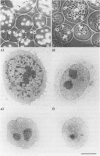
Abstract
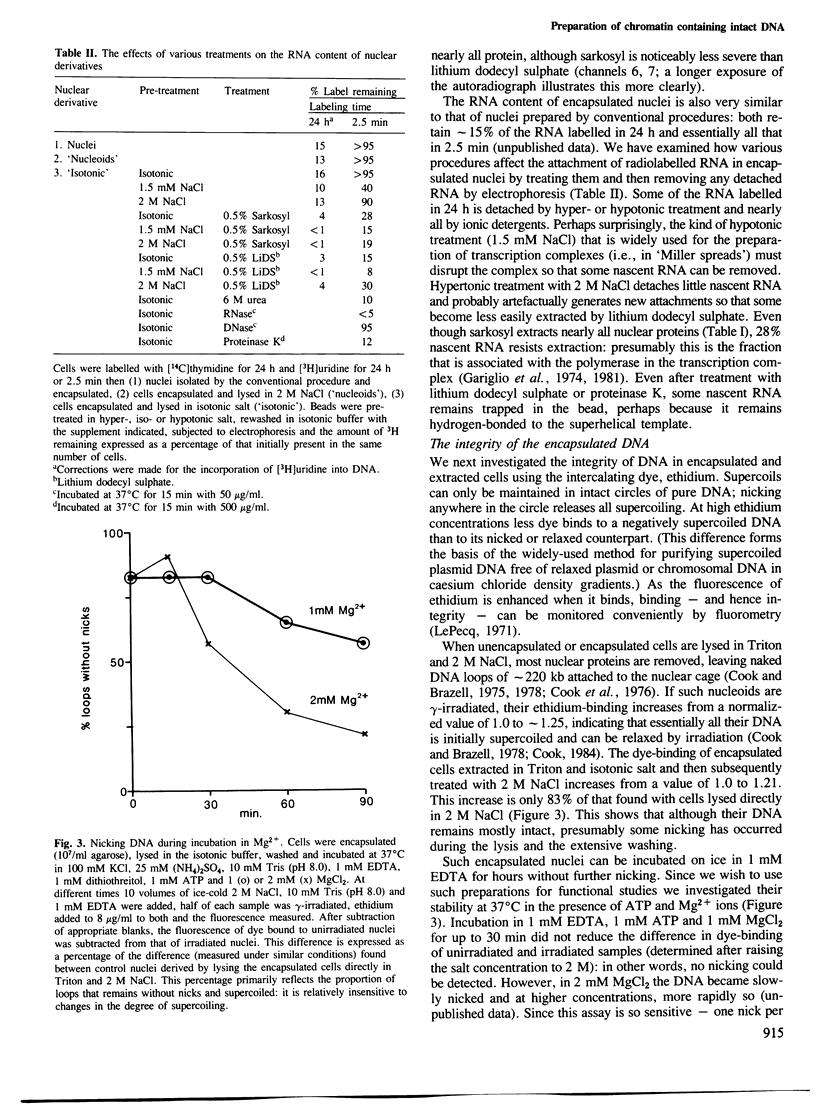
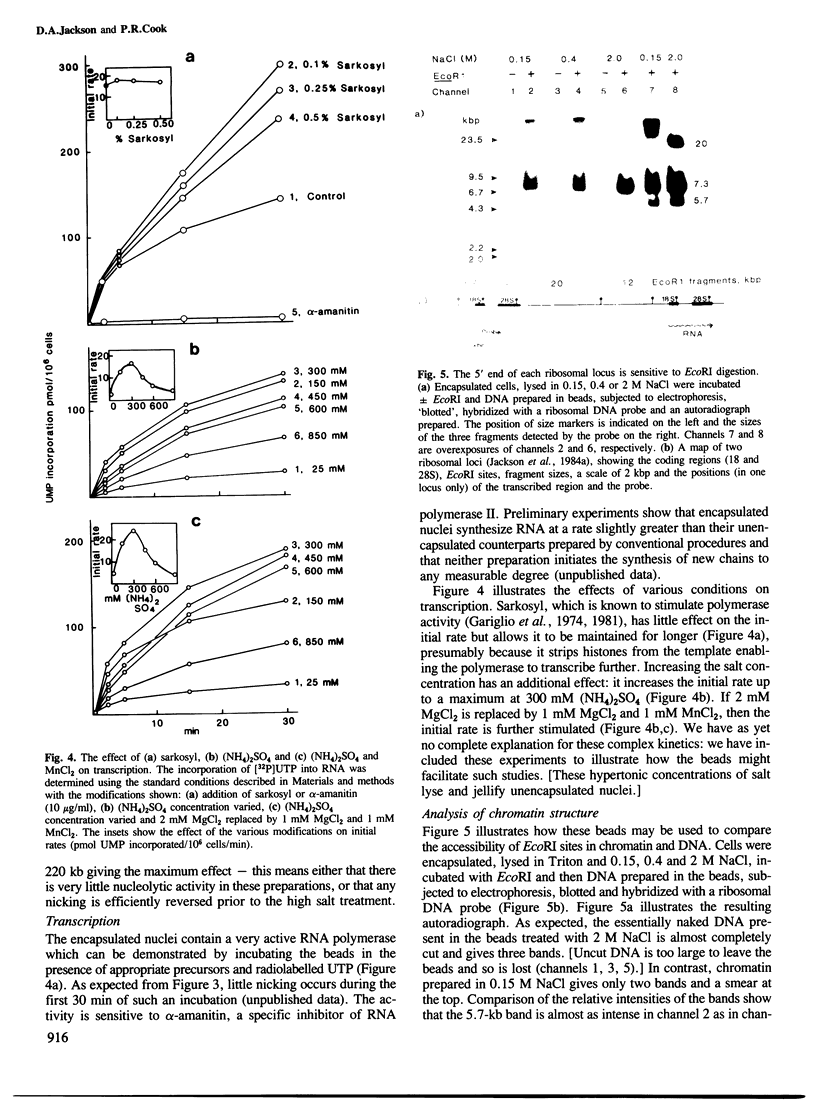
A simple and general method is described for preparing chromatin from eukaryotic cells using isotonic conditions. First, cells are encapsulated in agarose microbeads and then lysed using Triton X-100 in the presence of a chelating agent and a physiological concentration of salt. Most cytoplasmic proteins and RNA diffuse rapidly out through pores in the beads to leave encapsulated chromatin which is nevertheless completely accessible to enzymes and other probes. This chromatin can be manipulated freely without aggregation in a variety of different salt and detergent concentrations. It also contains intact DNA since removal of the histones releases superhelical DNA. Conditions are described for incubating this chromatin at 37 degrees C in the presence of Mg2+ ions without any nicking of the DNA. We illustrate the usefulness of this chromatin in investigations on the attachment of nascent RNA to the nucleoskeleton, the accessibility of the ribosomal locus to EcoRI and the properties of the endogenous RNA polymerase II. This type of chromatin preparation should prove useful for both structural and functional studies.
Full text
PDF
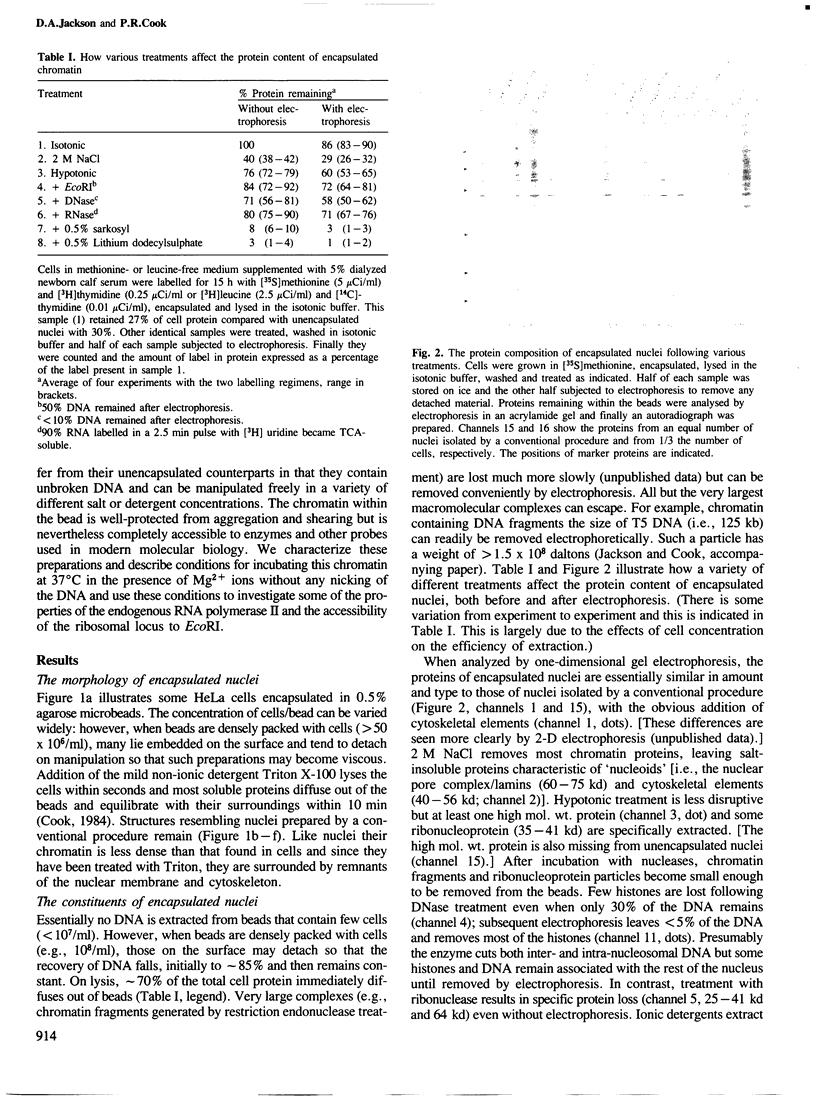


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colman A., Cook P. R. Transcription of superhelical DNA from cell nuclei. Eur J Biochem. 1977 Jun 1;76(1):63–78. doi: 10.1111/j.1432-1033.1977.tb11570.x. [DOI] [PubMed] [Google Scholar]
- Cook P. R. A general method for preparing intact nuclear DNA. EMBO J. 1984 Aug;3(8):1837–1842. doi: 10.1002/j.1460-2075.1984.tb02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A., Jost E. Characterization of nuclear structures containing superhelical DNA. J Cell Sci. 1976 Nov;22(2):303–324. doi: 10.1242/jcs.22.2.303. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Spectrofluorometric measurement of the binding of ethidium to superhelical DNA from cell nuclei. Eur J Biochem. 1978 Mar 15;84(2):465–477. doi: 10.1111/j.1432-1033.1978.tb12188.x. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Supercoils in human DNA. J Cell Sci. 1975 Nov;19(2):261–279. doi: 10.1242/jcs.19.2.261. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Bellard M., Chambon P. Clustering of RNA polymerase B molecules in the 5' moiety of the adult beta-globin gene of hen erythrocytes. Nucleic Acids Res. 1981 Jun 11;9(11):2589–2598. doi: 10.1093/nar/9.11.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariglio P., Buss J., Green M. H. Sarkosyl activation of RNA polymerase activity in mitotic mouse cells. FEBS Lett. 1974 Aug 30;44(3):330–333. doi: 10.1016/0014-5793(74)81170-1. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R., Patel S. B. Attachment of repeated sequences to the nuclear cage. Nucleic Acids Res. 1984 Sep 11;12(17):6709–6726. doi: 10.1093/nar/12.17.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., McCready S. J., Cook P. R. Replication and transcription depend on attachment of DNA to the nuclear cage. J Cell Sci Suppl. 1984;1:59–79. doi: 10.1242/jcs.1984.supplement_1.5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Pecq J. B. Use of ethidium bromide for separation and determination of nucleic acids of various conformational forms and measurement of their associated enzymes. Methods Biochem Anal. 1971;20:41–86. doi: 10.1002/9780470110393.ch2. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Post-replicative nonribosomal transcription units in D. melanogaster embryos. Cell. 1979 Jul;17(3):551–563. doi: 10.1016/0092-8674(79)90263-0. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Beatty B. R. Visualization of nucleolar genes. Science. 1969 May 23;164(3882):955–957. doi: 10.1126/science.164.3882.955. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Scheirer W., Merten O. W., Ostberg L., Liehl E., Katinger H. W., Mosbach K. Entrapment of animal cells for production of monoclonal antibodies and other biomolecules. Nature. 1983 Apr 14;302(5909):629–630. doi: 10.1038/302629a0. [DOI] [PubMed] [Google Scholar]
- Ohlenbusch H. H., Olivera B. M., Tuan D., Davidson N. Selective dissociation of histones from calf thymus nucleoprotein. J Mol Biol. 1967 Apr 28;25(2):299–315. doi: 10.1016/0022-2836(67)90143-x. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]