Abstract
Background
CPX-351 is a liposome-encapsulated fixed-molar ratio formulation of cytarabine and daunorubicin that exploits molar ratio-dependent drug-drug synergy to enhance anti-leukemic efficacy.
Methods
This Phase II study randomized 125 patients 2:1 to CPX-351 or investigator’s choice of first salvage chemotherapy. Patients with acute myeloid leukemia in first relapse after initial CR lasting ≥1month were stratified per European Prognostic Index (EPI) into favorable, intermediate, and poor-risk groups based upon duration of first CR, cytogenetics, age, and transplant history. Control salvage treatment was usually based on cytarabine and anthracycline, often with one or more additional agents. Survival at 1-year was the primary efficacy endpoint.
Results
Patient characteristics were well balanced between the two study arms. Improvements in efficacy outcomes were observed following CPX-351, but did not meet prospectively defined statistical criteria for 1-year survival improvement in the overall population. Subset analyses of the EPI-defined poor-risk strata demonstrated higher response rates (39.3% vs. 27.6%), improvements in event-free survival (HR=0.63, p=0.08) and overall survival (HR=0.55, p=0.02). Also, 60-day mortality was lower in the CPX-351 study arm for poor-risk patients (16.1% vs. 24.1%).
Conclusion
Taken together, the data suggest possible improved outcome in CPX-351-treated first relapse AML patients with EPI-defined poor-risk disease.
Introduction
Patients with acute myeloid leukemia (AML) who relapse have poor prognosis marked by reduced response rates, short response duration, and short survival. Absent effective salvage treatment experimental therapy is considered appropriate.1
Addition of one or more new agents, e.g. gemtuzumab ozogamicin, fludarabine, or etoposide, to cytarabine and anthracycline-based treatment regimens, has had limited success, particularly in patients with poor risk characteristics.2 An alternative approach would be to optimize the cytarabine and anthracycline treatment backbone itself. In vitro observations suggest that drug-drug interactions between cytarabine and daunorubicin may be exploited to improve efficacy for this chemotherapy doublet.3 The ratiometric dosing hypothesis postulates that efficacy for certain chemotherapy doublets may depend on the molar ratio of the two drugs and that drug delivery at molar ratios found in vitro to be consistently synergistic may enhance efficacy.
CPX-351 is a liposomal formulation of cytarabine and daunorubicin encapsulated at a 5:1 molar ratio, which was found to be maximally synergistic and minimally antagonistic in vitro3. Liposomal encapsulation markedly increases the plasma half-life of cytarabine and daunorubicin4, 5 and leads to drug accumulation within the bone marrow6. There is evidence that drug-containing liposomes may preferentially enter leukemic blasts within the marrow.6, 7 CPX-351 was markedly active in preclinical leukemia models and in relapsed leukemia patients. In the phase I study, response was first documented at one-third of the maximum tolerated dose (MTD) in patients, most of them previously treated with cytarabine and daunorubicin.4
CPX-351 was compared against investigator’s choice of first salvage treatment in adult patients with first relapse AML. We hypothesized that CPX-351 would produce better clinical outcomes than available salvage treatment. No single regimen was acceptable to all investigators as control, reflecting the absence of effective therapy. Investigators choice of treatment was expected to include regimens with relatively intensive cytarabine and anthracycline often with addition of one or more new agents not used in first-line treatment. Consequently, this study compares liposome encapsulated fixed molar ratio cytarabine and daunorubicin against salvage regimens administering cytarabine and anthracycline often with other agents.
Patients and Methods
Study Design
This is a multi-center, randomized, open-label study conducted in compliance with the Declaration of Helsinki and the Code of Good Clinical Practices (ClinicalTrials.gov identifier: NCT00822094). The primary efficacy endpoint was survival at 12-months and hypothesis generating subset analyses were performed to identify subjects for later study. This study was powered to reveal trends (P<0.1) warranting further study in subsequent clinical trials.
Patients were stratified using the European Prognostic Index (EPI)8, then randomized 2:1 to receive either CPX-351 or investigator’s choice of salvage therapy. Up to two inductions and two consolidation courses were to be administered. Allogeneic stem cell transplant (HSCT) for consolidation was allowed. Monitoring for safety continued until 30 days after treatment completion and for relapse and survival until 1-year after randomization.
Treatment
CPX-351 100 units/m2 (1 unit=1.0 mg cytarabine + 0.44 mg daunorubicin) was administered by 90-minute infusion on days 1, 3, and 5 (first induction) and days 1 and 3 (second induction and consolidation). Control therapy consisted of cytarabine (97.7%) and anthracycline (77.3%) usually with additional agents (79.4%), such as etoposide (54.5%) or gemtuzumab ozogamicin (18.2%) (Table 1).
Table 1.
Intensive Salvage Regimens in Control Arm
| n | |
|---|---|
| Mitoxantrone/Etoposide/Cytarabine | 23 |
| Idarubicin/Cytarabine | 8 |
| Other Cytarabine-based* induction: | |
| +Fludarabine ± gemtuzumab ozogamicin | 5 |
| +Amsacrine | 2 |
| +Mitoxantrone ± gemtuzumab ozogamicin | 2 |
| +Gemtuzumab ozogamicin | 1 |
| +Cladribine | 1 |
| Cytarabine* alone | 1 |
| Mitoxantrone + Etoposide | 1 |
Cytarabine was usually administered at doses >1gm/m2/dose.
Supportive care included chemotherapy premedication, infection prophylaxis (antibiotic, antifungal, and antiviral), and hematopoietic growth factors per local practice.
Patient Eligibility
AML patients with pathologically confirmed previously untreated first relapse, age 18–65, were eligible. Eligibility required ECOG performance status 0–2, serum creatinine <2.0 mg/dL, total bilirubin <2.0 mg/dL, serum ALT/AST <3 times the upper limit of normal, and LVEF >50% by echocardiography or MUGA scan. Patients with active second malignancies, acute promyelocytic leukemia, prior cumulative daunorubicin-equivalent exposure >368 mg/m2, inability to give full informed consent, active CNS leukemia, New York Heart Association Class III/IV cardiac impairment, uncontrolled bacterial or invasive fungal infection, history of copper handling disorder (e.g. Wilson’s disease), and pregnant or breast feeding women were ineligible.
Assessments
Survival was assessed until 1-year after randomization, event-free survival (EFS) until documentation of persistent AML, relapse, or death and response was assessed according to International Working Group criteria.9
Safety assessments complied with GCP/ICH guidelines, used CTCAE V3.0 criteria, and included early mortality (at day 30, 60, and 90), serious adverse events, hematological and non-hematological adverse events, and laboratory assessments.
Statistical Analysis
Chi-square, Fisher’s exact, and Kruskal-Wallis tests were used to assess baseline patient characteristics. EFS and OS analyses were performed by Cancer Research And Biostatistics, Seattle, WA using the method of Kaplan and Meier (K-M). Transplanted patients were censored at the start of conditioning and progression-free patients were censored at last contact for EFS. Patients alive at last contact were censored for survival. This study had 83.6% power (with a one sided alpha=0.1) to detect an absolute increase of 23% (from 27% to 50%) in survival rate at 1 year.
Results
Patient Characteristics
Eighty-one patients were randomized to CPX-351 and 44 to control over a period of 21 months. The CPX-351 group had younger median age (52 vs. 56 years) and more patients with secondary AML (12.3% vs. 6.8%). Patient characteristics (Table 2) were well balanced except for a higher rate of prior transplant in the CPX-351 group (27.2% vs. 15.9%). Most patients (68%) were in the poor-risk strata.
Table 2.
Patient Characteristics and Outcomes
| All Patients | Poor Risk Patients | |||||
|---|---|---|---|---|---|---|
| Demographics | CPX-351 | Salvage | CPX-351 | Salvage | ||
| n (%) | n (%) | n (%) | n (%) | |||
| n=81 | n=44 | n=56 | n=29 | |||
| Gender | Male | 38 (46.9) | 19 (43.2) | 25 (44.6) | 11 (37.9) | |
| Age Group | 18 – 60 yr | 68 (84.0) | 35 (79.5) | 44 (78.6) | 21 (72.4) | |
| 61 – 65 yr | 13 (16.0) | 9 (20.5) | 12 (21.4) | 8 (27.6) | ||
| Median (yr) | 52 | 56 | 56 | 58 | ||
| Race | Caucasian | 70 (86.4) | 36 (81.8) | 50 (89.3) | 26 (89.7) | |
| AML Type | De novo AML | 71 (87.7) | 41 (93.2) | 47 (83.9) | 28 (96.6) | |
| Secondary AML | 10 (12.3) | 3 (6.8) | 9 (16.1) | 1(3.4) | ||
| WBC at Baseline | < 20 × 109/L | 65 (80.2) | 38 (86.4) | 45 (80.4) | 23 (11.5) | |
| 20 – 100×109/L | 16 (19.8) | 5 (11.4) | 11 (19.6) | 5 (17.2) | ||
| > 100×109/L | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (3.4) | ||
| European Prognostic Index Factors | Points | |||||
| Risk Group | Favorable | 1–6 | 9 (11.1) | 6 (13.6) | -- | -- |
| Intermediate | 7–9 | 16 (19.8) | 9 (20.5) | -- | -- | |
| Poor | 8–14 | 56 (69.1) | 29 (65.9) | 56 (100) | 29 (100) | |
| Relapse-free Interval from CR1 (months) | > 18 | 0 | 11 (13.6) | 8 (18.2) | 0 (0.0) | 1 (3.4) |
| 6–18 | 3 | 43 (53.1) | 20 (45.5) | 30 (53.6) | 12 (41.4) | |
| ≤ 6 | 5 | 27 (33.3) | 16 (36.4) | 26 (46.4) | 16 (55.2) | |
| Cytogenetics at Diagnosis | Inv(16) or t(16;16) | 0 | 7 (8.6) | 4 (9.1) | 0 (0.0) | 0 (0.0) |
| t(8;21) | 3 | 2 (2.5) | 1 (2.3) | 0 (0.0) | 0 (0.0) | |
| Other | 5 | 72 (88.9) | 39 (88.6) | 56 (100) | 29 (100) | |
| Age at First Relapse (years) | ≤ 35 | 0 | 10 (12.3) | 4 (9.1) | 4 (7.1) | 2 (6.9) |
| 36 – 45 | 1 | 14 (17.3) | 7 (15.9) | 5 (8.9) | 1 (3.4) | |
| > 45 | 2 | 57 (70.4) | 33 (75.0) | 47 (83.9) | 26 (89.7) | |
| HSCT before Relapse | No | 0 | 59 (72.8) | 37 (84.1) | 39 (69.6) | 24 (82.8) |
| Yes | 2 | 22 (27.2) | 7 (15.9) | 17 (30.6) | 5 (17.2) | |
| Outcomes | ||||||
| Response (CR+CRi) | MLFS* | 58/75* (77.3) | 25/42* (59.5) | 37/56 (66.1) | 13/29 (44.8) | |
| CR+CRi | 40 (49.4) | 18 (40.9) | 22 (39.3) | 8 (27.6) | ||
| CR | 30 (37.0) | 14 (31.8) | 16 (28.6) | 6 (20.7) | ||
| CRi | 10 (12.3) | 4 (9.1) | 6 (10.7) | 2 (6.9) | ||
| Favorable | 7 (8.6) | 5 (11.4) | -- | -- | ||
| Intermediate | 11 (13.6) | 5 (11.4) | -- | -- | ||
| Poor | 22 (27.2) | 8 (18.2) | 22 (39.3) | 8 (27.6) | ||
| Early Mortality | 30 days | 6 (7.4) | 2 (4.5) | 5 (8.9) | 2 (6.9) | |
| 60 days | 12 (14.8) | 7 (15.9) | 9 (16.1) | 7 (24.1) | ||
| 90 days | 15 (18.5) | 13 (29.5) | 12 (21.4) | 11 (37.9) | ||
| EFS (median, mos.) | -- | 4.0 | 1.5 | 2.0 | 1.2 | |
| OS (median, mos.) | -- | 8.5 | 6.3 | 6.6 | 4.2 | |
| Post Induction HSCT | ALL | 38 (46.9) | 21 (47.7) | 23 (41.1) | 11 (37.9) | |
| CR | 22 (27.2) | 12 (27.3) | 10 (17.6) | 5 (17.2) | ||
| CRi | 6 (7.4) | 4 (9.1) | 4 (7.1) | 2 (6.9) | ||
| NR | 10 (12.3) | 5 (11.4) | 9 (16.1) | 4 (13.8) | ||
SixCPX-351 patients and two Control patients did not have a Day 14-21 bone marrow. MLFS= morphologic leukemia-free state: bone marrow blasts <5% absence of Auer rods and extramedullary disease on or after Day 14
Treatment Received
Few patients required two inductions (10/81, 12.3% vs. 3/44, 6.8%). Most CPX-351 patients achieving CR or CRi had post remission therapy (85%) including 19 (47.5%) with one (15) or two (4) courses of CPX-351 consolidation, and 15 with allogeneic transplant in place of CPX-351 consolidation and 13 with transplant following consolidation. All 18 control arm responders had post-remission therapy: 7 with one (3) or two (4) chemotherapy courses and 11 with HSCT in place of chemotherapy consolidation and 5 with transplant following consolidation. Similar proportions of patients were transplanted after induction failure (24.4% vs. 19.2%). Patients transplanted after induction failure had similar outcomes in both study arms with similar proportions alive >300 days (60% vs. 60%) alive at 201–300 days (10% vs. 20%), alive 101–200 days (20% vs 20%), and alive <100 days (10% vs. 0%). Study treatment did not appear to have an effect on the outcomes of patients transplanted after induction failure.
Efficacy
Response was more frequent following CPX-351 (30CR (37%) + 10CRi (12.3%) than control (14CR (31.8%) + 4CRi (9.1%). Factors including duration of first CR, age and adverse cytogenetics that might have affected the CR rate were examined by multivariate logistic regression. Duration of first CR of 7+ months (p=0.0001) and adverse cytogenetic risk (p=0.0022) had a significant correlation with induction response. Age was not a significant factor.
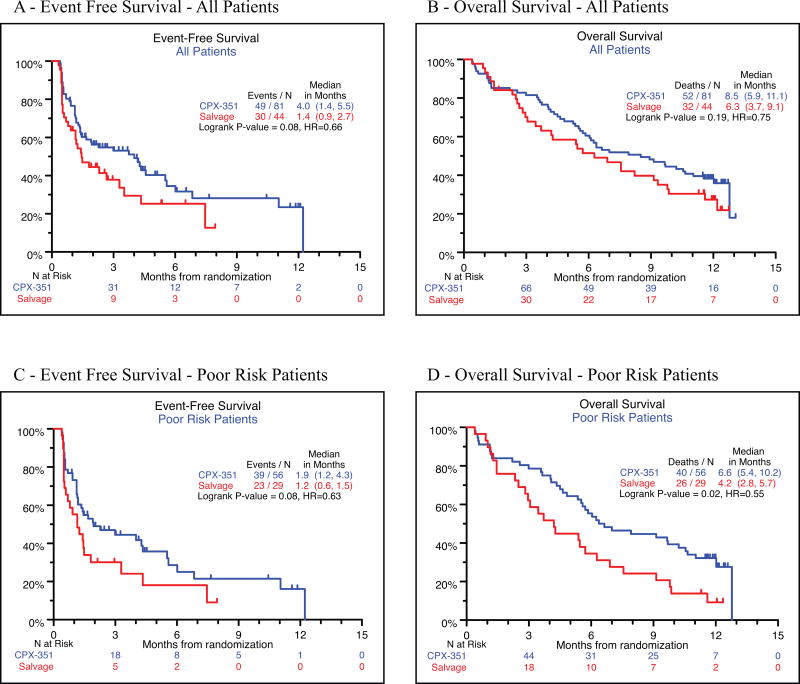
Kaplan Meier analysis of overall survival (HR=0.75, p=0.33), and EFS (HR=0.66, p=0.08) did not demonstrate significant differences. (Figures 1A and 1B). Survival at 1-year was 36% vs. 27% (p=0.33).
Figure 1.
CONSORT diagram.
Although outcomes of favorable vs. intermediate vs. poor-risk groups by EPI scoring were not compared formally due to small numbers in the favorable (n=15, 12%) and intermediate (n=25, 20%) risk groups, poor-risk patients tended to have lower rates of CR (all patients: 37.0% vs. 31.8%; poor-risk 28.6% vs. 20.7%) and CRi (all patients: 12.3% vs. 9.1%; poor-risk 10.7% vs. 6.9%), higher rates of 60-day mortality (all patients: 14.8% vs. 15.9%; poor-risk 16.1% vs. 24.1%), and median survival was slightly shorter as well (all patients: 8.5 mos. vs. 6.3 mos.; poor-risk 6.6 mos. vs. 4.2 mos.). Outcomes assessed by a number of measures tended to be slightly but consistently worse for the EPI poor risk group.
The EPI-defined poor-risk strata accounted for 68% (85/125) of all patients and efficacy differences favoring CPX-351 were observed for this group (Table 2). This CPX-351 subgroup had higher rates of Day 14 morphologic leukemia free bone marrow (66.1% vs. 44.8%, P = 0.07), with higher CR (28.6% vs. 20.7%) and CRi (10.7% vs. 2.9%) rates, a favorable trend for EFS (HR=0.63, p=0.08), and a statistically significant improvement in OS (HR=0.55, p=0.02). (Figures 1C and 1D). Survival at 12 months was 28% (19–46%) for the CPX-351 group and 9% (0–20%) for the control group. A test for the level of heterogeneity between different strata treated on this study was performed comparing the CPX-351 poor-risk, CPX-351 non-poor risk, Control poor risk, and Control non-poor risk strata. Log-rank testing of differences among strata for OS was highly significant (P=0.0003) and for EFS was significant (p=0.009).
Safety
Early mortality was similar at 30-days (7.4% vs. 4.5%) and at 60-days (14.8% vs. 15.9%). By 90-days deaths were more frequent in the control arm (21.4% vs. 37.9%). Sixty day deaths on the CPX-351 arm were due to infections (8), progressive AML (1), acute respiratory failure (1), CNS hemorrhage after a fall with head trauma (1), and sudden cardiac death (1). Sixty day deaths among control arm patients were due to infections (4), progressive AML (2), and unknown causes (1). CPX-351-treated patients had slower neutrophil recovery (time to ANC >1000/μL, 42d vs. 34d) and platelet recovery (time to platelets >100,000/μL, 45d vs. 35d) compared to control. Delayed hematologic recovery was associated with more infection-related events including: febrile neutropenia, bacteremia, pneumonia, sepsis, urinary tract infection, pyrexia, and cellulitis but did not increase the rate of infection-related deaths by day 60 (9.9% vs. 9.1%). The rate of grade 5 adverse events, regardless of causality, was similar between the two arms (23.5% vs. 20.5%).
Impact of Prior HSCT on Outcomes
Ninety-six patients (77%) had no history of transplant at study entry with CPX-351 treated patients having higher CR+CRi rates (54.2% vs. 37.8%) and similar 30-day (3.4% vs. 5.4%), and 60-day mortality (10.2 vs. 16.2%). In contrast, 22 of 29 patients (75.9%) with prior HSCT were randomized to CPX-351 and had lower response rates (36.4% vs. 57.1%) and higher 30-day (18.2% vs. 0) and 60-day mortality (27.3% vs. 14.3%) compared with control (Table 4). Notably, 6 of the 12 CPX-351 patients dying by day 60 had prior HSCT and the causes included infection (5) and progressive AML (1). One of 7 patients with prior HSCT randomized to control arm therapy died within 60 days of infection.
Table 4.
Outcome by Prior Exposure to HSCT
| Prior HSCT | No Prior HSCT | |||
|---|---|---|---|---|
| n=29 | (n=96) | |||
|
|
||||
| CPX-351 n=22 |
Salvage n=7 |
CPX-351 n=59 |
Salvage n=37 |
|
| n (%) | n (%) | n (%) | n (%) | |
|
|
||||
| CR+ CRi | 8 (36.4) | 4 (57.1) | 32 (54.2) | 14 (37.8) |
|
| ||||
| EPI Favorable Risk | 0/0 | 0/0 | 7/9 (77.8) | 5/6 (83.3) |
|
| ||||
| EPI Intermediate Risk | 3/5 (60.0) | 1/2 (50.0) | 8/11 (72.7) | 4/7 (57.1) |
|
| ||||
| EPI Poor Risk | 5/17 (29.4) | 3/5 (60.0) | 17/39 (43.6) | 5/24 (20.8) |
|
| ||||
| 30 Day Mortality | 4 (18.2) | 0 | 2 (3.4) | 2 (5.4) |
|
| ||||
| 60 Day Mortality | 6 (27.3) | 1 (14.3) | 6 (10.2) | 6 (16.2) |
Factors Potentially Influencing Outcomes
Post induction transplants were common and equally frequent in the two study arms (46.9% vs. 47.7%) but were somewhat more frequent in the control arm after CR (73% vs. 86%) and CRi (60% vs. 100%) (Table 2). Non-responding patients were also transplanted (24.4% vs. 19.2%). Survival after response and transplant was similar in both study arms. CR patients and CRi patients across both study arms also had similar survival after transplant. Transplants had similar impact on both study arms.
The duration of first CR was well balanced between the two study arms with similar proportions of patients with CR1 duration lasting <6 months (33.3% vs. 36.4%), 7–18 months (53.1% vs. 45.5%), and >18 months (13.6% vs. 18.2%). This result suggests that differences in study outcomes were not likely due to differences in first CR duration.
Treatments administered to induce and consolidate first CR were similar between CPX-351 and control groups. Only one patient in each arm received induction treatment with less than the intensity of conventional 7+3 (1.2% vs. 2.3%) and most patients received 7+3 induction (55.6% vs. 63.6%) with a substantial minority treated with greater intensity (43.2% vs. 34.1%), usually in the form of another drug added to 7+3. A small minority of patients received post remission therapy with lower intensity regimens (17.3% vs. 13.6%), while most patients received HiDAC (66.7% vs. 70.5%) or HiDAC regimens with another drug added (13.6% vs. 13.6%). Only 2 (2.5%) of CPX-351 patients and 1 (2.3%) control arm patient received no post remission therapy whatsoever. Somewhat more patients on the CPX-351 arm had prior HSCT (27.2% vs. 15.9%). Although the numbers are small, there does not appear to be a marked difference in the amount of chemotherapy administered to patients in either arm to induce or consolidate their initial response.
The profile of cytarabine dosing in the control arm was examined. One of 44 patients received no cytarabine, 4 received 100 mg/m2 (3 patients) or 200 mg/m2 (1 patient) 7 day infusions of cytarabine, 30 received 1gm/m2/day x5-6 days usually with mitoxantrone etoposide or fludarabine + gemtuzumab ozogamicin, and the remaining 9 patients received more intensive cytarabine regimens (>1gm/m2/day x6 days up to 12gm/m2/day×3 days). Control arm response rates were similar between those receiving the highest dose cytarabine regimens (9 patients, 33% CR) and those receiving lower doses of cytarabine (30 patients 40% CR). Investigator’s choice of salvage therapy relied mostly on intermediate and higher doses of cytarabine.
Discussion
Patients with first relapse AML have limited likelihood of response and short expected survival following salvage treatment. For example, mitoxantrone, etoposide, and cytarabine (MEC) induces response in 23% of patients with median overall survival of only 2 months.10 Modulation of deoxycitidine kinase by fludarabine, led to the combination of fludarabine and cytarabine, resulting in a 36% CR rate with median remission duration of 39 weeks.11 First salvage gemtuzumab ozogamicin induces CR+CRp response in 30% of patients with CD33+ AML12 and, for patients with short 1st CR durations, appeared to be superior to cytarabine-based therapy.13 The need for improved salvage therapy remains evident.
CPX-351 delivers the two most active single agents at a fixed 5:1 molar ratio, shown to be consistently synergistic in vitro and in preclinical leukemia models3,14 In the current study patients were prospectively selected, stratified, randomized and treated. Breems, et al8 created a scoring system (EPI) based on duration of first remission, cytogenetics at diagnosis, age at relapse and history of transplant, which was used to stratify patients on this study. The rate of morphologic leukemia free-state assessed at the time of early (Day 14) response assessment was clearly higher following CPX-351 (76.0% vs. 51.2%) and the rate of subsequent CR+CRi response assessed at time of hematologic recovery was modestly improved among all patients (49.4% vs. 40.9%) compared to control. In spite of these observations, significant improvements in 1-year survival, OS, and EFS were not achieved. Subset analyses however, indicated that patients in the poor risk strata as defined by the European Prognostic Index might benefit. Patients with poor-risk EPI scores constituted 66.7% of the patients in the Breems8 series and had an expected 1-year probability of survival of 16%, very similar to the poor risk strata in our study with 68% of the patients and 9% 1-year survival after control arm treatment. In contrast, the 1-year survival was 29% in the CPX-351 poor-risk group and the observed response rate was higher as well.
Improved 1-year survival following CPX-351 in the poor-risk strata is attributed to the higher rate of response and was associated with a lower rate of 90-day (21.4% vs. 37.9%) mortality. We speculate that the increased rate of leukemia clearance reflected in the day 14 bone marrow examination (77.3% vs. 59.5%) may reduce the impact of active leukemia on early mortality, which begins to be seen by day 60 and becomes fully evident by day 90. Early reduction of tumor burden may delay progression to death, even in patients who do not ultimately achieve a full CR.
The longer duration of bone marrow suppression in the CPX-351 arm can be interpreted as reflecting a more intensive treatment and this view is supported by preclinical evidence of cytarabine and daunorubicin accumulation within the marrow following CPX-351 treatment.6 Still, the 30 and 60-day induction mortality rates and the rates of non-hematologic adverse events were similar in both study arms, suggesting comparable overall treatment intensity.
Survival analyses were unlikely to have been affected by post-induction transplants because the overall rates of transplantation were similar in both arms and because there was no difference in survival after transplant.
An unexpected finding was that patients with stem cell transplants prior to CPX-351 treatment appeared to have poorer outcomes. A disproportionate number of patients with prior transplant were allocated to the study arm with 6 of 22 patients (27.3%) dying within 60-days after CPX-351 treatment. These six patients were half of all the 60-day deaths on the CPX-351 arm. While not definitive, these results suggest caution in future studies of CPX-351 if patients with prior transplant are considered for treatment. The poor outcomes could be due to leukemia persistence, increased treatment morbidity, or insufficient bone marrow reserve for timely recovery following CPX-351 therapy. It is premature to conclude that there is no role for CPX-351 in this population but additional study will be needed to confirm the safety of treatment for this group. The 96 patients without prior transplant performed considerably better following CPX-351 treatment with higher rates of response, similar rates of 60-day mortality and lower rates of mortality by 90 days.
Although both study arms produced similar outcomes in patients with lower risk disease, the poor-risk strata tended to respond more often and have better outcomes when treated with CPX-351 compared to control. This differential response to CPX-351 is consistent with what is known about the pharmacological and pharmacodynamic actions of CPX-351. Patients with poor-risk first relapse AML often have adverse cytogenetics and early relapse (<6 months) following initial treatment with cytarabine and anthracycline and poor outcome is often associated with altered cellular drug transport, increased drug inactivation, and altered signaling pathways that inhibit cell cycle-dependent drug action. Poor-risk biology blunts the effect of treatment by reducing drug levels at sites of pharmacologic action and/or reducing susceptibility to killing after exposure of drugs to the leukemia cell. For example, leukemic cells from high-risk patients are more likely to over-express several membrane-associated drug transport proteins, such as MDR1 and MRP, resulting in reduced uptake and retention of anthracyclines such as daunorubicin and mitoxantrone.16 Evidence from leukemia cell lines and AML patient blasts obtained from marrow and peripheral blood indicate that intact CPX-351 liposomes are likely to be internalized by leukemia cells in an energy-dependent fashion followed by intracellular release of drug.6 These observations help explain the increased and prolonged cytarabine and daunorubicin drug concentrations within bone marrow following CPX-351 dosing and suggest that this approach may bypass membrane transporter-based resistance mechanisms which limit exposure of leukemic cells to drug. In addition, delivery of excess cytarabine relative to daunorubicin (the 5:1 molar ratio) may help to reduce the potential for daunorubicin-induced G2/M phase arrest, which could block leukemic cells from entering S-phase, potentially inhibiting cytarabine action due to its S-phase specificity. These mechanistic hypotheses need to be confirmed in subsequent trials.
Another consideration is that liposomal encapsulation of cytarabine and daunorubicin results in marked increases in plasma half-life (>24 hours) for both drugs, with a negligible distribution phase, where detectable plasma drug levels are extended deep into the second week after the start of induction treatment.4 It is possible that marked prolongation of exposure to cytarabine and daunorubicin could extend the duration of drug action and increase treatment-induced cytotoxicity to very slowly replicating leukemic cells. In addition, leukemic cells from high risk and secondary AML patients with mixed lineage leukemia (MLL) gene translocations block the functional response of P53 to chemotherapy in preclinical models, limiting induction of apoptosis to much slower P53-independent processes.17, 18 The marked increase in plasma half-life of cytarabine and daunorubicin following CPX-351 administration may extend the period of cytotoxic drug concentrations enabling effective treatment. In contrast, drug levels rapidly diminish after completing infusions of unencapsulated cytarabine and anthracycline, with rapid loss of cytotoxicity against leukemia cells with such phenotypes. These considerations may help to explain why the impact of CPX-351 treatment is greater on patient populations with more difficult to treat disease.
In summary, although this randomized study did not meet pre-specified goals for survival at 1-year in the overall patient population, it was able to generate a number of hypotheses based upon subgroup analyses. Possible clinical benefit after CPX-351 treatment was observed for patients stratified to the poor-risk subgroup defined by European Prognostic Index scoring. Preliminary findings also suggest that CPX-351-treated patients with prior stem cell transplant may be at risk for poorer outcomes and simultaneously raise the possibility that CPX-351 patients with no prior history of transplant may have improvements in response rate and 60-day mortality. All of these findings are preliminary but are consistent with observations from a companion randomized Phase II study suggesting that AML patients with poor risk characteristics may have improved outcomes in the newly diagnosed AML setting (In press, Blood). Observations from this study extend and support the rationale behind an ongoing randomized Phase III trial being conducted in patients with high risk (secondary) AML.
Figure 2.
EFS and OS Kaplan-Meier curves.
Table 3.
Non-Hematologic Treatment Emergent Adverse Events
| Grade 3 | Grade 4 | Grade 5 | Grades 3–5 | |||||
|---|---|---|---|---|---|---|---|---|
| CPX-351 n=81 n(%) |
Salvage n=44 n(%) |
CPX-351 n=81 n(%) |
Salvage n=44 n(%) |
CPX-351 n=81 n(%) |
Salvage n=44 n(%) |
CPX-351 n=81 n(%) |
Salvage n=44 n(%) |
|
| Febrile Neutropenia | 43 (53) | 14 (32) | 1 (1) | 1 (2) | 0 (0) | 0 (0) | 44 (54) | 15 (34) |
|
| ||||||||
| Bacteremia | 21 (26) | 17 (39) | 3 (4) | 1 (2) | 0 (0) | 1 (2) | 24 (30) | 19 (43) |
|
| ||||||||
| Pneumonia1 | 11 (14) | 2 (5) | 3 (4) | 0 (0) | 4 (5) | 2 (5) | 18 (22) | 4 (9) |
|
| ||||||||
| Sepsis | 2 (3) | 1 (2) | 5 (6) | 2 (5) | 4 (5) | 0 (0) | 11 (14) | 3 (7) |
|
| ||||||||
| Fatigue | 11 (14) | 1 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 12 (15) | 1 (2) |
|
| ||||||||
| Urinary Tract Infection2 | 5 (6) | 5 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (6) | 5 (11) |
|
| ||||||||
| Hypokalemia | 7 (9) | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (9) | 3 (7) |
|
| ||||||||
| Rash | 9 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (11) | 0 (0) |
|
| ||||||||
| Pyrexia | 7 (9) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (9) | 1 (2) |
|
| ||||||||
| Acute Renal Failure3 | 4 (5) | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (5) | 3 (7) |
|
| ||||||||
| Hypertension | 4 (5) | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (5) | 3 (7) |
|
| ||||||||
| Malignant Neoplasm Progressive4 | 2 (3) | 0 (0) | 1 (1) | 0 (0) | 2 (3) | 2 (5) | 5 (6) | 2 (5) |
|
| ||||||||
| Cellulitis | 4 (5) | 2 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (5) | 2 (5) |
includes Pneumocystis, Bacterial Pneumonia and Fungal Pneumonia
includes UTI Enterococcal
includes Renal Failure and Renal Tubular Necrosis
includes AML and Leukemic Infiltration
Acknowledgments
Grant: P30 CA016672
Funding: Celator Pharmaceuticals and the Leukemia and Lymphoma Society
Footnotes
Financial Disclosures: This research funded by Celator Pharmaceuticals
Conflict of Interest: None
Trial Registration: Clinicaltrials.gov: NCT00822094
References
- 1. [accessed December 2012];National Comprehensive Cancer Network. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- 2.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer LD, Harasym TO, Tardi PG, et al. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 4.Feldman EJ, Lancet JE, Kolitz JE, et al. First-In-Man Study of CPX-351: A Liposomal Carrier Containing Cytarabine and Daunorubicin in a Fixed 5:1 Molar Ratio for the Treatment of Relapsed and Refractory Acute Myeloid Leukemia. J Clin Oncol. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman EJ, Kolitz JE, Trang JM, et al. Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine:daunorubicin, in patients with advanced leukemia. Leuk Res. 2012;36:1283–1289. doi: 10.1016/j.leukres.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Lim WS, Tardi PG, Dos Santos N, et al. Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine:daunorubicin formulation, in bone marrow xenografts. Leuk Res. 2010;34:1214–1223. doi: 10.1016/j.leukres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Kim HP, Gerhard B, Harasym TO, Mayer LD, Hogge DE. Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp Hematol. 2011;39:741–750. doi: 10.1016/j.exphem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–1978. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien S, Kantarjian H, Estey E, et al. Mitoxantrone and high-dose etoposide for patients with relapsed or refractory acute leukemia. Cancer. 1991;68:691–694. doi: 10.1002/1097-0142(19910815)68:4<691::aid-cncr2820680404>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Estey E, Plunkett W, Gandhi V, Rios MB, Kantarjian H, Keating MJ. Fludarabine and arabinosylcytosine therapy of refractory and relapsed acute myelogenous leukemia. Leuk Lymphoma. 1993;9:343–350. doi: 10.3109/10428199309148532. [DOI] [PubMed] [Google Scholar]
- 12.Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 13.Leopold LH, Berger MS, Cheng SC, Cortes-Franco JE, Giles FJ, Estey EH. Comparative efficacy and safety of gemtuzumab ozogamicin monotherapy and high-dose cytarabine combination therapy in patients with acute myeloid leukemia in first relapse. Clin Adv Hematol Oncol. 2003;1:220–225. [PubMed] [Google Scholar]
- 14.Tardi P, Johnstone S, Harasym N, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33:129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Estey E, Kornblau S, Pierce S, Kantarjian H, Beran M, Keating M. A stratification system for evaluating and selecting therapies in patients with relapsed or primary refractory acute myelogenous leukemia. Blood. 1996;88:756. [PubMed] [Google Scholar]
- 16.Seedhouse CH, Grundy M, White P, et al. Sequential influences of leukemia-specific and genetic factors on p-glycoprotein expression in blasts from 817 patients entered into the National Cancer Research Network acute myeloid leukemia 14 and 15 trials. Clin Cancer Res. 2007;13:7059–7066. doi: 10.1158/1078-0432.CCR-07-1484. [DOI] [PubMed] [Google Scholar]
- 17.Zuber J, Radtke I, Pardee TS, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23:877–889. doi: 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]