Abstract
Introduction
As the HIV+ population ages, the risk for and need to screen for HIV-associated neurocognitive disorders (HAND) increases. We aimed to determine the utility and ecological validity of the Montreal Cognitive Assessment (MoCA) among older HIV+ adults.
Method
One hundred HIV+ older adults (≥50 years) completed a comprehensive neuromedical and neurocognitive battery, including the MoCA and several everyday functioning measures.
Results
Receiver operating characteristic curve indicated ≤26 as the optimal cut-point balancing sensitivity (84.2%) and specificity (55.8%) compared to ”gold standard” impairment as measured on a comprehensive neuropsychological battery. Higher MoCA total scores were significantly (p-values < 0.01) associated with better performance in all individual cognitive domains except motor abilities, with the strongest association with executive functions (r = −0.49, p < 0.01). Higher MoCA total scores were also significantly (p-values < 0.01) associated with fewer instrumental activities of daily living declines (r = −0.28), fewer everyday cognitive symptoms (r = −0.25), and better clinician-rated functional status (i.e., Karnofsky scores; r = 0.28); these associations remained when controlling for depressive symptoms. HIV+ individuals who were neurocognitively normal demonstrated medium-to-large effect size differences in their MoCA performances than those with asymptomatic neurocognitive impairment (d=0.85) or syndromic HAND (mild neurocognitive disorder or HIV-associated dementia; d=0.78), while the latter two categories did not differ.
Conclusions
Although limited by less than optimal specificity, the MoCA demonstrated good sensitivity and ecological validity, which lends support to its psychometric integrity as a brief cognitive screening tool among older HIV+ adults.
Keywords: HIV/AIDS, MoCA, cognitive assessment, IADLs, external validity, HIV/AIDS
Introduction
HIV-infected (HIV+) individuals are reaching life expectancies nearly comparable to HIV-negative individuals (May et al., 2014). In 2012, ~40.1% of persons with HIV-infection in the U.S. were 50 years or older (Centers for Disease Control, 2015). Effective treatment has decreased the prevalence of HIV-associated dementia (Dore et al., 2003; Robertson et al., 2007; Sacktor et al., 2002); however, milder forms of HIV-associated neurocognitive disorders (HAND) are still observed in an estimated 30–50% of HIV+ individuals (Heaton et al., 2011). Older HIV+ adults show two to three times higher risk for neurocognitive impairment as compared to younger HIV+ adults (Valcour et al., 2004) and a seven-fold risk compared to healthy comparison groups (Sheppard et al., 2015). HIV-related impairments commonly affect executive functions (Iudicello, Woods, Deutsch, Grant, & Group, 2012), episodic memory (Sacktor et al., 2007), prospective memory (Doyle et al., 2012; Woods, Dawson, Weber, Grant, & Group, 2010), and processing speed (Fellows, Byrd, & Morgello, 2014), and are associated with poorer everyday outcomes (e.g., antiretroviral nonadherence, dependence on activities of daily living; (Hinkin et al., 2004; Moore et al., 2014; Morgan et al., 2012; Rodriguez-Penney et al., 2013).
Given the aging of the HIV+ population (Centers for Disease Control, 2015) and prevalence of HAND, psychometrically sound neurocognitive screeners are needed to detect those among this vulnerable population who are experiencing neurocognitive difficulties. Best practices for diagnosing HAND include a comprehensive neuropsychological evaluation (Antinori et al., 2007), which is often not feasible for highly-impacted primary care or first-line specialty clinics. Precise, broad, sensitive, specific, brief, and low resource burden neurocognitive screening tools are necessary for identifying patients that may require a more comprehensive evaluation (Finkel, 2003). The Montreal Cognitive Assessment (MoCA) was developed for this purpose (Nasreddine et al., 2005), and has been validated in numerous clinical populations, including Alzheimer’s disease (Freitas, Simoes, Alves, & Santana, 2013; Nasreddine et al., 2005), Parkinson’s disease (Dalrymple-Alford et al., 2010; Gill, Freshman, Blender, & Ravina, 2008), stroke (Burton & Tyson, 2015), substance use disorders (Copersino et al., 2009), and cardiovascular disease (McLennan, Mathias, Brennan, & Stewart, 2011). The MoCA is popular because it is free, brief, more sensitive (Damian et al., 2011; Tsoi, Chan, Hirai, Wong, & Kwok, 2015), and more accurate in detecting cognitively impaired patients at higher risk for developing dementia than other widely-used neurocognitive screeners such as the Mini-Mental State Exam (MMSE; (Dong et al., 2012).
Although it is an empirically supported screener, relatively limited research exists on the efficacy of the MoCA to detect neurocognitive impairment among HIV+ patients as compared to other populations, and there is little to no research on the association of the MoCA with everyday functioning outcomes among older HIV+ adults. The latter is particularly important in determining the clinical relevance and ecological validity of the MoCA. Some studies converge to support the MoCA as a practical and valid neurocognitive screening tool in HIV+ adults (Brouillette et al., 2015; Chartier et al., 2015; Hasbun et al., 2012; Koski et al., 2011; Ku et al., 2014; Overton et al., 2013; Robbins et al., 2013; Valcour, 2011), although not sufficient as a stand-alone tool for diagnosing HAND (Chartier et al., 2015; Janssen, Bosch, Koopmans, & Kessels, 2015). To our knowledge, only one study has examined the MoCA as a neurocognitive screener in older HIV+ adults and found the MoCA moderately sensitive and specific for HIV+ adults aged 60 and older, yielding 72% sensitivity and 67% specificity with a cut-off of ≤25 (Milanini et al., 2014). However, the utility of the MoCA in predicting real-world outcomes important for treatment planning is not well understood.
The purpose of this study was to expand the current literature by examining the MoCA’s ability to identify “gold standard” neurocognitive impairment in a representative and well-characterized cohort of HIV-infected adults aged 50 and older (Centers for Disease Control, 2008; Stoff, Khalsa, Monjan, & Portegies, 2004). Additionally, we assessed the external validity of the MoCA by examining its associations with several indices of everyday functioning: self-reported everyday functioning and cognitive symptoms, as well as clinician-rated functional performance. External validity is of critical importance in determining implications for real-world outcomes. By comparing MoCA with a comprehensive “gold-standard” neurobehavioral battery, more accurate analyses can be made to determine sensitivity, specificity, and its overall accuracy as a neurocognitive screening tool in older HIV+ adults.
Methods
Subjects and Procedure
This study included 100 community-dwelling HIV-infected adults aged 50 years and above from the Successfully Aging Seniors with HIV (SASH) study conducted at University of California, San Diego (UCSD) HIV Neurobehavioral Research Program. The study was approved by the UCSD Institutional Review Board, and all participants provided written informed consent. Given that the goal of the larger SASH study was to include a representative cohort of HIV+ subjects, exclusion criteria were generally minimal with the exception of acute intoxication (e.g., positive urine toxicology screen), other neurodegenerative conditions (e.g., Parkinson’s Disease) and psychotic disorders (e.g., schizophrenia). Participants were reviewed for severe confounding neuromedical conditions that might negatively affect neurocognitive functioning and thus preclude a true HAND diagnosis as one would be unable to attribute impairment to direct effects of HIV (using validated methods described in detail elsewhere; i.e., (Heaton et al., 2010). Severe confound status was determined by two independent raters blinded to neuropsychological status (Master’s- and Doctoral-level psychology trainees; KBC and PLF, respectively) and confirmed by a clinical neuropsychologist (DJM). Severely confounded comorbidities in this sample were generally operationalized as: stroke, myocardial infraction, neurosyphilis, and/or severe head injury (e.g., coma with sequelae). Given the relatively small subset of participants classified as severely confounded (n = 16) as well as the high prevalence of neurological comorbidities in the general HIV population (and the need for screening for neurocognitive dysfunction in these individuals), we chose to include the entire cohort to enhance the ecological validity and generalizability of our study to the broader HIV population. Nonetheless, we examined whether exclusion of these 16 participants meeting severe confounding criteria impacted the psychometrics of the MoCA in detecting “gold standard” impairment. All subjects completed the MoCA, a comprehensive neurocognitive and neuromedical assessment, and everyday functioning questionnaires. Depressive symptoms were measured using the Beck Depression Inventory-II (BDI-II; (Beck, Steer, & Brown, 1996). Substance use disorders (i.e., including any current or past diagnosis of substance abuse and/or dependence) and major depressive disorder (MDD) diagnoses were assessed via the computer-assisted Composite International Diagnostic Interview, version 2.1 (Wittchen, 1994).
Measures
Montreal Cognitive Assessment (MoCA)
The MoCA is a nonproprietary, paper-and-pencil, brief (~10 minutes) cognitive screener used to assess major cognitive domains (i.e., attention, concentration, executive functions, memory, language, visuospatial skills, abstraction, calculation and orientation; (Nasreddine et al., 2005). The 10 items summed to create the total score include: visuospatial/executive (trail making, clock drawing, and cube drawing), naming (animals), memory (delayed recall), attention (digit span, vigilance, serial 7’s), language (sentence reading, fluency), abstraction, and orientation. The MoCA is scored out of 30 possible points, with higher scores indicating better functioning. Per MoCA guidelines, an education correction of 1 point was added to the total score for subjects with ≤ 12 years of education.
Neuropsychological and Everyday Functioning Measures
Neurocognitive Impairment
“Gold standard” neurocognitive impairment was classified via clinical ratings (CRs) using consensus research-based criteria (i.e., Frascati criteria; (Antinori et al., 2007) and using an approach consistent with the large multi-site CHARTER studies (Heaton et al., 2010; Heaton et al., 2011). The neurocognitive battery assessed the following seven domains commonly affected by HIV (Antinori et al., 2007): verbal fluency, abstraction/executive functioning, speed of information processing, visual and verbal learning, visual and verbal delayed recall (memory), attention/working memory, and motor skills (see Table 1 for a list of tests comprising each domain as well as sources of normative data). Raw neurocognitive test scores were converted into T-scores (standard scores with a mean of 50 and SD of 10) using demographically adjusted norms to control for the effects of age, education, gender, and where available race/ethnicity (Cherner et al., 2007; Heaton, Miller, Taylor, & Grant, 2004; Heaton, Taylor, & Manly, 2002; Norman et al., 2011). Demographically-corrected T-scores were used to assign algorithm-derived CRs for each of the seven neurocognitive domains (≥55 = CR 1 [above average]; 45–54 = CR 2 [average]; 40–44 = CR 3 [low average]; – = CR 4 [borderline]; 35–39 = CR 5 [definite mild impairment]; 30–34 = CR 6 [mild-to-moderate impairment]; 25–29 = CR 7 [moderate impairment]; 20–24 = CR 8 [moderate-to-severe impairment]; ≤19 = CR 9 [severe impairment]) (Woods et al., 2004). A global CR of ≥5 is indicative of neurocognitive impairment and requires at least two domains in the impaired range. Although there are nuances based on the number and types of measures in a domain, as well as pattern of impairment, in general, a global CR is calculated as the lowest domain CR minus one. In this manner, impaired domains are given particular weight in CR calculations (i.e., not necessarily an average). See Woods et al. (2004) for a more detailed discussion of the application and validation of clinical ratings. Thus, the “gold standard” CR impairment outcome included the Frascati criteria for HAND, which necessitates mild impairment in at least two cognitive domains vs. neurocognitively normal.
Table 1.
Tests and Sources of Normative Data for the Neuropsychological Battery
| Cognitive Domain and Test | Normative Data |
|---|---|
| Speed of Information Processing | |
| WAIS-III Digit Symbol | Heaton, Taylor, & Manly |
| WAIS-III Symbol Search | Heaton, Taylor, & Manly |
| Trail Making Test, Part A | Heaton, Miller, Taylor, & Grant |
| Stroop Color Trial | Norman et al. |
|
| |
| Learning and Memory (2 domains) | |
| Hopkins Verbal Learning Test-Revised | Norman et al. |
| Brief Visuospatial Memory Test-Revised | Norman et al |
|
| |
| Abstraction/Executive Functioning | |
| Wisconsin Card Sorting Test (64-item) | Norman et al. |
| Trail Making Test, Part B | Heaton, Miller, Taylor, & Grant |
| Stroop Color Word Trial | Norman et al. |
|
| |
| Verbal Fluency | |
| Controlled Oral Word Association Test | Heaton, Miller, Taylor, & Grant |
| Category Fluency (Animals) | Heaton, Miller, Taylor, & Grant |
| Category Fluency (Actions) | Woods et al |
|
| |
| Attention/Working Memory | |
| WAIS-III Letter-Number Sequencing | Heaton, Taylor, & Manly |
| PASAT (1st channel only) | Heaton, Miller, Taylor, & Grant |
|
| |
| Motor | |
| Grooved Pegboard Test (Dominant & Non-dominant Hands) | Heaton, Miller, Taylor, & Grant |
WAIS III – Wecshler Adult Intelligence Scale 3rd Edition
PASAT – Paced Auditory Serial Addition Task
For subsequent analyses, we also included everyday functioning measures to differentiate among ANI (asymptomatic neurocognitive impairment), mild neurocognitive disorder (MND) and HIV-associated dementia (HAD) again following the Frascati criteria (Antinori et al., 2007). Neurocognitively normal was defined as those with CR ≤4; ANI included those classified as globally impaired (CR ≥5) but without everyday functioning impairment; MND included those with global CR ≥5 who additionally demonstrated functional impairment quantified by at least two of the following: IADL dependence (details below), at least three significant everyday cognitive symptoms (Patient’s Assessment of Own Functioning Inventory [PAOFI] explained further below), and/or employment problems (participant must be unable to work due to cognitive problems and/or express difficulty with work due to cognitive problems); HAD included those with a CR ≥7 who also demonstrated IADL dependence, at least four significant everyday cognitive symptoms, and were unemployed due to cognitive problems.
Everyday Functioning Measures
Instrumental activities of daily living (IADL) dependence was measured using a revised version of the Lawton and Brody (1969) self-report measure of everyday functioning (Heaton, Marcotte, et al., 2004; Woods et al., 2008). On the IADL questionnaire, participants rated current abilities compared to previous levels of functioning across 12 domains: housekeeping, home repairs, laundry, managing finances, managing medications, shopping, buying groceries, cooking, working, transportation, understanding written material/television, and using the telephone. Total number of IADL declines was derived as a continuous score (possible range: 0–12), and was used as the main outcome for this measure in the current study; participants who endorsed ≥2 declines and indicated that the decline was at least partially attributable to cognitive problems were classified as “IADL dependent” (a criterion for syndromic HAND). In the current cohort, the most common IADL declines were in the following domains: working (58%), housekeeping (22%), home repairs (18%), and understanding written material/television (16%).
The Patient’s Assessment of Own Functioning Inventory (PAOFI) is a self-report measure used to measure perceived cognitive symptoms in everyday life across the domains of memory, language and communication, use of hands, sensory-perceptual, higher level cognitive and intellectual function, and work (if applicable) (Chelune, Heaton, & Lehman, 1986). A sample item from the memory section includes: “How often do you forget something that has been told to you within the last day or two?” (Likert-type scale responses: “Almost always”, “Very often”, “Fairly often”, “Once in a while”, “Very infrequently”, and “Almost never”). Items endorsed as “fairly often” or greater were classified as “significant” cognitive symptoms. The primary outcome on the PAOFI was the number of significant everyday cognitive symptoms (possible range: 0–34).
Last, overall clinician-rated daily functioning was assessed via the Karnofsky Scale of Performance Status, in which a certified nurse assigned an overall functional impairment rating ranging from 100 to 0 (e.g., 100 = normal, no complaints, and no evidence of disease; 50 = requires occasional assistance, but is able to care for most of his/her personal needs; 0 = death; (Karnofsky & Burchenal, 1949).
Statistical Analyses
Given the heterogeneity and complex medical histories of many HIV+ patients, primary analyses included the full sample, as our goal was to examine the MoCA’s utility in detecting neurocognitive impairment regardless of etiology. A receiver operating characteristic (ROC) curve was plotted for the MoCA and was used to determine the optimal cut-off score by producing a Yuden’s index value (one minus specificity subtracted from sensitivity; (Fluss, Faraggi, & Reiser, 2005; Loong, 2003). The area under the curve (AUC) with 95% confidence intervals (CI) was used as an indicator of the utility of the MoCA to differentiate between subjects with and without “gold standard” CR neurocognitive impairment. We then re-conducted this analysis excluding the 16 participants with severe neuromedical confounds to determine if this impacted the psychometrics of our initial analysis (i.e., we examined whether the MoCA was comparably sensitive and specific to HIV-related impairment [i.e., HAND], which cannot be ascertained in those with severe confounds). We then used Pearson’s r correlation analyses to examine the association between MoCA total scores and each of the following: neurocognitive domain performance (CRs); clinico-demographic factors; and the three everyday functioning outcomes (IADL declines, cognitive symptoms [i.e., PAOFI], and Karnofsky score). Given the important role of depressive symptoms in everyday functioning outcomes (Thames et al., 2011), in order to determine whether depressive symptoms influenced our univariate associations between MoCA and the everyday functioning outcomes, we conducted multivariable linear regressions controlling for BDI-II. Finally, among those in whom an HIV-related neurocognitive diagnosis could be made (i.e., excluding n=16 with severe neuromedical confounds), we conducted analysis of variance (ANOVAs) to compare the utility of the MoCA in differentiating neurocognitively normal versus non-syndromic (i.e., ANI), and syndromic (i.e., MND or HAD) HAND.
Results
See Table 2 for full sample descriptive statistics. ROC analysis for the MoCA revealed a cut-point of ≤26 as the most optimal balance of sensitivity (84.21%) and specificity (55.81%) (AUC = 70.42 [95% CI −0.49 to −0.14, p < 0.01]; PPV = 71.64%; NPV = 72.73%; accuracy = 72.00%; Yuden’s index = 40.02; Kappa = 0.41, p < 0.001). (Figure 1). This cut-point yielded 48 true positives (dually impaired, 48%), 24 true negatives (dually normal, 24%), 9 false negatives (gold standard impaired only, 9%), and 19 false positives (MoCA impaired only, 19%). MoCA impaired subjects comprised 67% of the sample while the “gold standard” impairment rate was 57%. See Figure 1 for data illustrating several MoCA cut-points. A subanalysis was conducted restricting the sample to participants without severe contributing neuromedical comorbidities (and thus allowing for a HAND diagnosis to be assigned as impairments were likely due to HIV) (n = 84) and the ROC analysis yielded the same cut-off of ≤26 as within the full sample and very similar values as those in the larger sample: sensitivity = 86.36%; specificity = 57.50%; AUC = 71.19 (95% CI −0.52 to −0.14 p< 0.01); PPV = 69.09%; NPV = 79.31%; accuracy = 72.62%; Yuden’s index = 43.86; Kappa = 0.44, p < 0.001. This cut-point yielded 38 true positives (dually impaired, 45.24%), 23 true negatives (dually normal, 27.38%), 6 false negatives (gold standard impaired only, 7.14%), and 17 false positives (MoCA impaired only, 20.24%). Furthermore, within the sample excluding severely confounded subjects, the HAND prevalence was 52% (39% [n = 33] ANI, 12% [n = 10] MND, 1% [n = 1] HAD). We then examined the association between MoCA total scores and each of the domain-level as well as global gold standard neurocognitive performances (CRs). All of the domain and the global neurocognitive performances demonstrated medium-sized associations with MoCA total scores (p-values < 0.01), with the exception of motor (r = −0.11, p = 0.26) (verbal: r = −0.34; working memory: r = −0.38; executive functions: r = −0.49; speed of information processing: r = −0.31; learning: r = −0.39; recall: r = −0.30; global: r = −0.44).
Table 2.
Sample Demographic, Psychiatric, and HIV-Disease Characteristics (N=100)
| Variable | Mean (SD) or % | Range |
|---|---|---|
| Demographics | ||
| Age | 58.2 (6.5) | 50 – 79 |
| Sex (% Male) | 88% | – |
| Education | 14.3 (2.6) | 8 – 20 |
| Race (% White) | 82% | – |
| HIV Characteristics | ||
| Current CD4* | 597 (365.0 – 776.0) | 6 – 1,606 |
| Nadir CD4* | 135.5 (39.5 – 300.0) | 0 – 850 |
| AIDS Status (% Yes) | 66% | – |
| ART status (% On) | 98% | – |
| Plasma Viral Load (% Undetectable) | 92% | – |
| Est. Duration HIV Infection (yrs) | 18.0 (8.0) | 1 – 30 |
| Comorbidities | ||
| Hypertension (% with) | 50% | |
| Diabetes (% with) | 26% | |
| HCV (% with) | 22% | |
| Mental Health | ||
| Beck Depression Inventory Score* | 8 (3 – 16.8) | 0 – 44 |
| Lifetime MDD Diagnosis (% Yes) | 60% | – |
| Current MDD Diagnosis (% Yes) | 14% | – |
| Lifetime Substance Diagnosis (% Yes) | 70% | – |
| Current Substance Diagnosis (% Yes) | 6% | – |
| Gold Standard Neurocognitive Impairment (% Yes) | 57% | – |
| MoCA Impaired (<=26) | 67% | |
| MoCA Total Score | 25.2 (3.0) | 15 – 30 |
| Functional Measures | ||
| IADL Declines* | 1 (0 – 2) | 0 – 9 |
| Cognitive Symptoms* | 2 (0 – 9) | 0 – 31 |
| Karnofsky Score | 87.2 (10.6) | 50 – 100 |
| IADL Dependent ** | 20% |
Notes
ART=antiretroviral therapy; IADL = instrumental activities of daily living; MDD=Major Depressive Disorder.
Median (IQR) reported for these variables.
Component for the everyday functioning impairment criteria for HIV-associated neurocognitive disorders.
Figure 1.
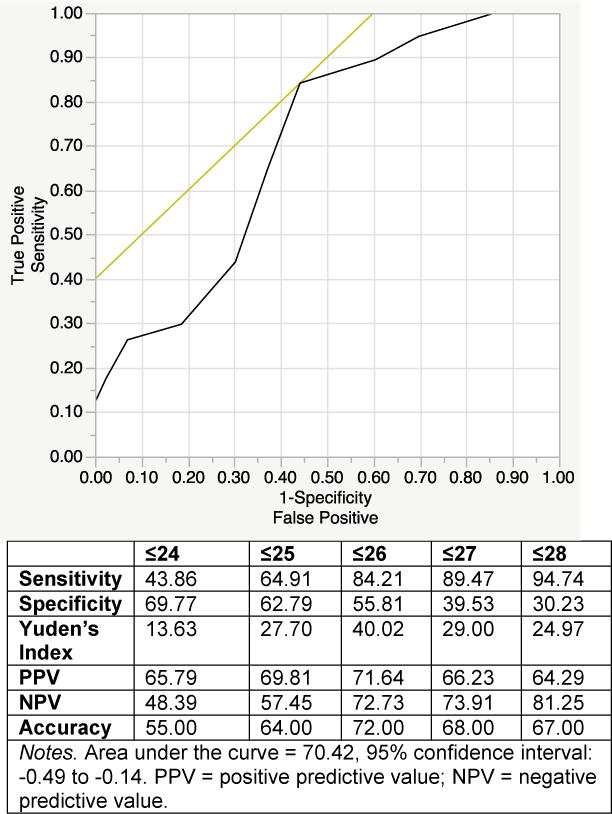
Receiver Operating Curve for MoCA Predicting HAND
To assess external and ecological validity of the MoCA, we examined associations between MoCA total scores and clinico-demographic factors and everyday functioning outcomes. Better total MoCA scores were associated with higher educational levels (r = 0.35, p < 0.001) and current CD4 (r = 0.23, p = 0.02), as well as White race (Cohen’s D = 0.83, p < 0.01), and HCV seronegativity (Cohen’s D = 0.54, p = 0.03). Regarding everyday functioning, MoCA total scores demonstrated small-to-medium effect sizes with all three functional outcomes: IADL declines (r = −0.28); everyday cognitive symptoms (PAOFI total; r = −0.25); Karnofsky total (r = 0.28) (p-values<0.01).
Given the strong association between BDI-II scores and all three functional outcomes (all p-values < 0.001) and the established association between affective distress and self-report measures of everyday function in the literature, we conducted independent multiple linear regression analyses examining the relationship between the MoCA and each of the functional outcomes covarying for BDI-II scores. In all three models, MoCA total remained an independent predictor of everyday outcomes (IADL declines: R2=0.21, F(2,97)=13.26, p < 0.001; MoCA: β = −0.27, partial correlation = −0.29, tolerance = 0.999, CI = −0.06 to −0.32, p = 0.004; BDI-II: β = 0.37, partial correlation = 0.38, tolerance = 0.999, CI = 0.04 to 0.12, p < 0.001; Karnofsky: R2=0.22, F(2,90)=12.50, p < 0.001; MoCA: β = 0.26, partial correlation = 0.28, tolerance = 0.998, CI = 0.26 to 1.53, p = 0.006; BDI-II: β = −0.37, partial correlation = −0.39, tolerance = 0.998; CI = −0.59 to −0.20, p = 0.0001; cognitive symptoms: R2=0.44, F(2,97)=37.55, p < 0.001; MoCA: β = −0.23, partial correlation = −0.29, tolerance = 0.999, CI = −0.18 to −0.88, p = 0.003; BDI-II: β = 0.61, partial correlation = 0.63; tolerance = 0.999, CI = 0.32 to 0.53, p < 0.001). Notably, the MoCA was not univariably associated with current depressive symptoms (r = − 0.04, p = 0.72).
Finally, we assessed the validity of the MoCA in differentiating between syndromic and non-syndromic HAND in the subset of HIV individuals without severely confounding conditions (n = 84). First, we found that there was a significant omnibus difference (F[2,81] = 7.11, p = 0.001) between those subjects classified as neurcognitively normal, non-syndromic, and syndromic HAND on MoCA total scores (Mean (SDs): 26.48 (2.68), 24.12 (2.88), 24.36 (2.91), respectively). Tukey’s pairwise tests showed that neurcognitively normal subjects demonstrated large effect size differences on the MoCA compared to non-syndromic (Cohen’s D = 0.85, p < 0.01) and syndromic (Cohen’s D = 0.78, p = 0.07) HAND individuals, while the non-syndromic and syndromic HAND did not differ (p = 0.97).
Discussion
We found a cut-off of ≤26 as the most optimal balance of sensitivity and specificity, which is somewhat higher than the ≤25 cut-point found in other studies in HIV (Chartier et al., 2015; Hasbun et al., 2012; Janssen et al., 2015; Ku et al., 2014; Milanini et al., 2014; Overton et al., 2013). Moreover, the optimal MoCA cut-off remained the same when participants with significant confounding neuromedical factors (e.g., history of stroke, severe head injury) were excluded from analyses. This latter finding is important, as it suggests that the MoCA is not only able to appropriately detect impairment in those with complex medical backgrounds and HIV, but does an equally effective job of detecting specific HIV-related impairment (i.e., impairment can be attributed to HIV rather than severe confounds that would preclude a true HAND diagnosis).
We also showed that MoCA total scores demonstrated medium-sized associations with performance in all of the individual neurocognitive domains with the exception of motor abilities, with the strongest association found between MoCA and executive functions. These associations support the convergent validity of the MoCA with traditional neurocognitive measures. The lack of association for motor abilities was not surprising given that the MoCA does not include a motor or speed component. The strongest association emerging with MoCA and executive abilities is consistent with the fact that executive items are more widely represented and demanding on this measure compared to other screeners such as the MMSE, which may at least in part explain its superior sensitivity (Nasreddine et al., 2005). We also found that better performance on the MoCA was associated with higher current CD4 counts, HCV seronegativity, higher education levels, and White race, which are commonly reported correlates of neurocognitive performance measured with traditional measures.
A novel aspect of our study was that we found the MoCA total scores were associated with self-report everyday functioning indices and well as clinician-administered functional abilities, supporting the external validity of this measure. Specifically, we found that lower MoCA scores were associated with poorer everyday functioning on all three indices, including a greater number of total self-reported IADL declines, lower clinician-rated functional performance, and a higher number of cognitive symptoms. The association between MoCA scores and these functional measures remained when accounting for depressive symptoms. Similarly, we found that the MoCA discriminated between those with normal neurocognitive performance and those with syndromic and non-syndromic HAND, demonstrating large effect size differences. However, the MoCA did not differ between those with non-syndromic and syndromic HAND, suggesting that while the MoCA is able to at least adequately predict “gold-standard” neurocognitive impairment and is associated with everyday functioning indices, on its own MoCA is not sufficient to distinguish those patients at risk for syndromic HAND. Thus, future work might benefit from establishing cut-offs on validated everyday functioning measures (both self-report and performance based) to combine with the MoCA to improve the MoCA’s ability to approximate levels of HAND as well as its’ specificity.
Although our results support the ecological validity of the MoCA, results of the MoCA should be interpreted with caution and should not replace formal neurocognitive evaluation when necessary. If the goal of the clinician is to comprehensively identify HIV+ patients with possible cognitive impairment, then employment of the recommended cut-off will achieve satisfactory sensitivity. However, although a cognitive screener with high sensitivity is preferable in order to inclusively detect any brain-related changes, this will importantly come at the cost of high false positive rate. Therefore, patients identified as having possible impairment by the MOCA should be referred for a comprehensive evaluation to more accurately characterize and diagnose neurocognitive abilities. By some criteria (as used in the Janssen et al., 2015 study), sensitivities less than 80% and specificities less than 60% are not considered adequate (Blake, McKinney, Treece, Lee, & Lincoln, 2002; Janssen et al., 2015). Thus, using those criteria, while our sensitivity (i.e., 84%) would be considered adequate, our specificity (i.e., 56%) was below the threshold. In contrast, the other published study examining the MoCA in older HIV+ adults (Milanini et al., 2014) found suboptimal sensitivity (i.e., 72%) and adequate specificity (i.e., 67%) with a cut-off of ≤25. Similarly, in a sample of adults with HIV (mean age = 48), the cut-off of ≤26 also yielded suboptimal sensitivity (i.e., 56%) but acceptable specificity (i.e., 63%; (Janssen et al., 2015). Some of the differences in results across studies may at least partly be explained by sample differences in terms of age ranges, MoCA ranges (which were not provided for previous studies), and education/cohort differences. Importantly, our “gold standard” neurocognitive impairment prevalence was 57% (52% when excluding those with severe confounds), as compared to ~40% in both of the aforementioned studies (Janssen et al., 2015; Milanini et al., 2014), which may have resulted in our higher sensitivity. Even in the context of inadequate specificity, its high sensitivity and associations with daily functioning outcomes suggests that the MoCA is a useful clinical and research marker of neurobehavioral functioning. Future work identifying a brief cognitive screening tool that may yield even better sensitivity and specificity than the MoCA continues to be warranted; however, in the absence of better alternatives, the MoCA is a psychometrically sound tool.
While it is low burden, efforts to ensure MoCA administration adheres to provided guidelines are also critical to obtain valid patient data. Other limitations of the MoCA, include: 1) lack of motor and speed of processing components, 2) many items are similar or identical to items in other neurocognitive screens and tests (e.g., trail making), potentially resulting in practice effects, and 3) poor specificity. There is also the potential ethical risk of false positive impairment classifications when using the MoCA, which may cause unnecessary psychological stress or limitations in daily activities. Indeed, the false positive rate was high in this study, at 45%. Thus, we caution clinicians to consider the limitations of such cognitive screening tools and avoid using these measures as stand-alone diagnostic tools, but instead as a means to refer patients for comprehensive neuropsychological evaluation to clinically diagnose HAND. Additionally, our study is not without limitations. The relatively small sample size for a measurement validation study may reduce power and limit generalizability. Furthermore, our cohort was relatively healthy with regard to HIV disease indices, and may further limit the generalizability of our findings to diverse patients aging with HIV. Finally, although our study used well-validated self-reported everyday functioning measures consistent with the current nosology for HAND diagnoses (i.e., Frascati criteria; (Antinori et al., 2007), future studies might benefit from examining MoCA in the context of multimodal assessment of everyday functioning (i.e., both performance-based and self-report measures) (Blackstone et al., 2012), including examining whether the combination of these scores (e.g., establishing cut-offs) to the MoCA may improve the specificity of the MoCA.
Summary
Consistent with previously published studies, we found that the MoCA may serve as an adequate cognitive screening tool in older HIV+ adults. Most importantly, our study extends the current literature by showing the association of the MoCA with several everyday functioning outcomes, enhancing the ecological validity and clinical utility of this brief cognitive screener. Future research would benefit from adapting the MoCA or other screening tests to improve specificity for this population.
Acknowledgments
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH. * The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, National Institutes of Health, nor the United States Government.
Funding: This work was primarily supported by ID10-SD-057 from California HIV/AIDS Research Program (CHRP) (Determinants of Successful Aging Among Older HIV+ Persons, D.J. Moore, PI) and the University of California San Diego (UCSD) Stein Institute for Research on Aging Faculty Pilot Research Grant, with additional support from the following National Institutes of Health (NIH) grants: P30MH062512 (The HIV Neurobehavioral Research Center [HNRC]); N01 MH22005, HHSN271201000036C, and HHSN271201000030C (The CNS HIV Anti-Retroviral Therapy Effects Research [CHARTER]); P50DA026306 (The Translational Methamphetamine AIDS Research Center [TMARC]); U01MH083506 and R24MH59745 (California NeuroAIDS Tissue Network [CNTN]). Dr. Fazeli is supported by 1K99 AG048762-01 from NIA (A Novel Neurorehabilitation Approach for Cognitive Aging with HIV, P. Fazeli, PI). Dr. R.C. Moore is supported by K23MH107260 from NIMH (Real-Time Mobile Assessment of Daily Functioning Among Older HIV-Infected Adults, R.C. Moore, PI).
Footnotes
Conflicts of Interest: The authors declare that they have no conflicts of interest to disclose.
Reference List
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Vol. 1. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blackstone K, Moore DJ, Heaton RK, Franklin DR, Woods SP, Clifford DB CHARTER Group. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. Journal of the International Neuropsychological Society. 2012;18(1):79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake H, McKinney M, Treece K, Lee E, Lincoln NB. An evaluation of screening measures for cognitive impairment after stroke. Age and Ageing. 2002;31(6):451–456. doi: 10.1093/ageing/31.6.451. [DOI] [PubMed] [Google Scholar]
- Brouillette MJ, Mayo N, Fellows LK, Lebedeva E, Higgins J, Overton ET, Koski L. A better screening tool for HIV-associated neurocognitive disorders: is it what clinicians need? AIDS. 2015;29(8):895–902. doi: 10.1097/qad.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton L, Tyson SF. Screening for cognitive impairment after stroke: A systematic review of psychometric properties and clinical utility. Journal of Rehabilitation Medicine. 2015;47(3):193–203. doi: 10.2340/16501977-1930. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. HIV/AIDS among persons aged 50 and older. 2008 Retrieved from http://www.cdc.gov/hiv/topics/over50/
- Centers for Disease Control. Diagnoses of HIV infection in the United States and dependent areas, 2013. HIV Surveillance Report. 2015;25 [Google Scholar]
- Chartier M, Crouch PC, Tullis V, Catella S, Frawley E, Filanosky C, Wong JK. The Montreal Cognitive Assessment: A Pilot Study of a Brief Screening Tool for Mild and Moderate Cognitive Impairment in HIV-Positive Veterans. Journal of the International Association of Providers of AIDS Care. 2015;14(3):197–201. doi: 10.1177/2325957414557270. [DOI] [PubMed] [Google Scholar]
- Chelune GJ, Heaton RK, Lehman RA. Neuropsychological and Personality Correlates of Patients’ Complaints of Disability. In: Goldstein G, Tarter R, editors. Advances in Clinical Neuropsychology. Third. New York: Plenum Press; 1986. pp. 95–126. [Google Scholar]
- Cherner M, Suarez P, Lazzaretto D, Fortuny LA, Mindt MR, Dawes S, group, H Demographically corrected norms for the Brief Visuospatial Memory Test-revised and Hopkins Verbal Learning Test-revised in monolingual Spanish speakers from the U.S.-Mexico border region. Archives of Clinical Neuropsychology. 2007;22(3):343–353. doi: 10.1016/j.acn.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Fals-Stewart W, Fitzmaurice G, Schretlen DJ, Sokoloff J, Weiss RD. Rapid cognitive screening of patients with substance use disorders. Experimental and Clinicial Psychopharmacology. 2009;17(5):337–344. doi: 10.1037/a0017260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, Anderson TJ. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- Damian AM, Jacobson SA, Hentz JG, Belden CM, Shill HA, Sabbagh MN, Adler CH. The Montreal Cognitive Assessment and the mini-mental state examination as screening instruments for cognitive impairment: item analyses and threshold scores. Dementia and Geriatric Cognitive Disorders. 2011;31(2):126–131. doi: 10.1159/000323867. [DOI] [PubMed] [Google Scholar]
- Dong Y, Lee WY, Basri NA, Collinson SL, Merchant RA, Venketasubramanian N, Chen CL. The Montreal Cognitive Assessment is superior to the Mini-Mental State Examination in detecting patients at higher risk of dementia. International Psychogeriatrics. 2012;24(11):1749–1755. doi: 10.1017/S1041610212001068. [DOI] [PubMed] [Google Scholar]
- Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ, National HIVSC. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 2003;17(10):1539–1545. doi: 10.1097/01.aids.0000076282.54156.c3. [DOI] [PubMed] [Google Scholar]
- Doyle K, Weber E, Atkinson JH, Grant I, Woods SP, Group H.I.V.N.R.P Aging, prospective memory, and health-related quality of life in HIV infection. AIDS and Behavior. 2012;16(8):2309–2318. doi: 10.1007/s10461-011-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows RP, Byrd DA, Morgello S. Effects of information processing speed on learning, memory, and executive functioning in people living with HIV/AIDS. Journal of Clinical and Experimental Neuropsychology. 2014;36(8):806–817. doi: 10.1080/13803395.2014.943696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel SI. Cognitive screening in the primary care setting. The role of physicians at the first point of entry. Geriatrics. 2003;58(6):43–44. [PubMed] [Google Scholar]
- Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biometrical Journal. 2005;47(4):458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- Freitas S, Simoes MR, Alves L, Santana I. Montreal cognitive assessment: validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Disease and Associated Disorders. 2013;27(1):37–43. doi: 10.1097/WAD.0b013e3182420bfe. [DOI] [PubMed] [Google Scholar]
- Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Movement Disorders. 2008;23(7):1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- Hasbun R, Eraso J, Ramireddy S, Wainwright DA, Salazar L, Grimes R, Strutt A. Screening for Neurocognitive Impairment in HIV Individuals: The Utility of the Montreal Cognitive Assessment Test. Journal of AIDS and Clinical Research. 2012;3(10):186. doi: 10.4172/2155-6113.1000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Group, C HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Group, H HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, Group, H The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources 2004 [Google Scholar]
- Heaton RK, Taylor MJ, Manly J. Demographic Effects and Use of Demographically Corrected Norms with the WAIS-III and WMS-III. In: Tulsky DS, Saklofske DH, Heaton RK, et al., editors. Clinical Interpretation of the WAIS-III and WMS-III. San Diego: Academic Press; 2002. pp. 181–210. [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Deutsch R, Grant I, Group H.I.V.N.R.P.H Combined effects of aging and HIV infection on semantic verbal fluency: a view of the cortical hypothesis through the lens of clustering and switching. Journal of Clinical and Experimental Neuropsychology. 2012;34(5):476–488. doi: 10.1080/13803395.2011.651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen MA, Bosch M, Koopmans PP, Kessels RP. Validity of the Montreal Cognitive Assessment and the HIV Dementia Scale in the assessment of cognitive impairment in HIV-1 infected patients. Journal of Neurovirology. 2015;21(4):383–390. doi: 10.1007/s13365-015-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemo-therapeutic agents in cancer. In: Maclead CM, editor. Evaluation of Chemotherapeutic Agents. New York: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- Koski L, Brouillette MJ, Lalonde R, Hello B, Wong E, Tsuchida A, Fellows L. Computerized testing augments pencil-and-paper tasks in measuring HIV-associated mild cognitive impairment(*) HIV Medicine. 2011;12(8):472–480. doi: 10.1111/j.1468-1293.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- Ku NS, Lee Y, Ahn JY, Song JE, Kim MH, Kim SB, Choi JY. HIV-associated neurocognitive disorder in HIV-infected Koreans: the Korean NeuroAIDS Project. HIV Medicine. 2014;15(8):470–477. doi: 10.1111/hiv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Loong TW. Understanding sensitivity and specificity with the right side of the brain. BMJ. 2003;327(7417):716–719. doi: 10.1136/bmj.327.7417.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, Study, U. K. C. H. C Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28(8):1193–1202. doi: 10.1097/QAD.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan SN, Mathias JL, Brennan LC, Stewart S. Validity of the montreal cognitive assessment (MoCA) as a screening test for mild cognitive impairment (MCI) in a cardiovascular population. Journal of Geriatric Psychiatry and Neurology. 2011;24(1):33–38. doi: 10.1177/0891988710390813. [DOI] [PubMed] [Google Scholar]
- Milanini B, Wendelken LA, Esmaeili-Firidouni P, Chartier M, Crouch PC, Valcour V. The Montreal cognitive assessment to screen for cognitive impairment in HIV patients older than 60 years. Journal of Acquired Immune Deficiency Syndromes. 2014;67(1):67–70. doi: 10.1097/QAI.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Fazeli PL, Jeste DV, Moore DJ, Grant I, Woods SP. Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS and Behavior. 2014;18(6):1186–1197. doi: 10.1007/s10461-014-0743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Iudicello JE, Weber E, Duarte NA, Riggs PK, Delano-Wood L, Woods SP. Synergistic effects of HIV infection and older age on daily functioning. Journal of Acquired Immune Deficiency Syndromes. 2012;61(3):341–348. doi: 10.1097/QAI.0b013e31826bfc53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D, Jr, Cysique L, Ake C, Group, H Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. Journal of Clinical and Experimental Neuropsychology. 2011;33(7):793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton ET, Azad TD, Parker N, Demarco Shaw D, Frain J, Spitz T, Ances BM. The Alzheimer’s disease-8 and Montreal Cognitive Assessment as screening tools for neurocognitive impairment in HIV-infected persons. Journal of Neurovirology. 2013;19(1):109–116. doi: 10.1007/s13365-012-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins RN, Joska JA, Thomas KG, Stein DJ, Linda T, Mellins CA, Remien RH. Exploring the utility of the Montreal Cognitive Assessment to detect HIV-associated neurocognitive disorder: the challenge and need for culturally valid screening tests in South Africa. The Clinical Neuropsychologist. 2013;27(3):437–454. doi: 10.1080/13854046.2012.759627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Penney AT, Iudicello JE, Riggs PK, Doyle K, Ellis RJ, Letendre SL, Group, H. I. V. N. R. P. H Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care and STDs. 2013;27(1):5–16. doi: 10.1089/apc.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. Journal of Neurovirology. 2002;8(2):136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, Valcour V. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. Journal of Neurovirology. 2007;13(3):203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- Sheppard DP, Iudicello JE, Bondi MW, Doyle KL, Morgan EE, Massman PJ, Woods SP. Elevated rates of mild cognitive impairment in HIV disease. Journal of Neurovirology. 2015 doi: 10.1007/s13365-015-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoff DM, Khalsa JH, Monjan A, Portegies P. Introduction: HIV/AIDS and Aging. AIDS. 2004;18(Suppl 1):S1–2. [PubMed] [Google Scholar]
- Thames AD, Becker BW, Marcotte TD, Hines LJ, Foley JM, Ramezani A, Hinkin CH. Depression, cognition, and self-appraisal of functional abilities in HIV: an examination of subjective appraisal versus objective performance. The Clinical Neuropsychologist. 2011;25(2):224–243. doi: 10.1080/13854046.2010.539577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi KK, Chan JY, Hirai HW, Wong SY, Kwok TC. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Internal Medicine. 2015 doi: 10.1001/jamainternmed.2015.2152. [DOI] [PubMed] [Google Scholar]
- Valcour VG. Evaluating cognitive impairment in the clinical setting: practical screening and assessment tools. Topics in Antiviral Medicine. 2011;19(5):175–180. [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63(5):822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU. Reliability and validity studies of the WHO–Composite International Diagnostic Interview (CIDI): a critical review. Journal of Psychiatric Research. 1994;28(1):57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I, Group H.I.V.N.R.C The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology. 2010;32(4):398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I, Group H.I.V.N.R.C HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22(1):110–117. doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. 2004;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]


