Abstract
Transplantation of hematopoietic stem cells (HSCs) with a naturally occurring CCR5 mutation confers a loss of detectable HIV-1 in the patient, making ablation of the CCR5 gene in HSCs an ideal therapy for an HIV-1 cure. Although CCR5 disruption has been attempted in CD4+ T cells and hematopoietic stem/progenitor cells (HSPCs), efficient gene editing with high specificity and long-term therapeutic potential remains a major challenge for clinical translation. Here, we established a CRISPR/Cas9 gene editing system in human CD34+ HSPCs and achieved efficient CCR5 ablation evaluated in long-term reconstituted NOD/Prkdcscid/IL-2Rγnull mice. The CCR5 disruption efficiency in our system remained robust in secondary transplanted repopulating hematopoietic cells. More importantly, an HIV-1 resistance effect was observed as indicated by significant reduction of virus titration and enrichment of human CD4+ T cells. Hence, we successfully established a CRISPR/Cas9 mediated CCR5 ablating system in long-term HSCs, which confers HIV-1 resistance in vivo. Our study provides evidence for translating CCR5 gene-edited HSC transplantation for an HIV cure to the clinic.
Keywords: CCR5, gene editing, HIV-1/AIDS, CRISPR, hematopoietic stem cell, gene therapy
Graphical Abstract
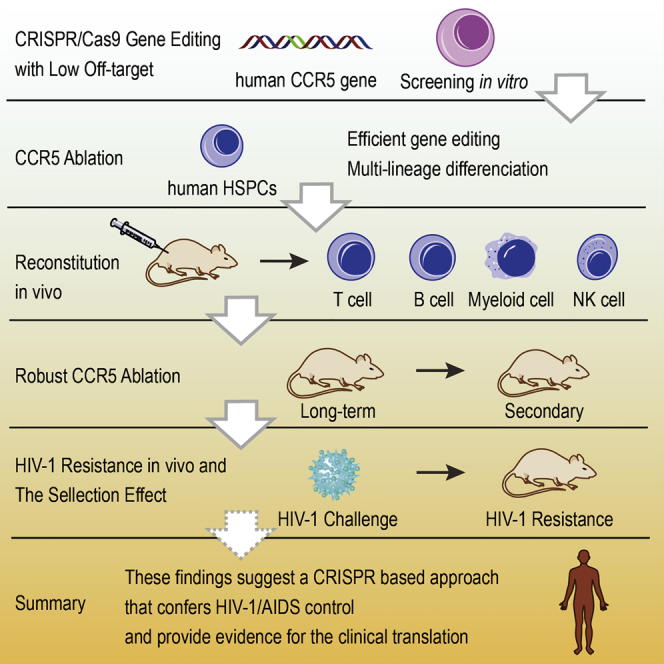
Xu et al. have used the CRISPR/Cas9 system to achieve CCR5 gene disruption in human hematopoietic stem/progenitor cells (HSPCs). The gene-ablated HSPCs could reconstitute in vivo long term and resist HIV-1 infection. This study provides evidence of CCR5 gene ablation in HSCs as a promising therapy for HIV-1.
Introduction
Hematopoietic stem cell (HSC) transplantation has been widely and successfully applied to treatments of blood diseases. CCR5 is the key chemokine receptor for HIV to enter targeted human hematopoietic cells.1 Individuals with a homozygous CCR5 mutation show resistance to HIV-1 infection.2, 3 The allo-transplantation of HSCs with naturally occurring CCR5 mutation into an HIV-1 patient resulted in a loss of detectable HIV-1.4, 5 These suggest that transplantation of CCR5-ablated HSCs would be a promising gene therapy approach for an HIV-1 cure.
Several studies have shown that zinc-finger nucleases (ZFNs) could be used to disrupt CCR5 in human CD34+ hematopoietic stem/progenitor cells (HSPCs) despite some off-target cleavage events.6, 7, 8 Moreover, immunodeficient mice reconstituted with CCR5-disrupted HSPCs show anti-HIV infection and CCR5 disruption enrichment after HIV-1 challenge.6 CRISPR/Cas9 has been used in an attempt to disrupt CCR5 in hematopoietic progenitor cells.9 However, CRISPR/Cas9 mediated CCR5 disruption in long-term repopulating HSCs has not been fully illustrated, and its HIV-1 prevention effect remains to be evaluated.
In this study, we established a CRISPR/Cas9 gene editing and non-viral transfection system in HSPCs with high cleavage efficiency and low off-target effect. Moreover, we achieved robust CCR5 disruption evaluated in both long-term reconstituted and secondary transplanted mice and observed a significant anti-viral effect in vivo.
Results
Development of an Efficient CCR5 Ablation System Based on CRISPR/Cas9 with a Minimal Off-Target Effect
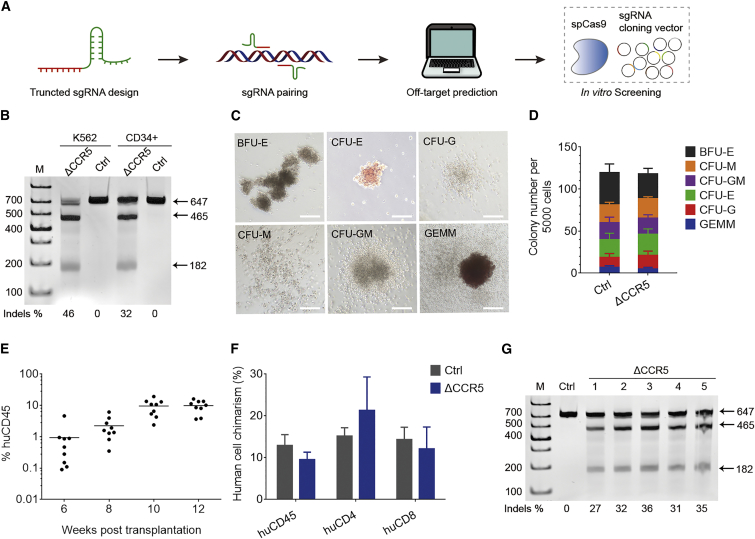
To efficiently disrupt the human CCR5 gene, we rationally designed and screened a series of single guide RNAs (sgRNAs) targeting the locus from the beginning of the first exon to the Δ32 mutation site in the human CCR5 gene (Figure 1A). These sgRNAs were paired and truncated into 17–18 bp,10 followed by construction into an optimized scaffold.11 Screening with multiple bioinformatic prediction tools12, 13 was performed to eliminate sgRNAs with high non-specific binding potential and improve gene editing efficiency. After removing those with high off-target potential, sgRNA pairs were co-nucleofected with Cas9 (Streptococcus pyogenes) into K562 cells to test the cleavage efficiency. Using the selected sgRNA pair, we achieved an average of 42% (±2.1%, n = 3) cleavage efficiency in K562 cells, detected using T7 endonuclease I (T7EI) assay (Figure 1B, representative data) and sequencing.
Figure 1.
Efficient CCR5 Ablation In Vitro and In Vivo
(A) Flowchart of sgRNA pair selection. The off-target effects of sgRNA pairs were predicted using multiple bioinformatic prediction tools, and high off-target pairs were eliminated. The remaining pairs were transfected with CRISPR/Cas9 into a cell line, and the cleavage efficiency was determined using T7 endonuclease I (T7EI) assay. (B) T7EI assay of CCR5 gene ablation in K562 cells and human CD34+ cells in a representative experiment. (C) Human CD34+ cells treated with the CRISPR/Cas9 system were analyzed in the CFU assay, and different types of colonies were presented. Scale bars, 200 μm. (D) Various types of colonies were counted for CRISPR/Cas9-treated or non-treated CD34+ cells. (E) Human CD45+ cell reconstitution was evaluated in peripheral blood in NPG mice transplanted with gene-edited HSPCs. Robust reconstitution was detected in mice from 6 to 12 weeks post-transplantation (mean values, 0.9%, 2.2%, 9.6%, and 9.9%; n = 9). (F) Human hematopoietic cell reconstitution of CCR5-disrupted or non-treated HSPCs in mouse peripheral blood lymphocytes collected at week 12 (mean ± SEM, n = 9). (G) Representative assay showing efficient human CCR5 disruption in peripheral blood of reconstituted mice 12 weeks after transplantation. The PCR products (647 bp) were digested into two fragments (465 and 182 bp), indicating effective CCR5 disruption. ΔCCR5, CCR5 gene ablation; Ctrl, non-treatment control.
Then, high-throughput whole-genome sequencing (100×) was performed to evaluate the non-specific gene targeting in K562 cells. At a genome-wide coverage, we observed only one potential non-specific site (chromosome 4 [chr4]: 18476075-18476173), which was not located in an annotated gene coding or functional region. Moreover, no off-target in human CCR2 gene locus was detected in our experiment, which has a sequence highly similar to CCR5.
CCR5 Disruption in CD34+ HSPCs without Impairing Differentiation Activity In Vitro
Using serum-free culture medium and nucleofection conditions, we achieved CCR5 ablation of 27% (±5.4%, n = 3) in human CD34+ HSPCs in vitro detected using T7EI assay (Figure 1B) and sequencing. Furthermore, colony-forming unit (CFU) assay was performed to examine the multi-lineage differentiation potential of CD34+ HSPCs after gene editing treatment, and various types of colonies (Figure 1C) were observed. Regardless of whether the CCR5 gene editing was performed, comparable colony types and numbers (Figure 1D) suggested that colony-forming potential was not affected by gene editing. In addition, we have detected as high as 45% of colonies with CCR5 disruption. The percentage of biallelic disruption was 44% in all edited colonies.
Treated HSPCs Produce CCR5-Ablated Hematopoietic Cells and Multi-lineage Progeny in Long-Term Reconstituted Mice
To evaluate the CCR5 disruption and the hematopoietic potential, human CD34+ HSPCs with CCR5-ablating treatment were transplanted into non-obese diabetic (NOD)/Prkdcscid/IL-2Rγnull (NPG) mice and resulted in rapid and efficient reconstitution in all mice (Figure 1E). In an average of 8.3% of human CD45+ cells, engraftment was detected 12 weeks post-transplantation, which was comparable with the percentage in control mice transplanted with non-edited CD34+ cells (Figure 1F). To determine the CCR5 editing efficiency, the genomic DNA extracted from the peripheral blood cells of five reconstituted mice 12 weeks post-transplantation was analyzed using T7EI assay (Figure 1G). The average CCR5 cleavage efficiency was 32.2% (±1.6%, n = 5), determined by sequencing.
Long-term HSCs, a rare population of CD34+ cells, support lifetime hematopoiesis by self-renewing and differentiating into all lineages. To confirm whether long-term repopulating HSCs were edited with our strategy, we sampled peripheral blood from 12 mice reconstituted for 30 to 47 weeks and conducted T7EI assay, followed by confirmation using Sanger sequencing. The average ablating efficiency at the CCR5 targeting locus was 31.2% (±4.9%), which was consistent with that of 12-week assay.
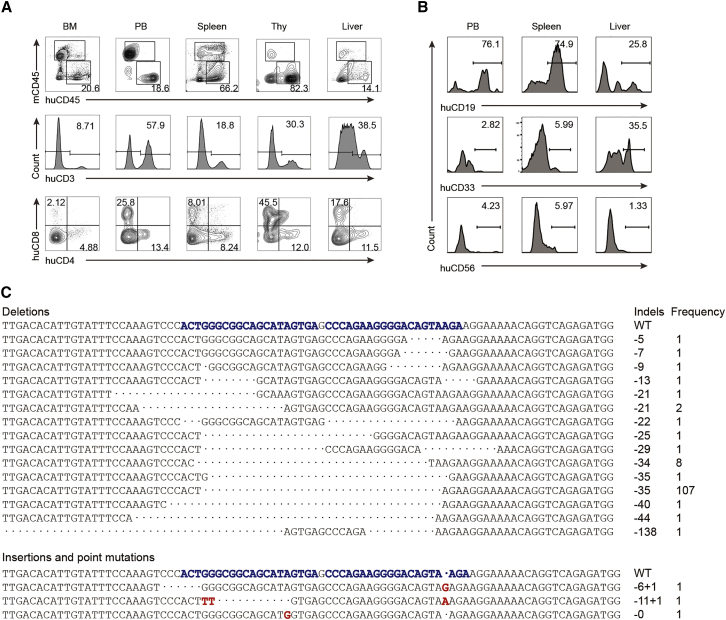
Next, we evaluated the multi-lineage reconstitution potential of human gene-edited HSPCs in long-term reconstituted NPG mice. High proportions of human cell chimerism and human CD3+, CD4+, and CD8+ T cells were detected in multiple hematopoietic and immune organs (Figure 2A), which was comparable with control mice. In addition, B cells, myeloid cells, and natural killer (NK) cells were observed (Figure 2B). These data indicated that treated HSPCs maintained the activity of long-term hematopoietic repopulating and differentiation into all hematopoietic lineages.
Figure 2.
Human Cell Chimerism and Feature of CCR5 Indels in Long-Term Reconstituted Mice
(A) Chimerism and human T cell reconstitution of HSPCs after gene editing in multiple organs from representative reconstituted mice. Human CD3+ (huCD3), human CD4+ (huCD4), and human CD8+ (huCD8) cells were gated from human CD45+ (huCD45) cells. (B) Representative T7EI data from one long-term reconstituted mouse show multi-lineage activity of human HSPCs in multiple organs. B lymphocytes (CD19+), myeloid cells (CD33+), and NK cells (CD56+) were detected. (C) Features of CCR5 indels in long-term reconstituted mice. The PCR products of human CCR5 gene in long-term reconstitution mice were sequenced and analyzed (n = 12). The predictable indel (35-bp deletion) was present at a dominant rate (107/122, 87.7%).
High Proportion of Frameshift Mutations in Disrupted CCR5 Locus
We then tested the ratio of frameshift mutations on human CCR5 locus in peripheral lymphocytes from long-term reconstituted mice, because this type of mutation has a higher probability to induce protein dysfunction. Sequencing data from the reconstituted mice implied a predictable 35-bp deletion in 107 of 122 insertion or deletion (indel) events (Figure 2C), confirming a high-frequency predictable deletion. The frequency of the CCR5 mutation with frameshift was 95%. These results indicate that CCR5 was efficiently disrupted and most alleles were modified with frameshift mutations in our gene editing system.
Robust CCR5 Ablation in Long-Term Repopulating HSCs
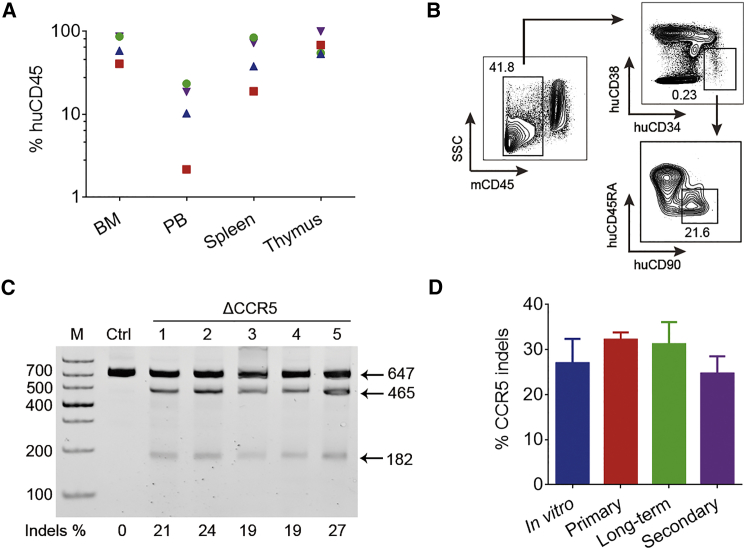
The gene modification in long-term and secondary reconstituted animal models, instead of short-term reconstitution, reveals the gene editing efficiency in HSCs. We performed secondary transplantation with bone marrow cells derived from long-term (30 to 47 weeks) reconstituted mice at a ratio of 1 to 3. Twelve weeks post-transplantation, we euthanized four secondary mice and observed robust human cell reconstitution in bone marrow, peripheral blood, spleen, and thymus (Figure 3A). Moreover, we stained bone marrow cells with human CD45 and CD34 markers for fluorescence-activated cell sorting (FACS) analysis. The representative result showed that human HSPCs (CD34+/38−/45RA−/90+) were still present among CD45+ cells (Figure 3B), suggesting that CCR5-modified HSPCs were retained in secondary transplanted mice. The genomic DNA from peripheral blood cells was analyzed by T7EI assay (Figure 3C), and the average CCR5 ablation efficiency was 24.7% (±3.8%, n = 9). In addition, gene editing in CD4+ T cells sorted from three secondary transplanted mice was evaluated. The average CCR5 ablation efficiency in human CD4+ T cells was 27.3% (±6.7%, n = 3), which was comparable to that of peripheral lymphocyte (25.4% ± 5.9%) from the same mice. The result indicated that CCR5 disruption in CD4+ T cells is as efficient as that in peripheral lymphocytes.
Figure 3.
CCR5 Ablation and Reconstitution in Secondary Transplanted Mice
(A) Proportion of huCD45 in cells from bone marrow (BM), peripheral blood (PB), spleen, and thymus of four secondary transplanted mice after 12 weeks of reconstitution. Data from the same mouse were shown in the same color and shape. (B) Human CD34+/38−/45RA−/90+ HSPCs were detected in the bone marrow from secondary transplantation mice. (C) T7EI assay showing efficient CCR5 disruption of human hematopoietic cells in secondary transplantation mice after 12 weeks of reconstitution. (D) CCR5 indel efficiency was summarized from an in vitro experiment (in vitro, 27.0 ± 5, n = 3), primary transplanted mice of 12 weeks (primary, 32.2 ± 1.6, n = 5), long-term reconstituted mice (long-term, 31.2 ± 4.9, n = 12), and secondary transplanted mice (secondary, 24.7 ± 3.8, n = 9). Data are represented as mean ± SEM.
The CCR5 disruption in our system remained at a high level and robust over time after transplantation in long-term and secondary transplanted repopulating HSCs (Figure 3D). Hence, efficient and robust CCR5 knockout was achieved with our developed system in HSCs that support long-term hematopoiesis.
HIV-1 Challenge in Reconstituted Mice and the Selection Effect
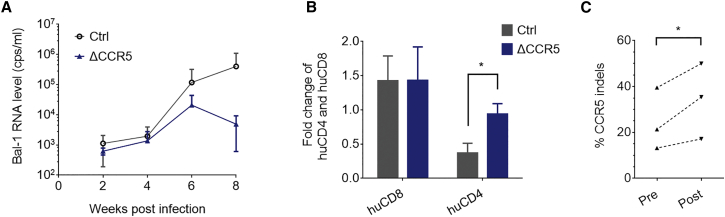
To test the HIV-1 resistance effect, after reconstitution for 10 to 12 weeks, Bal-1 virus (CCR5-tropic HIV-1 strain) challenge was conducted in NPG mice transplanted with CCR5-disrupted or non-edited human CD34+ HSPCs. We measured HIV RNA levels in mouse peripheral blood plasma biweekly post-infection and observed a significant decrease of HIV-1 RNA level in mice transplanted with edited HSPCs 8 weeks post-infection (wpi). By contrast, the HIV RNA level increased in the non-edited control group (Figure 4A).
Figure 4.
HIV-1 Resistance and the Selection Effect
(A) HIV-1 RNA level was detected after Bal-1 virus strain infection in the peripheral blood plasma of NPG mice, which were transplanted with CCR5-disrupted (CCR5−) or non-treated control (Ctrl) HSPCs (mean ± SEM, n = 3). (B) The fold change of human CD4+ and human CD8+ cells in the peripheral blood of mice was evaluated based on the ratio of reconstitution from 6 to 8 weeks post-infection (mean ± SEM; p = 0.026, n = 3). (C) CCR5 locus of human cells in mouse peripheral blood was analyzed pre- and post-HIV challenge. Percentage of indel alleles significantly increased 8 weeks after Bal-1 HIV-1 infection, indicating the enrichment of CCR5 disruption. p = 0.042, n = 3.
HIV-resistant cells could be enriched through the elimination of infected cells under the selection pressure of the virus.14 The reconstitution of human CD4+ cells at 8 wpi was 0.37-fold of that at 6 wpi in the control group, but the ratio was about three times higher (0.94-fold) in the CCR5-disrupted group, while the ratios of human CD8+ cell reconstitution in both groups were similar (Figure 4B). This result revealed that CCR5 disruption contributed to protection against the decline of CD4+ cells in reconstituted mice under HIV-1 infection pressure. In addition, the gene ablation efficiency was analyzed pre- and post-HIV challenge. The average rate of CCR5 gene disruption was higher after virus infection (Figure 4C), indicating that CCR5 edited cells were enriched during HIV-1 infection. Hence, CCR5-ablated human HSPCs, using the developed system, reconstituted human CD4+ cells in NPG mice, which conferred resistance against HIV-1 infection.
Discussion
In this study, we reported efficient gene modification in long-term repopulating HSCs targeting human CCR5 using the established CRISPR/Cas9 system. Human HSPCs with CCR5 ablating treatment reconstituted the human immune system and conferred resistance to HIV-1 infection after being transplanted into NPG mice.
Efficient and robust gene ablation in long-term HSCs targeting CCR5 has been achieved using our developed CRISPR/Cas9 system. Successful gene editing in long-term HSCs is the key aspect for translating gene-targeted therapy in HSCs to the clinic, because only CCR5-disrupted HSCs are responsible for sustainable generation of HIV-1-resistant cells. In a recent work, CCR5 ablation was attempted in HSPCs using CRISPR/Cas9 in vitro and short-term reconstituted mice.9 However, T lymphocyte reconstitution has not been reported, and the ability of gene editing in long-term HSCs remains uncertain. In previous reported works, gene editing efficiency decreased 5- to 20-fold after long-term transplantation in animal models.15, 16 Our results showed that human CCR5-ablated HSPCs reconstituted in mice for over 1 year. Robust CCR5 disruption was indicated by the similar efficiency detected in vitro and in vivo at various reconstitution periods. Human HSPCs (CD34+/38−/45RA−/90+) were still presented in bone marrow from secondary transplanted mice. Our achievement of robust and efficient CCR5 ablation in long-term HSCs was possible because of the optimized gene editing system and the pre-culture condition of CD34+ cells. CRISPR/Cas9 has been optimized to enhance cleavage efficiency and reduce non-specific targeting in recent years,10, 11, 17, 18 achieving better performance over ZFNs and transcription activator-like effector nucleases (TALENs). A serum-free culture condition was developed and adopted in various works to support gene manipulation or expansion of CD34+ HSPCs without impairing the all-lineage reconstitution property.19, 20, 21
The HIV-resistant efficacy of hematopoietic cells generated from CCR5-ablated HSPCs was confirmed in reconstituted mice in our gene editing system. In our study, HIV-1 RNA level decreased in mice transplanted with CCR5 modified HSPCs. Human CD4+ T cells remained at a stable level after HIV-1 challenge, while they declined dramatically in control mice. These results indicated that CCR5 gene modification in our system supports stable CD4+ cell counts that resist HIV-1 infection, which are attributed to the consistent generation of modified hematopoietic cells from CCR5-ablated HSPCs. The HIV-1-resistant effect in our CRISPR/Cas9-based strategy is comparable to that in the previous studies using ZFNs and lentivirus-based strategies.6, 15, 22 Therefore, CRISPR/Cas9-based CCR5-ablated HSPCs could consistently generate HIV-resisting hematopoietic cells in our system to support stable CD4+ cell counts, making our study evidence for the therapeutic potential of CRISPR/Cas9-ablated CCR5 in HSCs for an HIV cure.
In our non-viral CRISPR/Cas9 system targeting CCR5, a minimal off-target effect was observed. Because the sequence of the CCR2 gene has high similarity to that of CCR5, several studies have reported off-target cleavage events in CCR2 when disrupting CCR5.7, 8, 9, 14 However, a low off-target level at only one site was observed in a CRISPR mediated CCR5 editing study,9 which may be attributed to the flexible design of sgRNAs in the CRISPR system. To minimize the off-target effect and retain highly efficient gene disruption, we adopted optimized sgRNA strategies in our gene editing system, including truncated sgRNA, optimized scaffold, and sgRNA pairing strategies.9, 10, 11, 17 The high-throughput whole-genome sequencing result showed only one potential non-specific site located in a nonsense region. Although high specificity was detected using genome-wide sequencing, safety concerns of a gene therapy approach should be evaluated in further studies and clinical trials.
Overall, CCR5 ablation was achieved in human long-term HSCs using our highly specific CRISPR/Cas9 system and resulted in apparent HIV-1 resistance. Our findings suggest that CRISPR/Cas9 mediated CCR5 ablation in human long-term HSCs might confer long-term control of HIV-1 infection, which is an attractive stem cell-based gene therapy for an HIV-1 cure in future clinical studies. In addition, this study provides an alternative strategy for clinical translation of HSC-based gene therapy in treating other genetic and infectious diseases. However, a safety evaluation using adult HSPCs should be performed before clinical trials.
Materials and Methods
Screen of sgRNA Pairs
We cloned the SpCas9 gene with the pCMV promoter and nuclear localization sequence (SV40 in the front of SpCas9 and nucleoplasmin at the back of it). A series of sgRNAs targeting the locus from the beginning of the first exon to the Δ32 mutation site in the human CCR5 gene was truncated to 17 to 18 bp and cloned into a reported scaffold, respectively (see Chen et al.11). A dual sgRNA strategy was applied for sgRNA pairs. The sgRNAs with high off-target potential were eliminated with predicting tools. The sequences for the selected paired sgRNAs are 5′-GACTATGCTGCCGCCCAGT-3′ and 5′-GCAGAAGGGGACAGTAAGA-3′.
Isolation of Human CD34+ HSPCs
Fetal liver samples were obtained as donated samples through the approval of the Institutional Review Board of Peking University. Mononuclear cells were isolated from cell suspension using Ficoll (Sigma). Human CD34+ cells were isolated through anti-human CD34 antibody conjugation using microbeads (Miltenyi Biotec) and the magnetic activated cell sorting method (Miltenyi Biotec) according to the manufacturer’s instructions.
Cell Culture and Transfection
Human CD34+ cells were cultured in Stemspan serum-free medium II (STEMCELL Technologies) supplemented with stem cell factor (SCF; 100 ng/mL, PeproTech), fms-related tyrosine kinase 3 ligand (Flt-3L; 100 ng/mL, PeproTech), thrombopoietin (TPO; 100 ng/mL, PeproTech), interleukin-6 (IL-6; 20 ng/mL, PeproTech), and 1% penicillin-streptomycin solution (Gibco). K562 cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS, Gibco) plus 1% penicillin-streptomycin and passaged every 2 days through a complete culture medium change. For transfection, human CD34+ cells were pre-stimulated for 24 hr in culture medium. A total of 1 × 106 to 3 × 106 cells were transfected with 10 μg of Cas9 and 5 μg of each sgRNA plasmid in a final volume of 100 μL and nucleofected using a 4D-Nucleofector device (Lonza) according to the manufacturer’s instructions. The nucleofected cells were cultured for 3 days at 37°C in a 5% CO2 incubator for further analysis. K562 cells were nucleofected after two passages according to the manufacturer’s instructions.
Analysis of Genome Editing
Genomic DNA was extracted from cells using a DNA blood and tissue kit (QIAGEN) according to the manufacturer’s instructions. The target sequence was amplified using a high-fidelity DNA polymerase (Takara) with genomic DNA and the primers 5′- TGGACAGGGAAGCTAGCAGCAAA-3′ (forward) and 5′-TCACCACCCCAAAGGTGACCG-3′ (reverse). The PCR products were annealed after purification. Next, 200 ng of hybridized DNA were subjected to digestion with 0.5 μL T7EI (New England Biolabs) in NE Buffer 2 for 30 min at 37°C in a 15 μL final volume. Subsequently, the samples were loaded onto a PAGE gel with an equal amount of PCR product controls from non-edited samples. The indel percentage was calculated according to the gray value detected using ImageJ software (ImageJ 1.46r). The PCR products were analyzed through Sanger sequencing. For off-target assays, genomic DNA was extracted from different samples for whole-genome sequencing to identify the potential off-target loci. Whole-genome sequencing and data analysis were conducted at Novogene.
CFU Assay and Detection of Mutation Efficiency
CD34+ HSPCs (5,000 cells) suspended in 100 μL of Iscove’s modified Dulbecco’s medium supplemented with 2% FBS was mixed with 1 mL of methylcellulose (MethoCult H4034 Optimum, STEMCELL Technologies). After plating on a 35 mm cell culture dish, the cells were cultured for 14 days at 37°C in a 5% CO2 incubator. Individual colonies were derived from the CFU dish after culturing for 14 days, and genomic DNA was extracted from each colony. The mono- and biallelic disruption rates were analyzed by sequencing for each colony.
Transplantation and Engraftment Assay
NOD/Prkdcscid/IL-2Rγnull (NPG) mice were obtained from Beijing Vitalstar Biotechnology (NOD-Prkdcscid Il2rgtm1/Vst mice, stock No. VS-AM-001). Animal experiments were performed according to the Animal Protection Guidelines of Peking University, China. Within 24 hr, neonatal NPG mice were sub-lethally irradiated (1.0 Gy) and transplanted with 1 × 106 human CD34 cells in 0.9% NaCl solution at 20 μL per mouse liver injection. For secondary transplantation, bone marrow was harvested from the 12-week-old engrafted mice. Three 6- to 8-week-old NPG mice were sub-lethally irradiated (1.6 Gy) 4 hours before bone marrow injection with one-third bone marrow CD34 cells in 0.9% NaCl solution at 30 μL per mouse.
Mouse peripheral blood samples were collected every 2 weeks from 6 wpi, and human cell engraftment was determined using flow cytometry. The genomic DNA was extracted after erythrocyte lysis, and the CCR5 disruption efficiency was analyzed by T7EI assay, followed by sequencing. For multi-lineage engraftment analysis, the cells from the mice were stained with listed BioLegend antibodies: human CD45 (PerCP, HI30), CD3 (APC, HIT3a), CD4 (PE, OKT4), CD8 (APC/Cy7, SK1), CD19 (Brilliant Violet 711, HIB19), CD33 (Brilliant Violet 421, WM53), CD56 (Brilliant Violet 605, HCD56), CD34 (PE, 561), CD38 (PE/Cy7, HIT2), CD45RA (APC/Cy7, HI100), and CD90 (APC, 5E10) and mouse CD45 (FITC, 30-F11).
HIV-1 Challenge
Eleven weeks after transplantation with either CCR5-disrupted or unmodified CD34+ HSPCs, mice were housed in an animal biosafety level 3 (ABSL-3) laboratory for 1 week before intraperitoneal injection with Bal-1 virus (provided from the Chinese Center for Disease Control and Prevention [China CDC]). Mouse peripheral blood samples were collected biweekly. Human cell engraftment was detected using flow cytometry as previously described, and the viral load was determined based on the HIV-1 RNA level in mouse peripheral blood plasma performed by the China CDC. Eight weeks post-virus infection, all mice were euthanized, and the CCR5 disruption was analyzed. Statistical analyses were performed by unpaired t test or unpaired Student’s t test for pairwise comparison using GraphPad Prism 7.
Author Contributions
H.D., H.C., C.W., and B.Z. conceived and supervised the project. L.X., H.Y., and Y.G. designed the experiments, performed the experiments, and analyzed the data. Z.C., L.X., Y.L., X.W., H.L., Y.H., A.Y., and L.M. assisted with the experiments. W.L. and Y.S. provided technical assistance. L.X., Y.L., L.X., and Z.C. contributed to manuscript preparation.
Conflicts of Interest
The authors declare no competing financial interests.
Acknowledgments
We thank Jing Zhang, Jingwen Niu, Lina Zhou, Li Liao, Weichao Du, Jingyun Li, and Daomin Zhuang for technical assistance. We thank Jun Xu, Xu Zhang, and Ting Zhao for assistance with the written manuscript. We thank the Core Facilities at School of Life Sciences, Peking University, for assistance. This work was supported by the Beijing Science and Technology Major Project (D171100000517004), the National Natural Science Foundation of China (31521004), the National Science and Technology Support Project (2014BAI02B01), the National High Technology Research and Development Program of China (2013ZX10001003003), the Guangdong Innovative and Entrepreneurial Research Team Program (2014ZT05S216), the Science and Technology Planning Project of Guangdong Province (2014B020226001), the Science and Technology Program of Guangzhou (2016B030232001), and the Ministry of Education of China (111 Project). This work was supported in part by a grant from BeiHao Stem Cell and Regenerative Medicine Translational Research Institute.
Contributor Information
Hu Chen, Email: chenhu217@aliyun.com.
Hongkui Deng, Email: hongkui_deng@pku.edu.cn.
References
- 1.Deng H., Liu R., Ellmeier W., Choe S., Unutmaz D., Burkhart M., Di Marzio P., Marmon S., Sutton R.E., Hill C.M. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 2.Samson M., Libert F., Doranz B.J., Rucker J., Liesnard C., Farber C.M., Saragosti S., Lapoumeroulie C., Cognaux J., Forceille C. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 3.Liu R., Paxton W.A., Choe S., Ceradini D., Martin S.R., Horuk R., MacDonald M.E., Stuhlmann H., Koup R.A., Landau N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 4.Hütter G., Nowak D., Mossner M., Ganepola S., Müssig A., Allers K., Schneider T., Hofmann J., Kücherer C., Blau O. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 5.Allers K., Hütter G., Hofmann J., Loddenkemper C., Rieger K., Thiel E., Schneider T. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 6.Holt N., Wang J., Kim K., Friedman G., Wang X., Taupin V., Crooks G.M., Kohn D.B., Gregory P.D., Holmes M.C., Cannon P.M. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol. 2010;28:839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiGiusto D.L., Cannon P.M., Holmes M.C., Li L., Rao A., Wang J., Lee G., Gregory P.D., Kim K.A., Hayward S.B. Preclinical development and qualification of ZFN-mediated CCR5 disruption in human hematopoietic stem/progenitor cells. Mol. Ther. Methods Clin. Dev. 2016;3:16067. doi: 10.1038/mtm.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L., Krymskaya L., Wang J., Henley J., Rao A., Cao L.F., Tran C.A., Torres-Coronado M., Gardner A., Gonzalez N. Genomic editing of the HIV-1 coreceptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases. Mol. Ther. 2013;21:1259–1269. doi: 10.1038/mt.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandal P.K., Ferreira L.M., Collins R., Meissner T.B., Boutwell C.L., Friesen M., Vrbanac V., Garrison B.S., Stortchevoi A., Bryder D. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell. 2014;15:643–652. doi: 10.1016/j.stem.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Y., Sander J.D., Reyon D., Cascio V.M., Joung J.K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 2014;32:279–284. doi: 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B., Gilbert L.A., Cimini B.A., Schnitzbauer J., Zhang W., Li G.W., Park J., Blackburn E.H., Weissman J.S., Qi L.S., Huang B. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155:1479–1491. doi: 10.1016/j.cell.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Mateos M.A., Vejnar C.E., Beaudoin J.D., Fernandez J.P., Mis E.K., Khokha M.K., Giraldez A.J. CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat. Methods. 2015;12:982–988. doi: 10.1038/nmeth.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upadhyay S.K., Sharma S. SSFinder: high throughput CRISPR-Cas target sites prediction tool. BioMed Res. Int. 2014;2014:742482. doi: 10.1155/2014/742482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez E.E., Wang J., Miller J.C., Jouvenot Y., Kim K.A., Liu O., Wang N., Lee G., Bartsevich V.V., Lee Y.L. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 2008;26:808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson C.W., Wang J., Norman K.K., Norgaard Z.K., Humbert O., Tse C.K., Yan J.J., Trimble R.G., Shivak D.A., Rebar E.J. Long-term multilineage engraftment of autologous genome-edited hematopoietic stem cells in nonhuman primates. Blood. 2016;127:2416–2426. doi: 10.1182/blood-2015-09-672337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dever D.P., Bak R.O., Reinisch A., Camarena J., Washington G., Nicolas C.E., Pavel-Dinu M., Saxena N., Wilkens A.B., Mantri S. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–389. doi: 10.1038/nature20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J.P., Li X.L., Neises A., Chen W., Hu L.P., Ji G.Z., Yu J.Y., Xu J., Yuan W.P., Cheng T., Zhang X.B. Different effects of sgRNA length on CRISPR-mediated gene knockout efficiency. Sci. Rep. 2016;6:28566. doi: 10.1038/srep28566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tycko J., Myer V.E., Hsu P.D. Methods for optimizing CRISPR-Cas9 genome editing specificity. Mol. Cell. 2016;63:355–370. doi: 10.1016/j.molcel.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner J.E., Jr., Brunstein C.G., Boitano A.E., DeFor T.E., McKenna D., Sumstad D., Blazar B.R., Tolar J., Le C., Jones J. Phase I/II trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell. 2016;18:144–155. doi: 10.1016/j.stem.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Ravin S.S., Reik A., Liu P.Q., Li L., Wu X., Su L., Raley C., Theobald N., Choi U., Song A.H. Targeted gene addition in human CD34(+) hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat. Biotechnol. 2016;34:424–429. doi: 10.1038/nbt.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genovese P., Schiroli G., Escobar G., Di Tomaso T., Firrito C., Calabria A., Moi D., Mazzieri R., Bonini C., Holmes M.C. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson C.W., Haworth K.G., Burke B.P., Polacino P., Norman K.K., Adair J.E., Hu S.L., Bartlett J.S., Symonds G.P., Kiem H.P. Multilineage polyclonal engraftment of Cal-1 gene-modified cells and in vivo selection after SHIV infection in a nonhuman primate model of AIDS. Mol. Ther. Methods Clin. Dev. 2016;3:16007. doi: 10.1038/mtm.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]