Abstract
Background
ADVANCE (NCT01304836) was a phase 4, multicenter, prospectively randomized, open-label, 24-week study comparing the incidence of posttransplantation diabetes mellitus (PTDM) with 2 prolonged-release tacrolimus corticosteroid minimization regimens.
Methods
All patients received prolonged-release tacrolimus, basiliximab, mycophenolate mofetil and 1 bolus of intraoperative corticosteroids (0-1000 mg) as per center policy. Patients in arm 1 received tapered corticosteroids, stopped after day 10, whereas patients in arm 2 received no steroids after the intraoperative bolus. The primary efficacy variable was the diagnosis of PTDM as per American Diabetes Association criteria (2010) at any point up to 24 weeks postkidney transplantation. Secondary efficacy variables included incidence of composite efficacy failure (graft loss, biopsy-proven acute rejection or severe graft dysfunction: estimated glomerular filtration rate (Modification of Diet in Renal Disease-4) <30 mL/min per 1.73 m2), acute rejection and graft and patient survival.
Results
The full-analysis set included 1081 patients (arm 1: n = 528, arm 2: n = 553). Baseline characteristics and mean tacrolimus trough levels were comparable between arms. Week 24 Kaplan–Meier estimates of PTDM were similar for arm 1 versus arm 2 (17.4% vs 16.6%; P = 0.579). Incidence of composite efficacy failure, graft and patient survival, and mean estimated glomerular filtration rate were also comparable between arms. Biopsy-proven acute rejection and acute rejection were significantly higher in arm 2 versus arm 1 (13.6% vs 8.7%, P = 0.006 and 25.9% vs 18.2%, P = 0.001, respectively). Tolerability profiles were comparable between arms.
Conclusions
A prolonged-release tacrolimus, basiliximab, and mycophenolate mofetil immunosuppressive regimen is efficacious, with a low incidence of PTDM and a manageable tolerability profile over 24 weeks of treatment. A lower incidence of biopsy-proven acute rejection was seen in patients receiving corticosteroids tapered over 10 days plus an intraoperative corticosteroid bolus versus those receiving an intraoperative bolus only.
This large, multicenter, prospective and randomized study shows no difference in the incidence of posttransplantation diabetes mellitus but higher incidence of acute rejection with a steroid avoidance versus a steroid sparing regimen in kidney transplant recipients receiving basiliximab, prolonged-release tacrolimus and mycophenolate.
The prevalence of posttransplantation diabetes mellitus (PTDM) has increased in recent years and remains high, despite therapeutic advances that have significantly improved graft and patient survival outcomes.1-5 Development of PTDM occurs primarily in the first 3 to 6 months posttransplant and has been associated with an increased risk of cardiovascular events and poor graft and patient survival.2,4-7
In addition to demographic factors, such as increasing age and higher body mass index, risk factors associated with PTDM in de novo kidney transplantation include the type of immunosuppressive regimen used, which is estimated to account for 74% of the risk of PTDM during the first year posttransplant.2,4,5,8 Steroid use has been associated with an increased risk of PTDM, whereas steroid cessation or withdrawal protocols have been associated with a significant decrease.9-11 Some studies have reported that the absence of steroid treatment was associated with increased rates of acute rejection (AR), although others have found rates of rejection to be comparable.9,11-15
In the optimizing immunosuppression after kidney transplantation with Advagraf (OSAKA) study, patients who received perioperative corticosteroids only, together with once-daily, prolonged-release tacrolimus at an initial dose of 0.2 mg/kg per day, plus basiliximab and mycophenolate mofetil (MMF) had comparable biopsy-confirmed AR rates and received significantly less anti-diabetic and insulin treatment at week 24 compared with patients receiving corticosteroids tapered over 24 weeks, although the rates of PTDM were comparable between arms.15 Definitions of PTDM vary considerably between studies and, as in the OSAKA study, often include reporting of PTDM as an adverse event (AE) without predefined criteria.1,15 Consequently, this makes meaningful comparisons of the incidence of PTDM between studies difficult. Optimization of immunosuppressive regimens to reduce the risk of the occurrence of PTDM, while maintaining efficacy, remains an ongoing challenge for transplant physicians, especially as an increased risk of PTDM has been associated with the use of tacrolimus, sirolimus, and everolimus.9,15-18
Advagraf-based immunosuppression regimen examining new onset diabetes mellitus in kidney transplant recipients (ADVANCE) was designed specifically to investigate the incidence of PTDM with prolonged-release tacrolimus-based immunosuppression in de novo kidney transplant recipients. The 24-week study was designed to determine whether prolonged-release tacrolimus-based regimens with posttransplant steroids tapered over a 10-day period (arm 1) or no postoperative steroids (arm 2) affected the incidence of PTDM differently. In this study, PTDM was defined using the comprehensive American Diabetes Association (ADA) criteria as recommended by the Kidney Disease Improving Global Outcomes clinical practice guidelines.19,20
MATERIALS AND METHODS
Study Design (ClinicalTrials.gov; NCT01304836)
ADVANCE was a phase 4, multicenter, 24-week, prospectively randomized, open-label, parallel-group study conducted at 99 sites in 24 countries between January 2011 and May 2013. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonization guidelines and applicable laws and regulations. An independent ethics committee or institutional review board granted approval before shipment of medication to the study sites. Written informed consent was obtained from all participants.
Eligible patients (≥18 years old) underwent primary renal transplantation or retransplantation (unless the graft was lost within 1 year due to immunological reasons). Patients who received an organ transplant other than a kidney, or in cases where donation occurred after cardiac death, were also excluded. Other reasons for exclusion from the study included a positive test for hepatitis B or C, or a cold ischemia time greater than 30 hours. Patients were also excluded if they had a panel-reactive antibody (PRA) score greater than 20% (highest PRA value in 6 months before transplant used for all PRA measurements, measured according to individual center policy), significant liver disease, or a diagnosis of diabetes mellitus before transplantation. Patients were considered to have been diagnosed with diabetes mellitus if they had previously been treated with prescribed medication or controlled diet for diabetes mellitus, if there was evidence of a previous positive oral glucose tolerance test (OGTT), prebaseline glycated hemoglobin (HbA1c) of 6.5% or greater, or gestational diabetes recorded in the patients' medical history. Patients receiving ongoing systemic immunosuppressive drugs before transplantation (with the exception of minimal levels of immunosuppressant after a previous failed transplantation without nephrectomy) and those requiring long-term steroid treatment were also excluded.
Randomization
The randomization scheme was prepared by Pierrel Research Europe GmbH, Essen, Germany, under the responsibility of Astellas Pharma Europe BV, Netherlands. Randomization and distribution of the study medication was coordinated centrally using an interactive voice response system. Eligible patients were randomized (1:1) to 1 of 2 treatment arms and stratified according to study center.
Procedure
All patients received prolonged-release tacrolimus (Advagraf; Astellas Pharma Europe BV, Netherlands), intravenous basiliximab (20 mg on day 0 and day 4) and oral MMF (1 g twice-daily [BD] preoperatively and until day 14; 0.5 g BD thereafter). Prolonged-release tacrolimus (0.1 mg/kg) was administered preoperatively; 0.2 mg/kg was administered for the initial postoperative dose. Postoperative, prolonged-release tacrolimus was taken orally once a day in the morning with the dose adjusted on the basis of clinical evidence and tolerability (target trough levels 11-15 ng/mL on days 0-21, 8-12 ng/mL on days 22-42, and 5-9 ng/mL on days 43-168).
The use of an intraoperative, intravenous bolus of corticosteroid was permitted in both arms on day 0. The bolus dose was according to center policy (up to a maximum of 1000 mg of methylprednisolone or equivalent); all patients at a participating center received the same dose. In arm 1, postoperative prednisolone (or equivalent oral corticosteroid) was administered for 10 days (20 mg/day on days 1-4, 15 mg/day on days 5-6, 10 mg/day on days 7-8, and 5 mg/day on days 9-10) and then discontinued after day 10; the total cumulative mandatory corticosteroid dose per patient in arm 1 was 140 mg. In arm 2, routine steroid administration was not permitted postoperatively.
To summarize the treatment arms:
Arm 1: prolonged-release tacrolimus plus basiliximab and MMF (intraoperative corticosteroid bolus as per center policy; mandatory tapered corticosteroids to day 10; discontinued after day 10).
Arm 2: prolonged-release tacrolimus plus basiliximab and MMF (intraoperative corticosteroid bolus as per center policy only).
If a rejection episode was suspected, the onset of the clinical, laboratory, or histological signs of the rejection episode was considered AR; a biopsy was performed and evaluated by a local histopathologist, before intervention, to confirm the AR, and the Banff 2007 classification of renal allograft pathology used to evaluate the grade.21 Rejection episodes diagnosed as acute antibody-mediated rejection (AMR) (grades I, II, or III) or acute T cell-mediated rejection (TCMR) (grades I, II, or III) according to the Banff 2007 criteria, were considered biopsy-proven AR (BPAR).21 In both treatment arms, a first rejection episode was treated with intravenous steroids 500-1000 mg once daily for 3 days; additional oral steroids were not permitted during or after this period. Patients in arm 1 continued to receive oral steroids as per protocol. Patients receiving treatment for AR were permitted to remain in the study. Subsequent episodes of AR were treated according to center policy. Steroid resistance was identified according to each investigator. Cytomegalovirus and Pneumocystis jiroveci pneumonia prophylaxes were administered as per each center's local policy. Routine laboratory assessments were performed locally; urine analysis for protein concentration was undertaken at week 24 only.
Primary Efficacy Variable
The primary efficacy variable was the incidence of PTDM, diagnosed as per ADA-suggested criteria. The ADA criteria for diagnosis of diabetes mellitus include HbA1c of 6.5% or greater, fasting plasma glucose of 7.0 mmol/L or greater, 2-hour plasma glucose of 11.1 mmol/L or greater during an OGTT or, for a patient with classic symptoms of hyperglycemia, a random plasma glucose of 11.1 mmol/L or greater.19 HbA1c and plasma glucose were assessed on an ongoing basis throughout the study period. An OGTT was performed at weeks 8 and 24 only. The primary analysis compared the incidence of PTDM at week 24 between arms.
Secondary Efficacy Variables
Secondary efficacy variables relating to metabolic effects included incidence of 2-hour plasma glucose of 11.1 mmol/L or greater during the OGTT at weeks 8 and 24 and mean change from baseline in HbA1c levels at weeks 12 and 24. The incidence of composite efficacy failure (defined as graft loss, BPAR or severe graft dysfunction) and composite efficacy failure including PTDM were assessed at week 24. Other outcomes assessed included incidence of patient survival and AR. Renal function at week 24 was assessed by estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease-4 (MDRD4) formula and by creatinine clearance calculated using the Cockcroft–Gault formula. Delayed graft function (DGF) was defined as dialysis for more than 1 day during days 1 to 7, and severe graft dysfunction was defined as eGFR (MDRD4) less than 30 mL/min per 1.73 m2.
Additional Analyses
Mean and median exposure to steroids were reported in the subgroups of patients with and without PTDM and with and without AR. Post hoc Kaplan–Meier analyses were performed to assess the incidence of PTDM in the subgroups of patients with and without AR.
Statistical Analysis
The safety-analysis set (SAF) included all patients who received 1 dose or greater of study medication. The intention-to-treat (ITT) population included all patients who were randomized and transplanted. The full-analysis set (FAS) included patients who were randomized, received a transplant, received 1 dose or greater of study drug, and recorded 1 postbaseline or greater estimation of the primary variable (all other patients were excluded from this population). The per-protocol set (PPS) population included all patients from the FAS population who did not have any major protocol deviations. A 33% reduction in the incidence of PTDM was considered clinically relevant. Assuming an incidence of PTDM of 20% in arm 1 (reference arm), a sample size of 548 patients per arm provided 80% power to detect a clinically relevant 6.5% difference in PTDM incidence (that is, a reduction to 13.5% in arm 2) using a 2-sided type 1 error rate of 5%. The number of randomized and transplanted patients required to reach the planned number of evaluable patients, assuming a dropout rate of 6%, was 1166 patients (583 per arm). For all comparisons, a P value less than 0.05 was considered statistically significant.
For the primary efficacy variable, the incidence of PTDM was analyzed by Kaplan–Meier procedures using the FAS population; the differences between arms were assessed using 2-sided 95% confidence intervals (CIs), and P values were calculated using the Wilcoxon–Gehan test. The primary analysis was repeated using the PPS population.
Secondary efficacy variables were analyzed using the intention-to-treat population. Kaplan–Meier estimates of composite efficacy failure, composite efficacy failure including PTDM, graft and patient survival, and BPAR at week 24 were calculated for each treatment arm. Differences between arms (arm 1–arm 2 for event-free survival) were assessed, with the corresponding 95% CIs and P values calculated using the Wilcoxon–Gehan test, and were confirmed using the χ2 test. Differences between arms in eGFR (MDRD4), creatinine clearance (Cockcroft–Gault), and mean change in HbA1c from baseline were assessed by analysis of variance. The incidence of graft dysfunction was analyzed using a Cox proportional-hazards model. The incidence of patients with DGF and 2-hour plasma glucose ≥11.1 mmol/L during the OGTT was analyzed using the χ2 test.
For the additional post-hoc analyses, the incidence of PTDM in subgroups of patients defined by AR status was analyzed over 24 weeks using the FAS. P values were calculated using the Wilcoxon–Gehan test. Tolerability analyses based on adverse events, laboratory parameters, and vital signs were assessed using the SAF population.
RESULTS
Patient and Donor Demographics
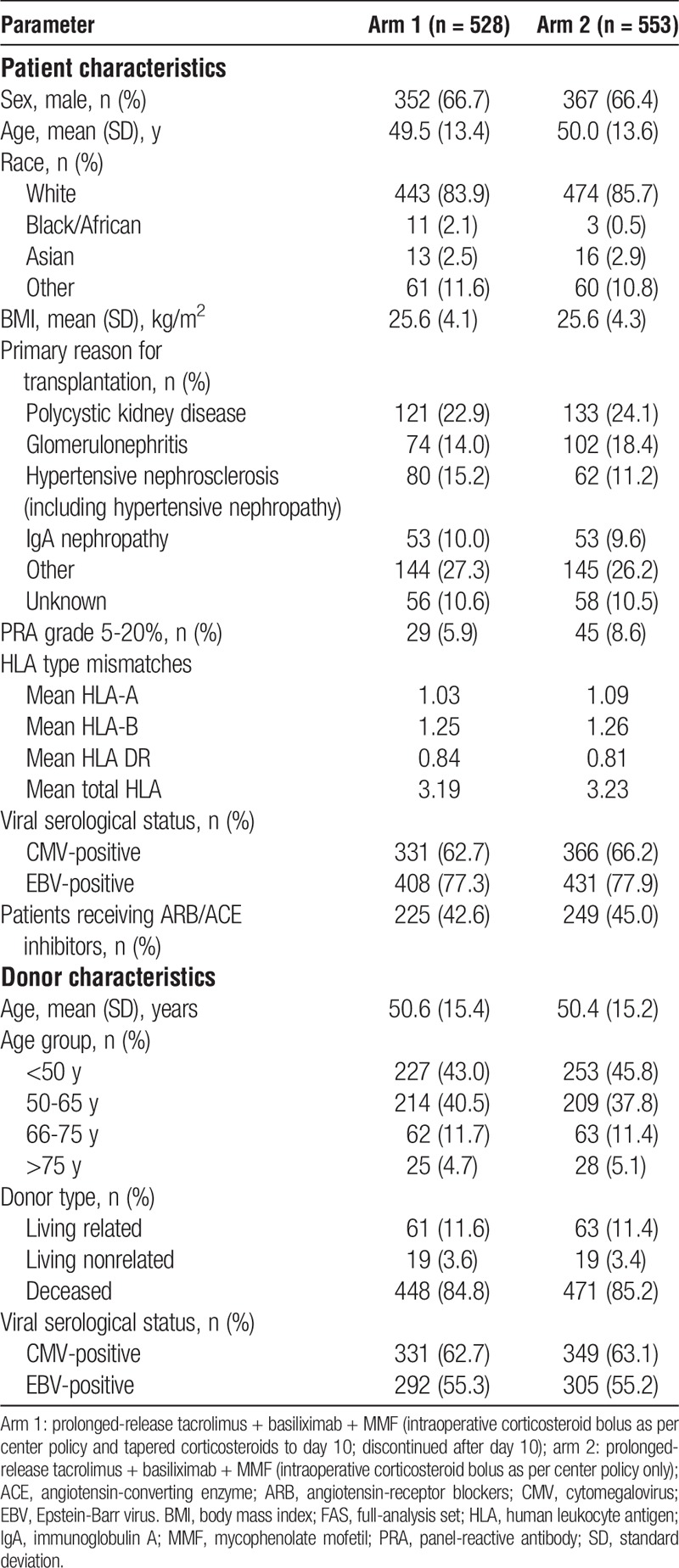
Overall, 1166 patients were randomized, and 1138 (97.6%) received 1 dose or greater of study medication. The FAS comprised 1081 patients; 14.6% and 16.5% in arms 1 and 2, respectively, discontinued study treatment prematurely, primarily due to AEs (Figure 1). Baseline patient and donor characteristics were comparable between arms (Table 1).
FIGURE 1.
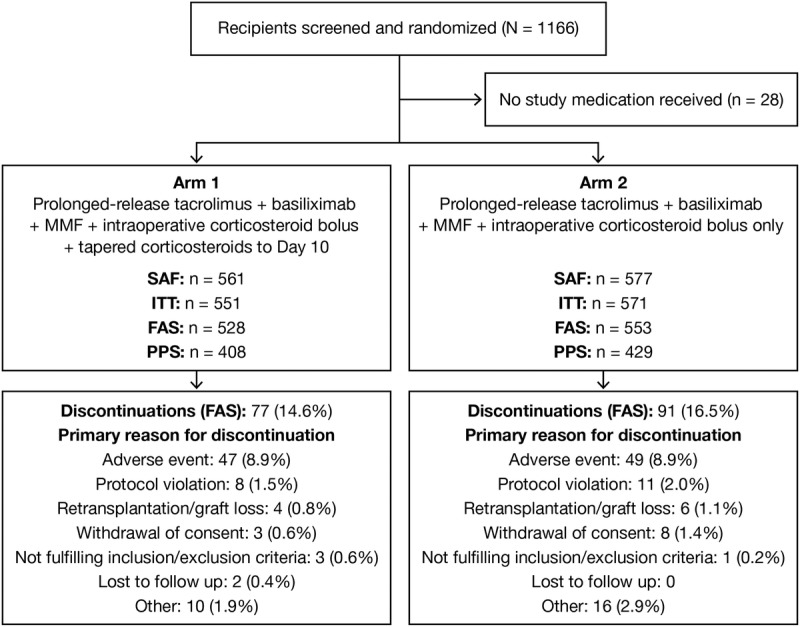
Flow of patients through the study to Week 24. SAF: randomized patients receiving 1 dose or greater of any study medication; ITT: patients randomized, transplanted and receiving 1 dose or greater of any study medication; FAS: patients enrolled in the study, transplanted, received 1 dose or greater of any study medication and 1 or greater postbaseline estimation of primary variable (all other patients were excluded from this population); PPS: all patients from FAS with no major protocol deviation. Patient discontinuations were analyzed using the FAS population; study completers: arm 1, 451; arm 2, 462. FAS, full-analysis set; ITT, intention-to-treat; PPS, per-protocol set; SAF, safety-analysis set.
TABLE 1.
Baseline patient and donor characteristics in each treatment arm (FAS)
Dosing and Exposure
Prolonged-release tacrolimus doses were similar between arms throughout the study, with mean (standard deviation [SD]) doses on day 0 of 0.13 (0.05) mg/kg in arm 1 and 0.14 (0.05) mg/kg in arm 2; and at week 24 of 0.09 (0.06) mg/kg in arm 1 and 0.09 (0.07) mg/kg in arm 2 (Figure 2A). Mean (SD) tacrolimus trough levels were also similar between arms throughout the study. Target trough levels were readily achieved early posttransplant (day 1: 10.5 (7.1) ng/mL in arm 1 and 11.3 (8.7) ng/mL in arm 2) (Figure 2B) and were generally within the recommended target range throughout the study. Mean doses of MMF and basiliximab were comparable between arms.
FIGURE 2.
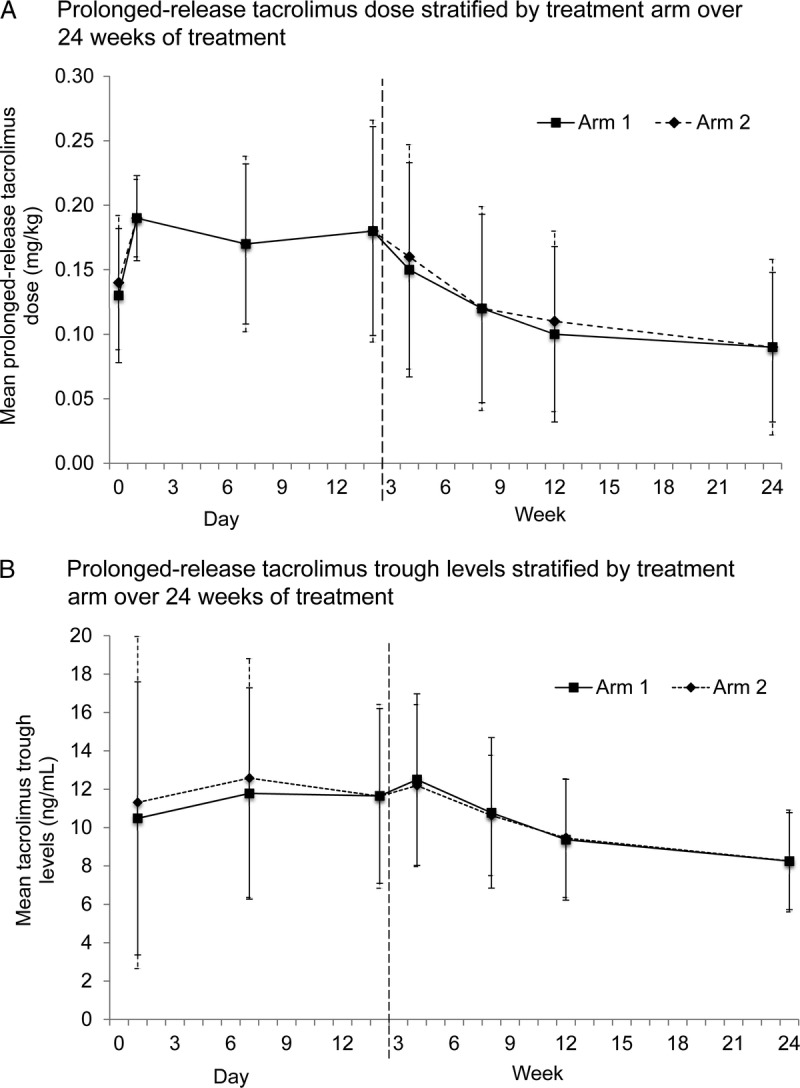
Prolonged-release tacrolimus (A) dose and (B) trough levels stratified by treatment arm over 24 weeks of treatment (FAS). Arm 1: prolonged-release tacrolimus + basiliximab + MMF (intraoperative corticosteroid bolus as per center policy and tapered corticosteroids to day 10; discontinued after day 10), arm 2: prolonged-release tacrolimus + basiliximab + MMF (intraoperative corticosteroid bolus as per center policy only); error bars represent standard deviation; dashed vertical line represents change from days to weeks. FAS, full-analysis set; MMF, mycophenolate mofetil.
Overall, 99.8% and 97.1% of patients in arms 1 and 2, respectively, received an intraoperative bolus of steroid on day 0 (1 patient in arm 1 and 16 patients in arm 2 did not receive intraoperative steroids). Median doses were comparable between arms (Table 2). On day 1, 99.2% versus 2.9% of patients received steroids in arm 1 versus arm 2; during days 1 to 10 up to 99.6% versus 10.5% received steroids. Of the patients in arm 2, 44 (8.1%) patients with AR and 13 (2.4%) without AR received steroids.
TABLE 2.
Summary of corticosteroid doses at week 24 (FAS)
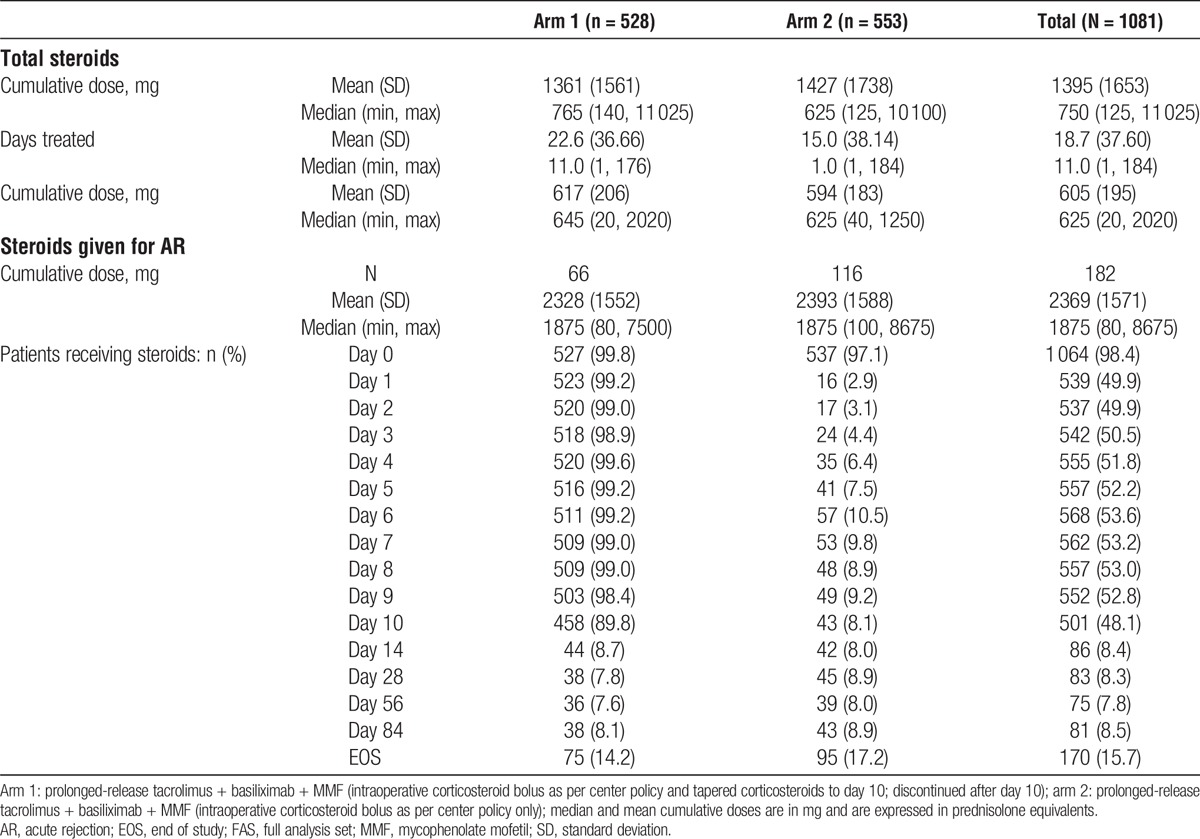
The median number of days treated with steroid was 11 days in arm 1 and 1 day in arm 2; the median cumulative dose of steroid at the end of the study was higher in arm 1 versus arm 2 (765 mg vs 625 mg; difference: 140 mg). Mean doses are also reported in Table 2. A higher number of patients received steroids for AR in arm 2 compared with arm 1 (116 [21.0%] vs 66 [12.5%]; Table 2). However, the median cumulative steroid dose given to patients with AR was comparable between arm 1 and arm 2 (2941 mg vs 2813 mg) (Table 3).
TABLE 3.
Cumulative corticosteroid doses in patients with and without PTDM, and patients with and without AR at week 24 (FAS)
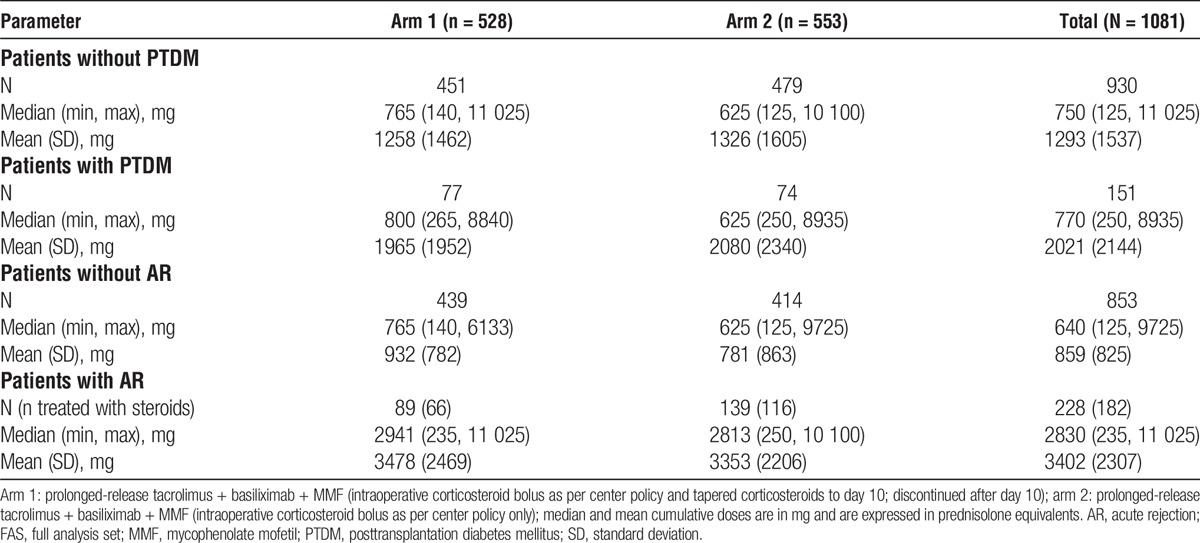
In total, 0.4% of patients received treatment for diabetes before the start of the study (0.5% vs 0.3% in arm 1 vs arm 2, respectively). By week 24, 28.1% of patients had received treatment for diabetes (29.1% vs 27.2%).
Primary Efficacy Variable
There were no significant differences between arms in the Kaplan–Meier estimates of PTDM at week 24 (17.4% vs 16.6% in arm 1 vs arm 2, respectively; difference, 0.8%; 95% CI, −6.0 to 4.0; P = 0.579) (Figure 3A). Secondary analyses using the PPS confirmed these findings (14.8% vs 16.1% in arm 1 vs arm 2; difference: −1.4%; 95% CI, −4.0 to 7.0; P = 0.514).
FIGURE 3.
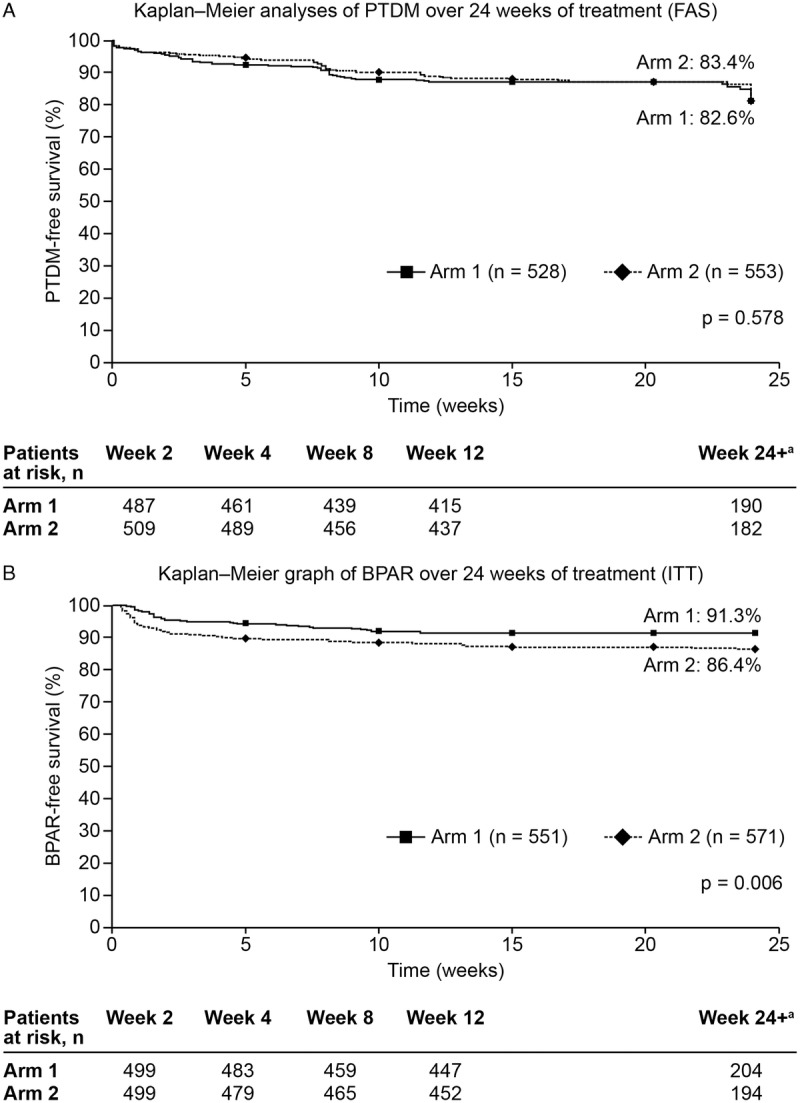
Kaplan–Meier analyses of (A) PTDM (FAS) and (B) BPAR (ITT) over 24 weeks of treatment. Arm 1: prolonged-release tacrolimus + basiliximab + MMF (intraoperative corticosteroid bolus as per center policy and tapered corticosteroids to day 10; discontinued after day 10); arm 2: prolonged-release tacrolimus + basiliximab + MMF (intraoperative corticosteroid bolus as per center policy only); aPatients who completed the 24-week follow-up period and discontinued at week 24 with no PTDM events recorded were not included in the “at risk” numbers for week 24+ (n = 61 and n = 63 in arms 1 and 2, respectively); events that occurred on or after week 24 were included in the week 24 timepoint; analyses of PTDM were performed on the FAS, whereas BPAR analyses were performed on the ITT. BPAR, biopsy-proven acute rejection; eGFR, estimated glomerular filtration rate; FAS, full-analysis set; ITT, intention-to-treat; MDRD4, Modification of Diet in Renal Disease-4; MMF, mycophenolate mofetil; PTDM, posttransplantation diabetes mellitus.
The most commonly first-met ADA criterion for diagnosis of PTDM was fasting plasma glucose ≥7.0 mmol/L (44.2% and 36.5% in arms 1 and 2, respectively). Two-hour plasma glucose of 11.1 mmol/L or greater during an OGTT was the next most commonly first-met ADA criterion (29.9% and 36.5%), followed by HbA1c of 6.5% or greater (19.5% and 20.3%) and then symptoms of hyperglycemia with random plasma glucose of 11.1 mmol/L or greater (6.5% and 6.8%).
Secondary Efficacy Variables
Glucose Metabolism
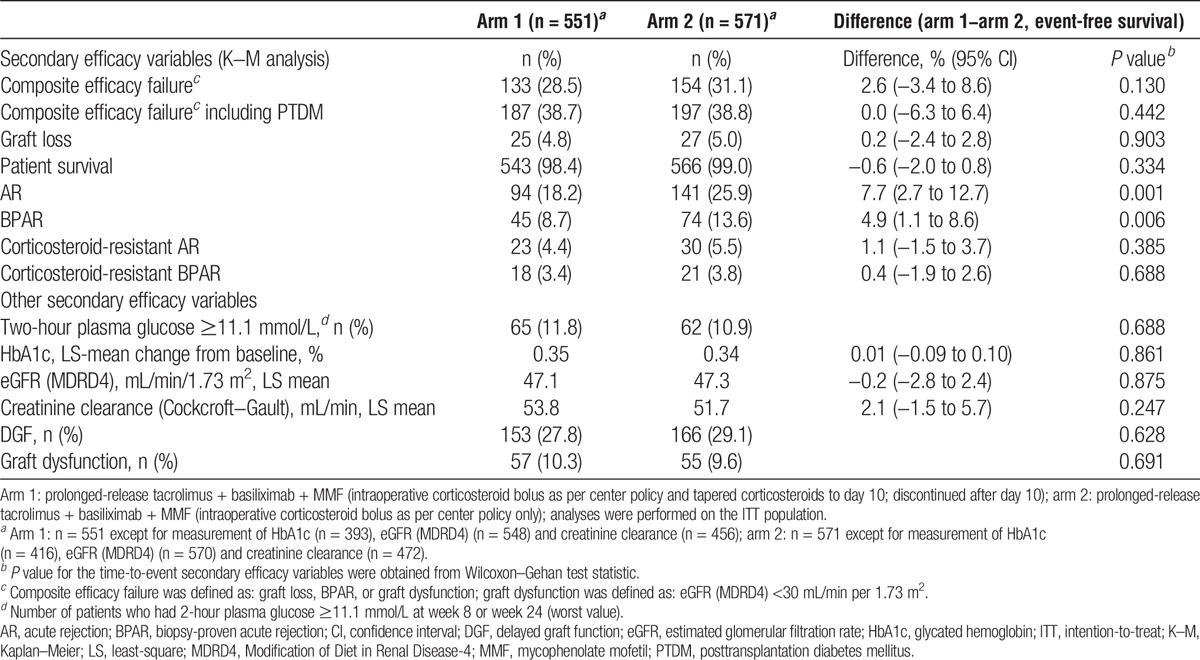
Mean (SD) 2-hour plasma glucose levels in arms 1 and 2 were similar at week 8 (8.15 [2.74] mmol/L vs 8.16 [2.83] mmol/L) and week 24 (7.26 [2.46] mmol/L vs 7.33 [2.50] mmol/L). There was no significant difference between arms in the proportion of patients with 2-hour plasma glucose levels of 11.1 mmol/L or greater at week 8 or week 24 (highest incidence; P = 0.688) (Table 4). The mean change from baseline in HbA1c levels was also comparable between arms at week 12 (0.15% vs 0.19%; difference, −0.04; 95% CI, −0.13 to 0.06; P = 0.445) and week 24 (P = 0.861) (Table 4).
TABLE 4.
Summary of secondary efficacy variables at week 24 (ITT)
Efficacy Measures
At week 24, there was no significant difference between arms in the incidence of composite efficacy failure (P = 0.130) or composite efficacy failure including PTDM (P = 0.442). Likewise, for the incidence of graft loss (P = 0.903) and patient survival (P = 0.334) (Table 4).
In the Kaplan–Meier analyses, significantly more patients in arm 2 versus arm 1 experienced AR (25.9% vs 18.2%; P = 0.001) and BPAR (13.6% vs 8.7%; P = 0.006) over 24 weeks of treatment (Figure 3B); the difference between arms was observed early posttransplant (AR at day 7: 9.6% vs 3.9% in arm 1 vs arm 2, respectively). However, the incidence of corticosteroid-resistant AR or BPAR was comparable between arms (Table 4). Among the 45 patients in arm 1 with BPAR, there were 34 (75.6%; 39 episodes) TCMRs and 15 (33.3%; 15 episodes) AMRs (TCMR: grade 2b, 4 [11.8%], grade 3: 1 [2.9%]; AMR: grade 2, 9 [60.0%], grade 3, 0). In arm 2, of the 74 patients with BPAR, 65 (87.8%, 75 episodes) experienced TCMR and 15 (20.3%, 17 episodes) experienced AMR (TCMR: grade 2b, 4 [6.2%]; grade 3, 2 [3.1%]; AMR: grade 2, 5 [33.3%]; grade 3, 2 [13.3%]).
Other Secondary Efficacy Variables
There were no significant differences between arms at week 24 in eGFR (MDRD4) (P = 0.875) or creatinine clearance (Cockcroft–Gault) (P = 0.247). The incidences of DGF and graft dysfunction were also comparable between arms (P = 0.628; P = 0.691) (Table 4).
Additional Analyses (FAS)
Median cumulative steroid doses were comparable, but mean doses were higher in patients who developed PTDM by week 24 versus those who did not (Table 3). In patients with AR, the Kaplan–Meier estimates of PTDM were 36.6% in arm 1 versus 30.5% in arm 2 (difference, −6.1%; 95% CI, −21.0 to 9.0; P = 0.446), and in patients without AR, the estimates of PTDM were 13.6% versus 12.4% (difference, −1.2%; 95% CI, −6.0 to 4.0; P = 0.439). Overall, patients who experienced AR received a higher median cumulative steroid dose versus those who did not experience AR (Table 3). In patients with BPAR, there was a higher incidence of PTDM in arm 1 versus arm 2 (47.5% vs 28.2%, respectively; difference, −19.4%; 95% CI, −41.0 to 3.0; P = 0.049); however, there was no significant difference in the incidence of PTDM between arms for patients without BPAR (14.9% vs 15.0%; difference, 0.2%; 95% CI, −5.0 to 5.0; P = 0.856).
Tolerability Outcomes (SAF)
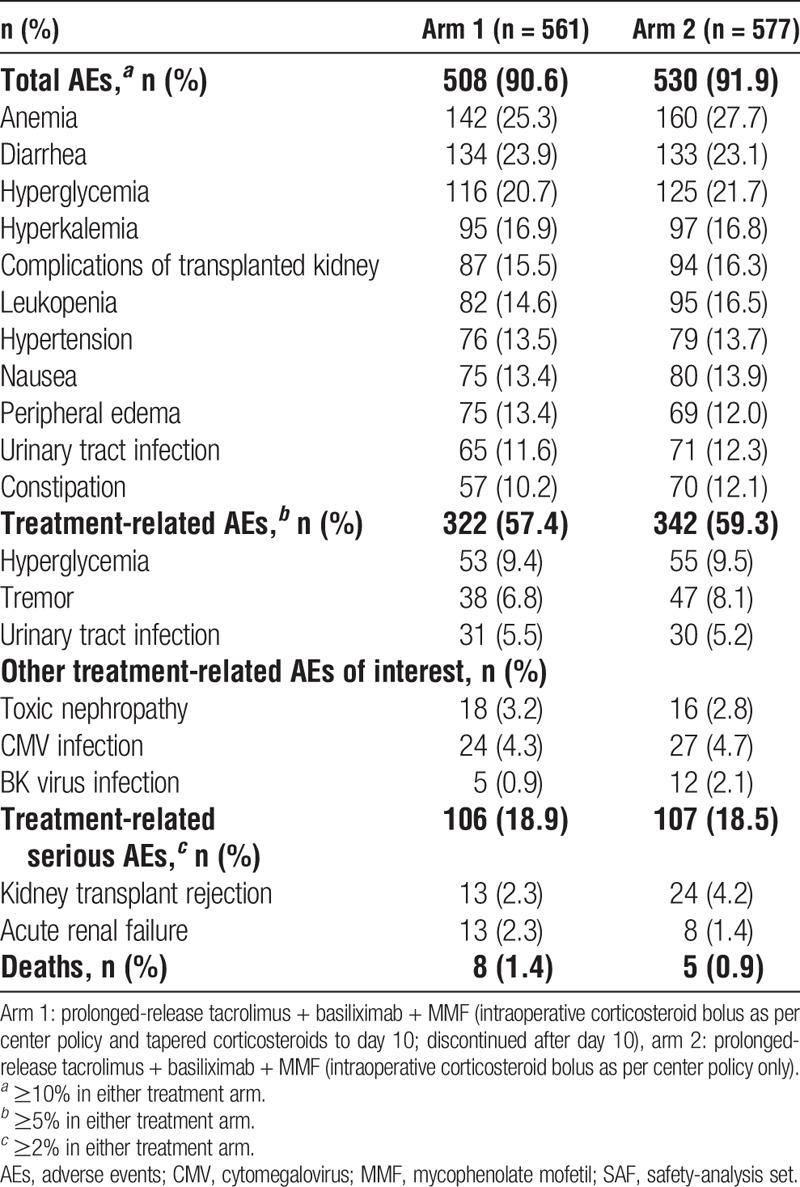
There was a comparable incidence of treatment-related AEs (57.4% vs 59.3%) and serious treatment-related AEs (46.9% vs 45.4%) in arm 1 versus arm 2, respectively (Table 5). Urine protein levels were similar in arms 1 and 2 at end of study (0.25 vs 0.26 mg/L, respectively). A total of 13 patients (1.1%) died during the study: 8 (1.4%) in arm 1 and 5 (0.9%) in arm 2. Seven of these deaths occurred during the study, and 6 occurred after premature withdrawal from the study during the follow-up period (until 24 weeks posttransplant). These deaths were attributed to cardiocirculatory failure, pulmonary embolism, intra-abdominal bleeding, acute hepatitis, acute infarction, heart infarction, pneumonia, respiratory insufficiency, and sepsis. In 1 patient, the cause of death was unknown.
TABLE 5.
Summary of adverse events to week 24 (SAF)
DISCUSSION
The results from the ADVANCE study showed that prolonged-release tacrolimus-based immunosuppression, administered with basiliximab and MMF, is efficacious and associated with a manageable tolerability profile and a low incidence of PTDM over 24 weeks of treatment. Prolonged-release tacrolimus plus MMF and a 10-day steroid tapering regimen had a lower incidence of BPAR, a specific risk factor for PTDM, versus prolonged-release tacrolimus plus MMF and a single bolus of steroid.
It has been reported previously that most PTDM occurs in the first 3 months after transplantation and that the choice of immunosuppressive regimen (including the use of steroids and tacrolimus) accounts for a large proportion of diabetes risk posttransplant.4 Previously, comparable AR rates were reported with tacrolimus plus MMF and induction therapy in a steroid-free regimen compared with tacrolimus plus MMF and corticosteroids.15 However, another study associated steroid withdrawal with an increased risk of AR and poorer long-term graft outcomes.22 The regimens in our study were designed to balance the benefit-to-risk ratio associated with steroid use and to assess optimal steroid administration in combination with prolonged-release tacrolimus plus MMF early posttransplant.
Overall, the incidence of PTDM in this study was lower than anticipated, and generally comparable to published data with prolonged-release tacrolimus-based regimens, although reported incidence varies depending on the definition of PTDM and length of follow-up.1,15,23,24 Previous studies have reported PTDM as an AE, often as a post hoc analysis,15,23 or use insulin 30 days or longer as a diagnostic criterion, which could underestimate the incidence of PTDM.25 In contrast, the use of 1 measurement or more of fasting plasma glucose of 7 mmol/L or more to diagnose PTDM in other trials could overestimate the incidence.1,24 ADVANCE was the first large, randomized controlled trial of its kind to use a stringent definition of PTDM including OGTT. Therefore, the low incidence of PTDM in this study, with prolonged-release tacrolimus-based immunosuppression, is likely to be reflective of the “real world.”
When assessing the impact of treatment on the study outcomes, it is interesting to note that the mean cumulative steroid dose was higher in arm 2 compared with arm 1. Post hoc analyses of those patients with PTDM showed a similar pattern between arms. It is likely that the higher incidence of AR and BPAR reported in arm 2 versus arm 1, necessitating additional steroid treatment could, at least in part, account for this observation. Additional analyses confirmed that patients with AR received a mean cumulative dose of steroid approximately 4-fold higher than patients without AR, regardless of treatment arm. Because the incidence of PTDM was comparable between arms at the end of the study, these data indicate that 3 boluses of corticosteroid represent a risk factor for the development of PTDM in kidney transplant recipients receiving prolonged-release tacrolimus and basiliximab plus MMF. The findings from this study are consistent with the previously described association between steroid use and PTDM occurrence2,4 and suggest that 10 days of tapered corticosteroids postkidney transplant reduces the risk of PTDM by reducing the incidence of AR and BPAR, and consequently the need for pulse steroids.
Overall, the incidences of BPAR and AR were low in both arms and consistent with previously published data.9,15,23 The incidence of BPAR in this study was lower than a study of kidney transplant recipients treated with tacrolimus BD-based regimens, with or without steroid treatment.9 Our results showed no significant differences in the incidence of corticosteroid-resistant BPAR between arms. However, AR and BPAR were significantly lower in arm 1 versus arm 2 at week 24, suggesting that a prolonged-release tacrolimus-based regimen with a short period of posttransplant steroid treatment could be effective while minimizing the risk of PTDM. Interestingly, a meta-analysis of 34 studies by Knight and Morris22 reported that steroid avoidance or withdrawal protocols postkidney transplant, used alongside various immunosuppressive regimens, increased the risk of AR but decreased cardiovascular risk.
The incidence of composite efficacy failure was comparable between arms. Graft and patient survival at week 24 was good, and comparable, both between arms and with previously published studies (despite low steroids limited to 10 days).15,23 There was also no significant difference in the incidence of DGF between arms; however, there was a higher incidence of AR by day 7 in arm 2, indicating that patients with DGF could benefit from steroid administration, beyond a single bolus, in the early posttransplant period. This observation is supported by a recent review of clinical trials that found a reduced risk of DGF when steroids were used for the first 3 to 7 days posttransplantation versus steroid-free protocols.26
This study had limitations, including the fact that the patients, the majority of whom were white, were generally at low immunological risk. Therefore, the population may not have been representative of other transplant populations. Moreover, the open-label design and the relatively short (6-month) duration of follow-up could have affected the results. It is important to note, however, that the study included the first 3 to 6 months after transplantation when PTDM typically develops. Intraoperative steroids were permitted as per center policy, that is, all patients in each center received the same intraoperative dose. However, the differences in the minimum and maximum values of intraoperative steroid dose suggest that there could have been a number of protocol violations. The more frequent use of steroid treatment for AR and BPAR in the minimized steroid arm (arm 2) meant that there was little difference between arms in the actual cumulative dose of steroid administered. Median cumulative doses are potentially more representative than the mean due to some patients requiring treatment for greater than 1 AR episode. It should be noted that at the time of the study design, the recommended tacrolimus trough levels were higher than those often used in clinical practice and in other reported studies, and bolus-only steroid was standard clinical practice for the treatment of AR, which is now not the case in all transplant centers. Renal function was estimated using MDRD4, Chronic Kidney Disease Epidemiology Collaboration, and Cockcroft–Gault methodologies as opposed to criterion standard measurements using iohexol clearance and isotope-dilution mass spectrometry. Donor-specific antibody data were not widely collected for patients with BPAR; however, this would be of interest to the transplant community and should be considered for future studies.
In conclusion, the ADVANCE study was the first study specifically designed to investigate the impact of posttransplant steroids on the development of PTDM in patients receiving prolonged-release tacrolimus-based immunosuppression. Although the rates of PTDM were low and comparable between arms, prolonged-release tacrolimus and basiliximab plus MMF and steroid tapering for 10 days might be the preferred regimen in de novo kidney transplant recipients due to a potential lower risk of AR and BPAR. It remains to be determined if there are subgroups of patients at particularly high risk of PTDM for whom total steroid avoidance may be advantageous.
ACKNOWLEDGMENTS
Participating Investigators: Argentina: M. del Carmen Rial; Australia: J. Eris, H. Markus, P. O'Connell; Belgium: D. Kuypers, M. Mourad, J. Sennesael, L. Weekers; Brazil: D. Carvalho, F. Contieri, I. de Lourdes Noronha, H. Silva Jr; Colombia: L. Mesa Ramirez; Czech Republic: P. Bachleda, M. Kuman, P. Navratil, O. Viklický; Estonia: A. Lõhmus; Finland: K. Salmela; France: L. Albano, E. Alamartine, E. Cassuto-Viguier, M. Delahousse, I. Etienne, P. Le Pogamp, P. Merville, A. Meurette, B. Moulin, G. Mourad, J.-P. Rerolle, E. Rondeau, F. Villemain; Germany: B. Banas, P. Fornara, O. Hakenberg, B. Krämer, A. Mühlfeld, T. Rath, P. Reinke, L. Renders, O. Vonend, O. Witzke, R. Woitas; Hungary: L. Asztalos, G. Lazar; Italy: U. Boggi, S. Chiaramonte, P. De Rosa, A. Famulari, G. Frascà, M.C. Maresca, E.E. Minetti, G.B. Piredda, P. Rigotti, A. Secchi, S. Stefoni, G. Tisone; Korea, Republic of: D.J. Han, M.K. Ju, S.-J. Kim, Y.S. Kim; Latvia: I. Folkmane; Lithuania: A. Zelvys; Mexico: B. Gabliondo Pliego, E. Mancilla Urrea, G.A. Mondragon; the Netherlands: M.H.L. Christiaans; Norway: T. Jenssen; Poland: K. Ciechanowski, M. Durlik, K. Dziewanowski, M. Glyda, Z.W. Lodarczyk, M. Klinger, B. Rutkowski; Portugal: M. Almeida, D. Machado, A. Mota, F. Nolasco, M.A. Santana; Romania: A. Covic, I. Sinescu; Russian Federation: I. Aleksandrov, A. Chernyavskiy, V. Dobronravov, M.M. Kaabak, N. Klimushveva, V. Medvedev, Y.G. Moysyuk, I. Nesterenko, S.A. Pavlov, A.V. Pinchuk, A. Salmayer, S.B. Semchenko, N. Tomilina, I. Ulyankina, A. Vatacin, V.E. Zagaynov; Slovakia: L. Bena, E. Lackova, Z. Zilinska; Spain: A. Andres, G. Bernal, J. Bustamante, A. Franco, J.M. Gonzalez, R. Lauzurica, R. Marcén, F. Moreso, A. Rodriguez, P. Sánchez; Sweden: M. Felldin, G. Tyden, B. von Zur-Mühlen; Switzerland: F.-I. Binet, U. Eisenberger; Turkey: S. Kacar, G. Moray, S. Sahin, G. Suleymanlar, M. Tuncer, A. Turkmen.
The authors would like to thank Amy MacLucas and Nina Kennard from iS LifeScience for their editorial support in the preparation of the article.
Footnotes
This study was funded by Astellas Pharma.
The authors of this manuscript have conflicts of interest to disclose as described by Transplantation. G.M. has received honoraria from Bristol-Myers-Squibb, Fresenius and Sanofi, and an academic grant from Amgen. O.V. has received consultancy and study fees from Astellas. P.M. has received research grants from Astellas. O.W. has received research grants for clinical studies, speaker's fees, honoraria and travel expenses from Amgen, Astellas, Bristol-Myers Squibb, Chiesi, Novartis, Roche, Pfizer and Sanofi. M.C. has received research grants from Astellas and Novartis and has lectured for, and received consultancy fees from Astellas. M.B. and N.U. are employees of Astellas. G.K. is a consultant statistician working on behalf of Astellas and has received support for travel from Astellas. D.K. has received research grants from Alexion, Astellas, Novartis and Roche and honoraria from Alexion, Astellas, BMS, Chiesi, Novartis, Pfizer, Roche and Opsona Therapeutics. All other authors have nothing to disclose.
The study was sponsored by Astellas Pharma, manufacturers of prolonged-release tacrolimus (Advagraf). Under the direction of the authors, Amy MacLucas and Nina Kennard from iS LifeScience drafted the initial version of the manuscript and provided editorial support throughout the development. Editorial support was funded by Astellas Pharma.
Clinical trials.gov registration: NCT01304836.
All authors met the ICMJE criteria for authorship. D.K., M.C., M.B. and N.U. were involved in the study conception and design. All authors were involved in the acquisition, analysis or interpretation of data. All authors were involved in revising the article critically for important intellectual content. All authors approved the final version of the article for submission. All others who contributed to this document, but who did not meet authorship criteria, are acknowledged.
Contributor Information
Collaborators: on behalf of the Advagraf-based immunosuppression regimen examining new onset diabetes mellitus in kidney transplant recipients (ADVANCE) study investigators
REFERENCES
- 1.First MR, Dhadda S, Croy R, et al. New-onset diabetes after transplantation (NODAT): an evaluation of definitions in clinical trials. Transplantation. 2013;96:58–64. [DOI] [PubMed] [Google Scholar]
- 2.Ghisdal L, Van Laecke S, Abramowicz MJ, et al. New-onset diabetes after renal transplantation: risk assessment and management. Diabetes Care. 2012;35:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller J, Mendez R, Pirsch JD, et al. Safety and efficacy of tacrolimus in combination with mycophenolate mofetil (MMF) in cadaveric renal transplant recipients. FK506/MMF Dose-Ranging Kidney Transplant Study Group. Transplantation. 2000;69:875–880. [DOI] [PubMed] [Google Scholar]
- 4.Montori VM, Basu A, Erwin PJ, et al. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care. 2002;25:583–592. [DOI] [PubMed] [Google Scholar]
- 5.Wauters RP, Cosio FG, Suarez Fernandez ML, et al. Cardiovascular consequences of new-onset hyperglycemia after kidney transplantation. Transplantation. 2012;94:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. [DOI] [PubMed] [Google Scholar]
- 7.Burroughs TE, Swindle J, Takemoto S, et al. Diabetic complications associated with new-onset diabetes mellitus in renal transplant recipients. Transplantation. 2007;83:1027–1034. [DOI] [PubMed] [Google Scholar]
- 8.Pham PT, Pham PM, Pham SV, et al. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes. 2011;4:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostaing L, Cantarovich D, Mourad G, et al. Corticosteroid-free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation. 2005;79:807–814. [DOI] [PubMed] [Google Scholar]
- 10.Luan FL, Steffick DE, Ojo AO. New-onset diabetes mellitus in kidney transplant recipients discharged on steroid-free immunosuppression. Transplantation. 2011;91:334–341. [DOI] [PubMed] [Google Scholar]
- 11.Woodle ES, First MR, Pirsch J, et al. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248:564–577. [DOI] [PubMed] [Google Scholar]
- 12.Vitko S, Klinger M, Salmela K, et al. Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation. 2005;80:1734–1741. [DOI] [PubMed] [Google Scholar]
- 13.Pascual J, Quereda C, Zamora J, et al. Steroid withdrawal in renal transplant patients on triple therapy with a calcineurin inhibitor and mycophenolate mofetil: a meta-analysis of randomized, controlled trials. Transplantation. 2004;78:1548–1556. [DOI] [PubMed] [Google Scholar]
- 14.Augustine JJ, Hricik DE. Steroid sparing in kidney transplantation: changing paradigms, improving outcomes, and remaining questions. Clin J Am Soc Nephrol. 2006;1:1080–1089. [DOI] [PubMed] [Google Scholar]
- 15.Albano L, Banas B, Klempnauer JJL, et al. OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation. 2013;96:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–1514. [DOI] [PubMed] [Google Scholar]
- 17.Johnston O, Rose CL, Webster AC, et al. Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol. 2008;19:1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peddi VR, Wiseman A, Chavin K, et al. Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation. Transplant Rev (Orlando). 2013;27:97–107. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1–S155. [DOI] [PubMed] [Google Scholar]
- 21.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. [DOI] [PubMed] [Google Scholar]
- 22.Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010;89:1–14. [DOI] [PubMed] [Google Scholar]
- 23.Krämer BK, Charpentier B, Bäckman L, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant. 2010;10:2632–2643. [DOI] [PubMed] [Google Scholar]
- 24.Dhadda S, Fitzsimmons W, Croy R, et al. Defining new-onset diabetes after transplantation (NODAT). Transplantation. 2010;90(Suppl 2):694. [DOI] [PubMed] [Google Scholar]
- 25.Bäckman LA. Post-transplant diabetes mellitus: the last 10 years with tacrolimus. Nephrol Dial Transplant. 2004;19(Suppl 6):vi13–vi16. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Huang H, Han S, et al. Is it safe to withdraw steroids within seven days of renal transplantation? Clin Transplant. 2013;27:1–8. [DOI] [PubMed] [Google Scholar]


