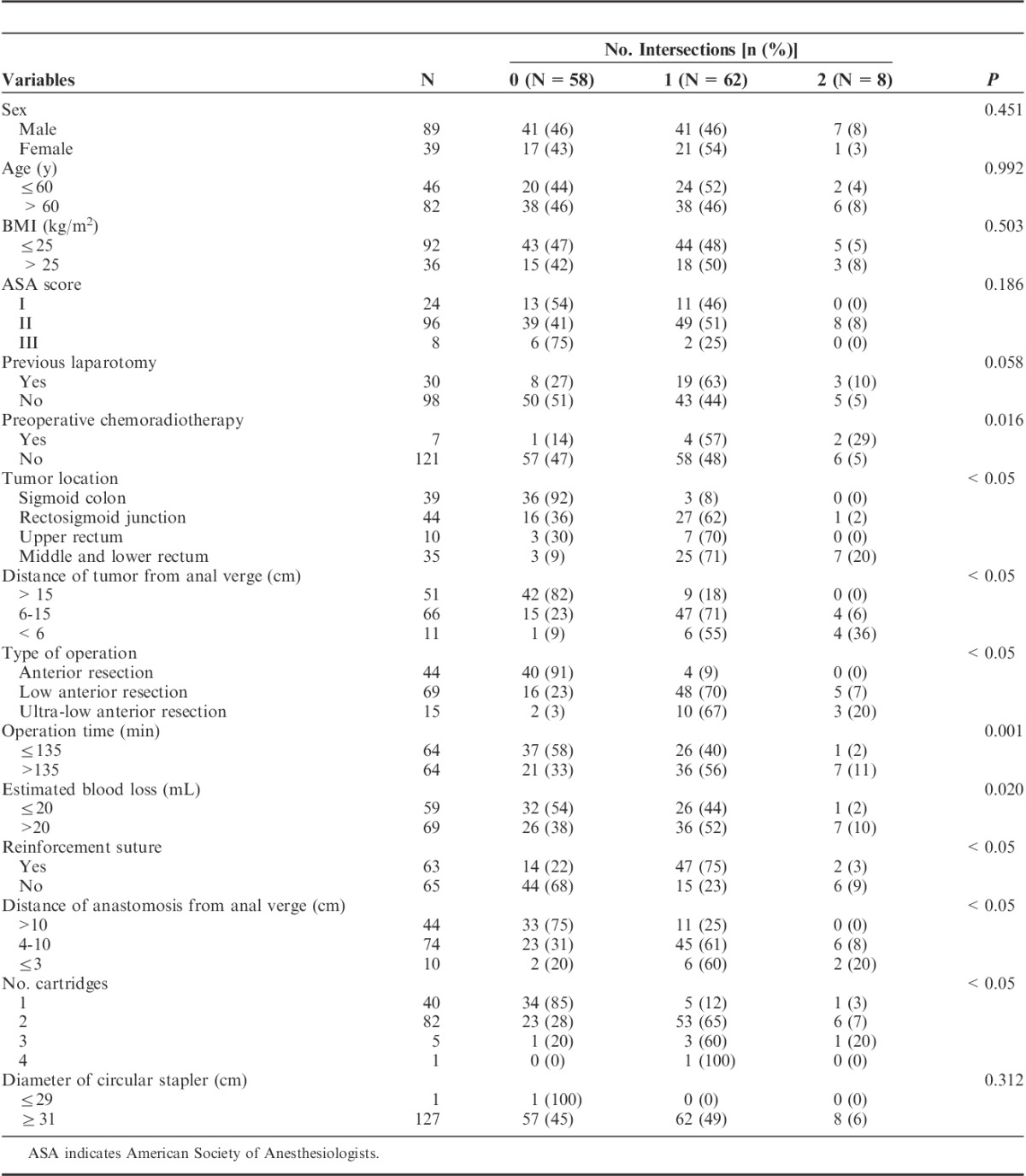
In total, 128 consecutive patients underwent laparoscopic colorectal surgery with an end-to-end double stapling technique. Of them 89 patients were male (69.5%). The median age was 63 years old (range, 39 to 84 y), and the median body mass index was 23.7 kg/m2 (range, 14.5 to 35.0 kg/m2). Preoperative chemoradiotherapy was performed in 7 patients (5.4%) and 30 patients had a previous laparotomy (23.4%). Three patients had synchronous multiple colorectal cancers; 1 patient had 3 lesions and the rest had 2 lesions in the left colon and rectum. Tumor-related factors of their distal lesion were used in this study. Thirty-nine patients (30.5%) had sigmoid colon cancer, 44 patients (34.4%) had rectosigmoid junction cancer, 10 patients (7.8%) had upper rectal cancer, and 35 patients (27.3%) had middle and lower rectal cancer. The median distance of the tumor from the anal verge was 14.5 cm (range, 2 to 45 cm); 51 patients had cancer >15 cm from the anal verge (39.8%), 66 patients had cancer 6 to 15 cm from the anal verge (51.6%), and 11 patients had cancer <6 cm from the anal verge (8.6%). The correlations between patient and tumor characteristics and anastomotic leakage are summarized in Table 1. The distance of the tumor from the anal verge was the only factor that was significantly associated with the development of clinical anastomotic leakage in univariate analysis (<6 cm from anal verge; P=0.035).
TABLE 1.
Patient Demographics and AL
Surgery-related Factors
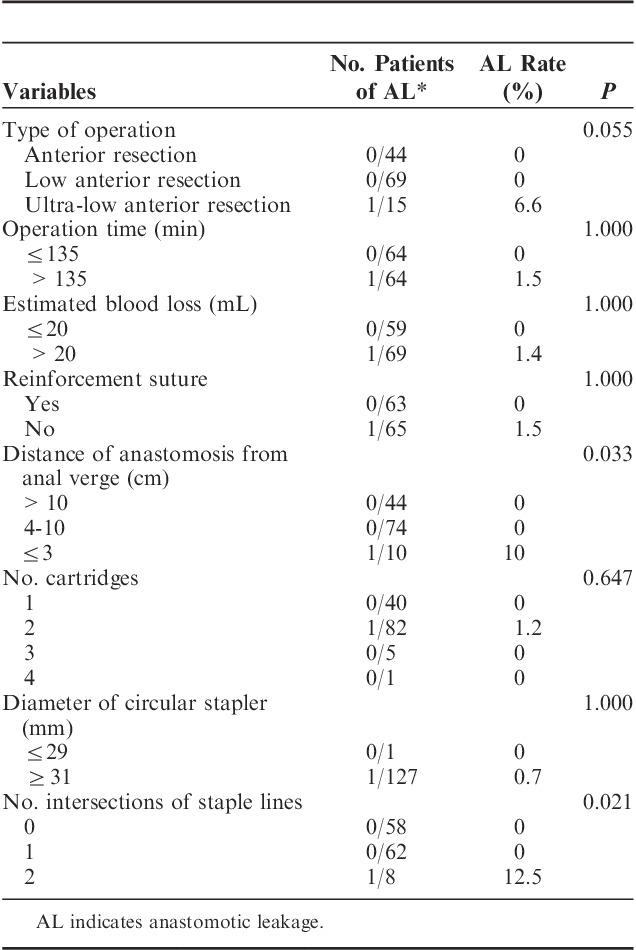
Surgical procedures were subdivided into 3 groups: anterior resection (colorectal anastomosis above the anterior peritoneal reflection), low anterior resection (colorectal anastomosis below the anterior peritoneal reflection), and ultra-low anterior resection (no rectum remains and the colon was connected directly to the anal canal, including intersphincteric resection for sphincter-saving operation). The median operation time was 137 minutes (range, 80 to 340 min). The median estimated blood loss was 30.0 mL (range, 10 to 350 mL). The median distance of anastomosis from the anal verge was 9.0 cm (range, 1 to 20 cm). A short distance of anastomosis from the anal verge (P=0.033) and multiple intersections of staple lines (P=0.021) were significant risk factors for anastomotic leakage in univariate analysis. Results of univariate analysis for correlations between surgery-related factors and anastomotic leakage are detailed in Table 2. In a multivariate analysis that included factors with a P-value <0.05, these factors did not remain significantly correlated with anastomotic leakage.
TABLE 2.
Surgery-related Factors of AL
Anastomotic Complication
Anastomotic leakage occurred in 1 patient (male, 62 y). The anastomotic leakage rate was 0.78% (1/128). Feculent discharge from the pelvic drain was noted on the third postoperative day with no symptoms or signs of peritonitis. Anastomotic leakage was confirmed by CT scan. The patient did not require surgical intervention and was treated by fasting, total parenteral nutrition, and antibiotics for 7 days. He left the hospital on the 13th postoperative day. Three patients with protective ileostomy did not have any anastomotic complications. In ultra-low anterior resection group, three patients who had received preoperative chemoradiotherapy were positive for pneumatic test. We performed fecal diversion in 1 patient whose point of air leakage could not be identified because of narrow pelvic cavity. Anastomotic stenosis occurred in 1 patient and we performed permanent colostomy 4 months after primary surgery. No patients had incomplete cut ring and bleeding of anastomotic site.
Factors Affecting the Number of Intersections of Staple Lines
Preoperative chemoradiotherapy, tumor location, operation time, distance of the tumor from the anal verge, type of operation, estimated blood loss, distance of the anastomosis from the anal verge, and number of cartridges for rectal division were significantly associated with the number of intersections of staple lines in univariate analysis (Table 3).
TABLE 3.
Correlation Between the Clinical Factors and Number of Intersections of Staple Lines
DISCUSSION
Anastomotic leakage is a major complication in patients with colorectal cancer undergoing laparoscopic surgery. It is associated with postoperative morbidity, mortality, functional defects, and oncologic outcomes.18–20 Despite technical improvements and instrumental developments, the anastomotic leakage rate remains at 6.3% to 13.7%.5,10–14 Recent reports have suggested that lower rectal cancer close to the anal canal should be treated with laparoscopic sphincter-saving rectal cancer resection with total mesorectal excision and preoperative chemoradiotherapy, instead of the traditional abdominoperineal resection.21,22 Potentially, the anastomotic leakage rate may be related to diversification of treatment strategies and an increased frequency of sphincter-saving rectal resection for lower rectal cancer.
An initial randomized controlled trial [the United Kingdom Medical Research Council Conventional versus Laparoscopic-Assisted Surgery in Colorectal Cancer (UK MRC CLASICC)] reported that laparoscopic-assisted surgery of the colon is as effective as open surgery in the short term and is likely to produce similar long-term outcomes and impaired short-term outcomes after laparoscopic anterior resection for rectal cancer.23 However, a number of studies have demonstrated that laparoscopic and open surgeries do not differ in terms of anastomotic leakage and that laparoscopic surgery for colorectal cancer is safe and feasible.24–27 Although laparoscopic surgery is well established for colonic and rectal cancers, several technical limitations are associated with anastomotic leakage. Unlike conventional open surgery, colorectal or coloanal anastomosis in laparoscopic surgery is performed in a restrictive and similar manner. Resection of the rectum after adequate mesorectal excision using intracorporeal stapling devices is technically difficult because of the narrow pelvic cavity and an inadequate cutting angle. Consequently, multiple firing of a linear endostapler, a long and tilted linear stapling line, lateral intersecting staple lines (dog-ears), multiple intersections of staple lines on the circular anastomotic plane, and size disparities between the two ends of the bowel are inherently different between laparoscopic colorectal surgery with the double stapling technique and conventional open surgery. Roumen et al7 reported that the lateral intersecting staple lines (dog-ears) of double stapling technique are structural weak spot and experimental study of Kawasaki et al8 demonstrated that the intersecting line of circular and linear stapler may be a dangerous point for the double stapling technique. Kuroyanagi et al9 reported technical efforts to remove the intersections of the linear staple lines, which may be a site of the anastomotic leakage. Asao et al28 introduced use of omega shape suture to eliminate the lateral intersecting lines in the double stapling technique. In this study, the operator tried to reduce these factors using adequate mesorectal excision, ensuring that the anastomotic plane was cleared of perirectal or pericolic fat, using the articulated endostapler adequately, and the inversion and invagination technique. In this study, the inversion and invagination technique was used to remove the intersections of staple lines and the lateral intersecting lines (dog-ears) on the anastomotic plane so that this plane becomes analogous to one produced by the single stapling technique (Figs. 4, 6A). Several recent studies have shown that multiple firing of the linear stapler increased the risk of anastomotic leakage after laparoscopic surgery for colorectal cancer.3–6 However, our results are not consistent with the conclusions of these studies; we found that the number of intersections of staple lines (P=0.021) was a risk factor for anastomotic leakage in univariate analysis (Table 2). Therefore, we hypothesize that it is the placement of intersecting staple lines in the double stapling technique that jeopardizes the outcome, and not the number of firings of the linear endostapler.
The anastomotic leakage rate was of 0.78% (1/128) in this study, and this is the lowest of reported rates, which have ranged from 6.3% to 13.7%.5,10–14 This may be because of standardization of all the laparoscopic steps, including the technique used for intracorporeal rectal transection and anastomosis. The performance of the individual surgeon is one possible important risk factor for anastomotic leakage,29 and all cases in this study were performed by a single skilled operator whose learning curves had already reached their plateau.
Tumors in the middle or lower rectum at lower anastomotic levels are generally accepted as important risk factors for anastomotic leakage,4,30,31 which was consistent with our data (distance of tumor from anal verge, P=0.035; distance of anastomosis from anal verge, P=0.033). However, parameters related to the level of tumor or operation were not risk factors for anastomotic leakage in univariate analysis (tumor location, P=0.154; type of operation, P=0.055) (Tables 1, 2). These may be related to a subjective point of view of the endoscopist or operator and contain biased information. This study suggests that a quantification of tumor localization and anastomotic level has significance for assessing the risk of anastomotic leakage.
A number of studies have reported that old age, male sex, obesity, and preoperative chemoradiotherapy are risk factors for anastomotic leakage after colorectal cancer surgery.12,32–35 However, we have identified no patient-related and tumor-related factors that were associated with anastomotic leakage, except for the distance of the tumor from the anal verge (Table 1). Kim et al4 reported that a larger diameter circular stapler decreases blood supply in the rectal remnant, and subsequent ischemia results in anastomotic leakage. Our results differ from their conclusion. Almost all anastomoses were performed by larger diameter circular staplers (≤29 mm; 1/128, 31 mm; 118/128, 33 mm; 8/128, 34 mm; 1/128) and the anastomotic leakage occurred in the 31 mm group. The diameter of the circular stapler was not associated with anastomotic leakage in univariate analysis (P=1.000) (Table 2).
We analyzed the relationship between clinical factors and the number of intersections of staple lines. Multiple intersections of staple lines were associated with preoperative chemoradiotherapy, lower tumor location, short distance between the tumor and the anal verge, lower type of operation, long operation time, blood loss, reinforcement suture, short distance of the anastomosis from the anal verge, and many cartridges for rectal division (Table 3). When compared with upper rectal cancer, laparoscopic surgery for middle and lower rectal cancer was expected to have a longer operating time because it requires of deeper dissection with tumor-specific mesorectal excision. These factors are associated with the level of the tumor and the difficulty of each surgery. This study suggests that laparoscopic colorectal surgery for middle and lower rectal cancer may itself be a risk factor for anastomotic leakage because of the technical difficulty, which can be affected by instrument factors and the performance of an individual surgeon.
In conclusion, because of a very low anastomotic leakage rate, our data did not suggest any independent risk factors for anastomotic leakage after laparoscopic colorectal surgery with a double stapling technique in multivariate analysis. However, we have shown that a short distance of the tumor from the anal verge with a subsequent low anastomosis and intersections of staple lines tends to be associated with anastomotic leakage. Technical efforts to reduce the intersection of staple lines with the inversion and invagination technique may help to avoid anastomotic leakage.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Knight CD, Griffen FD. An improved technique for low anterior resection of the rectum using the EEA stapler. Surgery. 1980;88:710–714. [PubMed] [Google Scholar]
- 2.Cohen Z, Myers E, Langer B, et al. Double stapling technique for low anterior resection. Dis Colon Rectum. 1983;26:231–235. [DOI] [PubMed] [Google Scholar]
- 3.Ito M, Sugito M, Kobayashi A, et al. Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis. 2008;23:703–707. [DOI] [PubMed] [Google Scholar]
- 4.Kim JS, Cho SY, Min BS, et al. Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg. 2009;209:694–701. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Choi GS, Kim SH, et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257:665–671. [DOI] [PubMed] [Google Scholar]
- 6.Kawada K, Hasegawa S, Hida K, et al. Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc. 2014;28:2988–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roumen RM, Rahusen FT, Wijnen MH, et al. “Dog ear” formation after double stapled low anterior resection as a risk factor for anastomotic disruption. Dis Colon rectum. 2000;43:522–525. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki K, Fujino Y, Kanemitsu K, et al. Experimental evaluation of the mechanical strength of stapling techniques. Surg Endosc. 2007;21:1796–1799. [DOI] [PubMed] [Google Scholar]
- 9.Kuroyanagi H, Oya M, Ueno M, et al. Standardized technique of laparoscopic intracorporeal rectal transection and anastomosis for low anterior resection. Surg Endosc. 2008;22:557–561. [DOI] [PubMed] [Google Scholar]
- 10.Kang CY, Halabi WJ, Chaudhry OO, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013;148:65–71. [DOI] [PubMed] [Google Scholar]
- 11.den Dulk M, Marijnen CA, Collette L, et al. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg. 2009;96:1066–1075. [DOI] [PubMed] [Google Scholar]
- 12.Peeters KC, Tollenaar RA, Marijnen CA, et al. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg. 2005;92:211–216. [DOI] [PubMed] [Google Scholar]
- 13.Paun BC, Cassie S, MacLean AR, et al. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010;251:807–818. [DOI] [PubMed] [Google Scholar]
- 14.Snijders HS, Wouters MW, van Leersum NJ, et al. Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur J Surg Oncol. 2012;38:1013–1019. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto S, Fujita S, Akasu T, et al. Risk factors for anastomotic leakage after laparoscopic surgery for rectal cancer using a stapling technique. Surg Laparosc Endosc Percutan Tech. 2012;22:239–243. [DOI] [PubMed] [Google Scholar]
- 16.Akiyoshi T, Ueno M, Fukunaga Y, et al. Incidence of and risk factors for anastomotic leakage after laparoscopic anterior resection with intracorporeal rectal transection and double-stapling technique anastomosis for rectal cancer. Am J Surg. 2011;202:259–264. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa S, Nagayama S, Nomura A, et al. Multimedia article. Autonomic nerve-preserving total mesorectal excision in the laparoscopic era. Dis Colon Rectum. 2008;51:1279–1282. [DOI] [PubMed] [Google Scholar]
- 18.Walker KG, Bell SW, Rickard MJ, et al. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell SW, Walker KG, Rickard MJ, et al. Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg. 2003;90:1261–1266. [DOI] [PubMed] [Google Scholar]
- 20.Hallbook O, Sjodahl R. Anastomotic leakage and functional outcome after anterior resection of the rectum. Br J Surg. 1996;83:60–62. [DOI] [PubMed] [Google Scholar]
- 21.Fielding LP, Stewart-Brown S, Blesovsky L, et al. Anastomotic integrity after operations for large-bowel cancer: a multicenter study. Br Med J. 1980;281:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karanjia ND, Corder AP, Bearn P, et al. Leakage from stapled low anastomosis after total mesorectal excision for carcinoma of the rectum. Br J Surg. 1994;81:1224–1226. [DOI] [PubMed] [Google Scholar]
- 23.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomized controlled trial. Lancet. 2005;365:1718–1726. [DOI] [PubMed] [Google Scholar]
- 24.Zhou ZG, Hu M, Li Y, et al. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc. 2004;18:1211–1215. [DOI] [PubMed] [Google Scholar]
- 25.Hartley JE, Mehigan BJ, Qureshi AE, et al. Total mesorectal excision: assessment of the laparoscopic approach. Dis Colon Rectum. 2001;44:315–321. [DOI] [PubMed] [Google Scholar]
- 26.Anthuber M, Fuerst A, Elser F, et al. Outcome of laparoscopic surgery for rectal cancer in 101 patients. Dis Colon Rectum. 2003;46:1047–1053. [DOI] [PubMed] [Google Scholar]
- 27.Feliciotti F, Guerrieri M, Paganini AM, et al. Long-term results of laparoscopic versus open resections for rectal cancer for 124 unselected patients. Surg Endosc. 2003;17:1530–1535. [DOI] [PubMed] [Google Scholar]
- 28.Asao T, Kuwano H, Nakamura J, et al. Use of a mattress suture to eliminate dog ears in double-stapled and triple-stapled anastomoses. Dis Colon Rectum. 2002;45:137–139. [DOI] [PubMed] [Google Scholar]
- 29.Tang R, Chen HH, Wang YL, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single-center prospective study of 2,809 consecutive patients. Ann Surg. 2001;234:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberl T, Jagoditsch M, Klingler A, et al. Risk factors for anastomotic leakage after resection for rectal cancer. Am J Surg. 2008;196:592–598. [DOI] [PubMed] [Google Scholar]
- 31.Martel G, Al-Suhaibani Y, Moloo H, et al. Neoadjuvant therapy and anastomotic leak after tumor-specific mesorectal excision for rectal cancer. Dis Colon Rectum. 2008;51:1195–1201. [DOI] [PubMed] [Google Scholar]
- 32.Jung SH, Yu CS, Choi PW, et al. Risk factors and oncologic impact of anastomotic leakage after rectal cancer surgery. Dis Colon Rectum. 2008;51:902–908. [DOI] [PubMed] [Google Scholar]
- 33.Law WI, Chu KW, Ho JW, et al. Risk factors for anastomotic leakage after low anterior resection with total mesorectal excision. Am J Surg. 2000;179:92–96. [DOI] [PubMed] [Google Scholar]
- 34.Moran BJ, Heald RJ. Risk factors for and management of anastomotic leakage in rectal surgery. Colorectal Dis. 2001;3:135–137. [DOI] [PubMed] [Google Scholar]
- 35.Alberts JC, Parvaiz A, Moran BJ. Predicting risk and diminishing the consequences of anastomotic dehiscence following rectal resection. Colorectal Dis. 2003;5:478–482. [DOI] [PubMed] [Google Scholar]


