Abstract
The glycocalyx covers the human mammalian cells and plays important roles in stroke, inflammation and atherosclerosis. It has also been shown to be involved in endothelial mechanotransduction of shear stress. Shear stress induces the remodelling of the major component of the glycocalyx including glypican‐1, a cell membrane heparan sulphate proteoglycan. Other factors, such as sphingosine‐1‐phosphate (S1P), protect the glycocalyx against syndecan‐1 ectodomain shedding and induce the synthesis of heparan sulphate. In this study, we reviewed the role of shear stress and S1P in glycocalyx remodelling and revealed that the glycocalyx is a critical signalling platform, integrating the extracellular haemodynamic forces and chemical signalling, such as S1P, for determining the fate of endothelial cells and vascular diseases. This review integrated our current understanding of the structure and function of the glycocalyx and provided new insight into the role of the glycocalyx that might be helpful for investigating the underlying biological mechanisms in certain human diseases, such as atherosclerosis.
Keywords: glycocalyx, sphingosine‐1‐phosphate, shear stress
Introduction
The glycocalyx mediates the endothelial mechanotransduction of shear stress and serves as a selective permeability, anti‐inflammatory and anti‐adhesive barrier at the luminal side of the endothelium 1, 2, 3, showing a protective effect on vascular functions. Endothelial cell (EC) injury in atherosclerosis (AS) causes reduction in the endothelial glycocalyx 4, 5. AS is the major pathological basis causing cardiovascular and cerebrovascular diseases. Thrombosis and atherosclerotic plaque rupture lead to acute coronary syndromes including unstable angina, acute myocardial infarction and heart attack 6, 7.
During AS, the changes in structure and function of ECs adapt to the local mechanical (such as shear stress) and chemical (such as vasoactive mediators and cytokines) microenvironments in vessels, which involve various cell activities, such as phenotypic conversion of cells 8 and remodelling of the extracellular matrix and endothelial glycocalyx 9, 10, 11, 12, 13. It is well known that different flow patterns and associated shear stresses are produced with the development of AS 14. Recently, it was reported that shear stress induces clustering of the major components of the endothelial glycocalyx including glypican‐1, a cell membrane heparan sulphate proteoglycan (HSPG) 9, 10.
Sphingosine‐1‐phosphate (S1P) is a lipid mediator produced by sphingolipid metabolism and mostly present in plasma that induces various cellular effects, including proliferation, differentiation, survival and migration 15. S1P is also emerging as a potent modulator of endothelial barrier function and vascular tone 16. The structure of the glycocalyx is also modulated by S1P, which protects the endothelial glycocalyx against shedding and induces its synthesis 9, 10. Thus, both shear stress and S1P play critical roles in modulating the structure and function of the glycocalyx and contribute to vascular homeostasis and remodelling.
This study reviews the research progress on the structure and function of the endothelial glycocalyx, their regulation by shear stress and S1P, and the roles of shear stress and S1P in AS. Finally, we conclude that the glycocalyx is a critical signalling platform for deciding the fate of ECs and vascular diseases, which may be helpful for elucidating the complicated pathological mechanism of AS.
Research progresses on the structure and function of the endothelial glycocalyx
Structure of the endothelial glycocalyx
The endothelial glycocalyx lines the luminal side of the vascular ECs and its soluble components exist in a dynamic equilibrium with the bloodstream. The structure of the endothelial glycocalyx has been investigated in depth 12, 17. It is mainly composed of glycoproteins bearing acidic oligosaccharides and terminal sialic acids (SA), proteoglycans (PG) like HSPG (including syndecans and glypican‐1 core proteins) and glycosaminoglycan (GAG) side chains (Fig. 1). The predominant GAGs in ECs are heparan sulphate (HS; >50% of the total GAG pool), chondroitin sulphate (CS) and hyaluronic acid (hyaluronan, HA). HS and CS are attached to PGs, whereas HA does not link to the PG core protein. HA is a kind of non‐sulphating GAG, which binds with receptor CD44.
Figure 1.
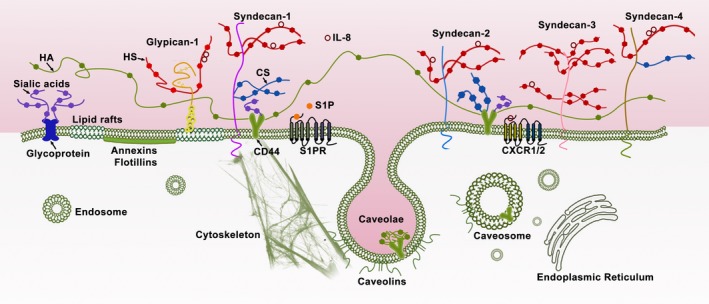
Structure of the endothelial glycocalyx. The endothelial glycocalyx is located at the luminal side of vascular endothelial cells and its soluble components are directly in contact with the bloodstream. The endothelial glycocalyx is mainly composed of glycoproteins bearing acidic oligosaccharides and terminal sialic acids (SA), proteoglycans (PG), such as heparan sulphate proteoglycans (HSPGs; syndecan family and glypican‐1), and glycosaminoglycan (GAG) side chains. The predominant GAGs in endothelial cells are heparan sulphate (HS), chondroitin sulphate (CS) and hyaluronic acid (hyaluronan, HA). HS and CS are attached to PGs. HA binds with receptor CD44. Syndecans (including syndecan‐1, syndecan‐2, syndecan‐3 and syndecan‐4) are single transmembrane domain proteins. Glypican‐1 is an extracellular glycosylphosphatidylinositol (GPI)‐anchored protein, which is localized in lipid rafts. Syndecan‐1 and CD44 interact with the cytoskeleton.
The HSPG syndecan family has four members: syndecan‐1, syndecan‐2, syndecan‐3 and syndecan‐4. Syndecan‐1 contains five potential GAG attachment sites, three near its NH2‐terminal ectodomain and two adjacent to the transmembrane domain near its COOH terminus. CS is only found near the COOH terminus of syndecan‐1 18. Syndecan‐3 contains eight potential GAG attachment sites, five near its NH2‐terminal ectodomain and three adjacent to the transmembrane domain near its COOH terminus. Both syndecan‐2 and syndecan‐4 contain three potential GAG attachment sites near their NH2‐terminal ectodomain 19, 20. Syndecan‐4 can also contain CS 21. In the HSPG glypican family, only glypican‐1 is expressed in ECs. Glypican‐1 is an extracellular glycosylphosphatidylinositol (GPI)‐anchored protein, which only binds with HS.
In resting conditions, syndecans and glypican‐1 mRNAs in human umbilical vein endothelial cells (HUVECs) are expressed in the order: syndecan‐1>syndecan‐4>syndecan‐3>syndecan‐2>glypican‐1 22. The endothelial glycocalyx is modified under several conditions including disturbed flow exposure in large vessels 23, protease degradation 23, 24, 25, and removal of plasma components, particularly albumin 26.
Function of the endothelial glycocalyx
The endothelial glycocalyx has various functions 5, 17. First, the negatively charged glycocalyx layer forms an electrostatic barrier for plasma cells and proteins, like albumin. Second, the glycocalyx layer also forms an anticoagulation barrier. Increased serum levels of syndecan‐1 are associated with acute coagulopathy following trauma 27.
The dominant mechanism that defines widespread endothelial dysfunction is impaired expression of constitutive endothelial nitric oxide synthase (eNOS) and production of nitric oxide (NO) 28. Knockdown of glypican‐1 inhibits the activation of eNOS under shear stress 29.
Syndecan‐1, syndecan‐2 and syndecan‐3 might contribute to angiogenesis. Syndecan‐1 plays important roles in EC survival, proliferation and organization into capillary‐like structures 30. Shed syndecan‐2 regulates angiogenesis by inhibiting EC migration via CD148 (PTPRJ) signalling 31.
Both HS‐ligand binding and interactions of the PG core protein with cytoskeletal and/or signalling molecules are required for cell adhesion and migration. Depletion of syndecan‐1 29 or syndecan‐4 32 has been shown to cause a failure to sense flow direction and inhibition of flow‐induced alignment in vitro. A recent study demonstrated that HS is essential for interleukin (IL)‐8‐induced cell migration 33. After enzymatic removal of HS, we observed significant suppression of the IL‐8‐up‐regulated Rho GTPases including Cdc42, Rac1 and RhoA, IL‐8‐increased Rac1/Rho activity, as well as IL‐8‐induced polymerization and polarization of actin cytoskeleton and an increase in stress fibres.
In cell recruitment, it has been thought that both chemokine oligomerization and binding to GAGs are required. Also, their interactions with GAGs facilitate the formation of the chemokine gradients, which provide directional cues for migrating cells 34. Thus, the glycocalyx could be a good platform to integrate various signals.
Shear stress induces remodelling of the endothelial glycocalyx
It was well known that a dysfunctional endothelium in the AS‐susceptible location is an early manifestation of AS 35. Vascular endothelial injury in the AS‐susceptible location was the prerequisite for AS formation, while the atherosclerotic plaque was the consequence of subsequent vascular repair induced by shear stress 36, 37, 38.
In the AS‐susceptible location, such as branches, bifurcation and curvatures (e.g. the aortic arch) of the arterial tree, the blood stream undergoes tremendous interference and the flow departs from pulsatile, unidirectional shear stress to create flow separation zones that include flow reversal, oscillatory shear stress and sometimes turbulence (chaotic flow) 28, 39. In contrast, flow in adjacent undisturbed flow regions of the arteries is pulsatile and has well‐defined directions. It can be speculated that low shear stress induces the initial lesion, and high shear stress promotes the formation of vulnerable plaques. In the vascular lesion location, a continuous exposure of EC to high shear stress induced an abnormal NO production 40, which might be associated with degradation of the glycocalyx 41 and extracellular matrix through matrix metalloproteinases (MMPs), as well as inflammation.
In a recent study, we detected that transcriptional expression of HSPGs (syndecan family and glypican‐1) in HUVECs responded to the distinct magnitudes of shear stress 22. During the initial 0.5 hr of exposure, syndecan‐1 mRNA was the most up‐regulated, by 4 dyn/cm2 of shear stress, and syndecan‐4 mRNA was significantly up‐regulated, by 10 and 15 dyn/cm2. After 24 hrs of exposure, the greatest increased HSPG mRNA was syndecan‐4 under 4 dyn/cm2, and syndecan‐3 under 15 dyn/cm2. These molecular changes that may be associated with vascular homeostasis and endothelial dysfunction revealed the potential candidate components of the glycocalyx in response to cardiovascular diseases.
The glycocalyx plays an important role in EC mechanotransduction of shear stress. Weinbaum et al. 42 pointed out that the existence of the endothelial glycocalyx could weaken the shear stress on the vascular EC surface to a negligible level by theoretical analysis. Thi et al. 43 further proved that the endothelial glycocalyx is required for the EC cytoskeleton to respond to shear stress. Furthermore, selective degradation of some specific components (such as HS) of the endothelial glycocalyx or silence of specific genes (such as glypican‐1) can inhibit the shear stress‐induced activation of eNOS 29 and the production of NO in ECs 44.
Using confocal microscopy, we discovered that 15 dyn/cm2 shear stress induces remodelling of the endothelial glycocalyx 9, 10. At an initial 30 min., 15 dyn/cm2 shear stress induced the junctional clustering of HS via mobility of GPI‐anchored glypican‐1 in lipid rafts (rapid change). After 24 hrs, 15 dyn/cm2 shear stress induced the recovery of HS (adaptive remodelling), which shows a similar distribution to that present in the aorta of rats and mice in vivo 45. The increases in syndecan‐3 and syndecan‐4 and glypican‐1 might contribute to the adaptive remodelling of the glycocalyx 22.
The remodelling of the glycocalyx might be associated with changes in various EC functions, such as proliferation, migration, adhesion, eNOS activation and NO production. Degradation of HS has been found to significantly inhibit the motility and proliferative responses of EC to shear stress 46 and greatly enhance the adhesion of leucocytes to the endothelium 47. The NO production increased significantly within minutes under 15 dyn/cm2 shear stress 9. Removal of glypican‐1 inhibited the 15 dyn/cm2 shear stress‐induced activation of eNOS and further reduced the 4 dyn/cm2‐inhibited eNOS activity 41. The glycocalyx damage might switch the role of high shear stress from protecting vessels to accelerating the rupture of an AS plaque. Thus, the glycocalyx could be a good platform to integrate the signals (i.e. chemokine and shear stress) that slow or prevent the development of AS by structure remodelling. Once the platform is impaired, the interplay of signals, as well as the involved mechanism might change.
S1P maintains the integrity of the endothelial glycocalyx
S1P is emerging as a potent modulator of endothelial function in response to injury 16. S1P exerts a variety of biological actions through binding with the specific G protein‐coupled receptor (S1P1‐5) on the cell surface to activate signalling cascades or serve as a second messenger 48. Receptor S1P1‐3 prevails among all kinds of tissues in the cardiovascular system 49, 50 and has been widely investigated. S1P and its receptor, S1P1, was required for embryonic angiogenesis and vascular stabilization 50. S1P can promote the formation of an actin ring around the vascular ECs and strengthen the cell–cell and cell–matrix interactions through S1P1, maintaining the permeability of the vascular wall 51. The specific agonist of S1P1 significantly inhibits the formation and development of AS but does not influence the S1P level in plasma 52. When mice were fed a high‐fat diet, abnormal vascular phenotype and development of plaque were obvious in the descending aorta in the Apoe−/− and EC‐specific S1PR1 null mice (S1PR1 f/f VE‐cadherin‐Cre‐ERT2), but was not evident in the Apoe−/− and S1PR1 wild‐type mice 53, showing S1P could maintain the vascular homeostasis and prevent the development of AS through S1PR1.
The glycocalyx was seen to be modified after removal of plasma components, particularly albumin 26. It was demonstrated that albumin‐bound S1P inhibits shedding of the syndecan‐1 ectodomain via activation of the S1P1 receptor in ECs 13 and, thus, maintains the normal vascular permeability in intact microvessels 54. In ECs depleted of plasma protein in a culture medium, the shedding of syndecan‐1 through MMP‐mediated proteolytic cleavage close to the plasma membrane on the external face was also recently demonstrated 13. The shedding of syndecan‐1 ectodomain also removes the attached HS and CS 13.
After complete shedding of the glycocalyx components (including syndecan‐1 with attached HS and CS) by depletion of plasma protein in a culture medium, the addition of S1P induced the recovery of the endothelial glycocalyx via the PI3K pathway 11, suggesting the synthesis of glycocalyx also contributes to the integrity of the endothelial barrier. Therefore, it can be concluded that S1P maintains the stability of the glycocalyx through collectively inhibiting the shedding and promoting the synthesis of the glycocalyx, thereby contributing to the maintenance of normal vascular permeability 54, and controlling the cardiovascular and immune functions 51. It is interesting to further investigate the exact intracellular signalling pathway involved in the S1P‐preserved/induced glycocalyx.
The glycocalyx is a signalling platform for integrating haemodynamic force and sphingosine‐1‐phosphate
Evidence shows the S1P receptor is associated with haemodynamic forces. It was demonstrated that ECs responded to haemodynamics via S1P1 in vivo 53. The vascular endothelial S1P1 receptor can respond to flow, transducing the signals into cellular chemical signalling to promote the stabilization of a newly formed vascular network 53. Knockout of S1P1 gene in mice manifests as injured vessel maturation and embryonic mortality. The change in S1P1 might further induce the remodelling of the glycocalyx.
The glycocalyx is a critical signalling platform that integrates the S1P, shear stress, chemokines and cytokines for maintaining vascular homeostasis (Fig. 2). Once the platform is destructed, the interplay among these factors and the underlying signalling pathways might be changed, which further contributes to the endothelial dysfunction and the development of AS.
Figure 2.
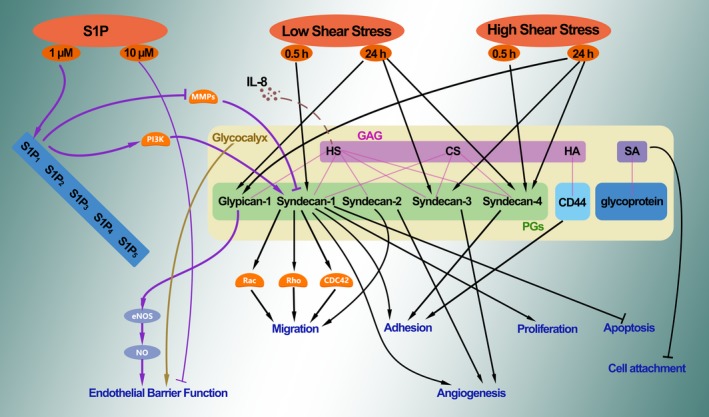
The glycocalyx acts as a signalling platform. Different signalling pathways involved in cellular function, in conditions with or without the glycocalyx. The glycocalyx acts as a signalling platform. Once the platform is destructed, the interplay among these factors and the underlying signalling pathways might be changed.
To elucidate the pivotal role of the glycocalyx under shear stress and its associated mechanism, a new field—mechanoglycobiology is gradually emerging. Importantly, investigations into the mechanoglycobiological mechanism underlying the remodelling of the glycocalyx could bridge the effects of shear stress, S1P, chemokines and cytokines in AS. We expect that innovations in the mechanoglycobiology field will provide new insight into developing novel prevention and treatment strategies for human diseases.
Conflict of interest
None declared.
Acknowledgements
Our work is supported by the National Natural Science Foundation of China (Grant no.11402153), and the Talent Introduction Scientific Research Projects Funded Start‐Up Funds (No. 2082204174089) and the Excellent Young Scientist Foundation (No. 2015SCU04A38) of Sichuan University of China.
References
- 1. Tarbell JM, Ebong EE: The endothelial glycocalyx: a mechano‐sensor and ‐transducer. Science signaling 1: pt8, 2008. [DOI] [PubMed]
- 2. Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng. 2012; 40: 828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reitsma S, Slaaf DW, Vink H, et al The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007; 454: 345–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becker BF, Chappell D, Bruegger D, et al Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010; 87: 300–10. [DOI] [PubMed] [Google Scholar]
- 5. Haeren RH, van de Ven SE, van Zandvoort MA, et al Assessment and imaging of the cerebrovascular glycocalyx. Curr Neurovasc Res. 2016; 13: 249–60. [DOI] [PubMed] [Google Scholar]
- 6. Virmani R, Burke A, Farb A. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. Eur Heart J. 1998; 19: 678–80. [PubMed] [Google Scholar]
- 7. Burke AP, Farb A, Malcom GT, et al Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997; 336: 1276–82. [DOI] [PubMed] [Google Scholar]
- 8. Coll‐Bonfill N, de la Cruz‐Thea B, Pisano MV, et al Noncoding RNAs in smooth muscle cell homeostasis: implications in phenotypic switch and vascular disorders. Pflugers Arch. 2016; 468: 1071–87. [DOI] [PubMed] [Google Scholar]
- 9. Zeng Y, Waters M, Andrews A, et al Fluid shear stress induces the clustering of heparan sulfate via mobility of glypican‐1 in lipid rafts. Am J Physiol Heart Circ Physiol. 2013; 305: H811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeng Y, Tarbell JM. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS One. 2014; 9: e86249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng Y, Liu XH, Tarbell J, et al Sphingosine 1‐phosphate induced synthesis of glycocalyx on endothelial cells. Exp Cell Res. 2015; 339: 90–5. [DOI] [PubMed] [Google Scholar]
- 12. Zeng Y, Ebong EE, Fu BM, et al The structural stability of the endothelial glycocalyx after enzymatic removal of glycosaminoglycans. PLoS One. 2012; 7: e43168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeng Y, Adamson RH, Curry FR, et al Sphingosine‐1‐phosphate protects endothelial glycocalyx by inhibiting syndecan‐1 shedding. Am J Physiol Heart Circ Physiol. 2014; 306: H363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Millon A, Sigovan M, Boussel L, et al Low WSS induces intimal thickening, while large WSS variation and inflammation induce medial thinning, in an animal model of atherosclerosis. PLoS One. 2015; 10: e0141880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu Rev Biomed Eng. 2014; 16: 505–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanchez T. Sphingosine‐1‐phosphate signaling in endothelial disorders. Curr Atheroscler Rep. 2016; 18: 31. [DOI] [PubMed] [Google Scholar]
- 17. Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med. 2016; 280: 97–113. [DOI] [PubMed] [Google Scholar]
- 18. Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006; 259: 339–50. [DOI] [PubMed] [Google Scholar]
- 19. Bernfield M, Gotte M, Park PW, et al Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999; 68: 729–77. [DOI] [PubMed] [Google Scholar]
- 20. Lopes CC, Dietrich CP, Nader HB. Specific structural features of syndecans and heparan sulfate chains are needed for cell signaling. Braz J Med Biol Res. 2006; 39: 157–67. [DOI] [PubMed] [Google Scholar]
- 21. Deepa SS, Yamada S, Zako M, et al Chondroitin sulfate chains on syndecan‐1 and syndecan‐4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J Biol Chem. 2004; 279: 37368–76. [DOI] [PubMed] [Google Scholar]
- 22. Liu JX, Yan ZP, Zhang YY, et al Hemodynamic shear stress regulates the transcriptional expression of heparan sulfate proteoglycans in human umbilical vein endothelial cell. Cell Mol Biol (Noisy‐le‐grand). 2016; 62: 28–34. [PubMed] [Google Scholar]
- 23. Brands J, Van Teeffelen JWGE, Van den Berg BM, et al Role for glycocalyx perturbation in atherosclerosis development and associated microvascular dysfunction. Future Lipidology. 2007; 2: 527–34. [Google Scholar]
- 24. Huxley VH, Williams DA. Role of a glycocalyx on coronary arteriole permeability to proteins: evidence from enzyme treatments. Am J Physiol Heart Circ Physiol. 2000; 278: H1177–85. [DOI] [PubMed] [Google Scholar]
- 25. Lipowsky HH. The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Ann Biomed Eng. 2012; 40: 840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michel CC, Phillips ME, Turner MR. The effects of native and modified bovine serum albumin on the permeability of frog mesenteric capillaries. J physiology. 1985; 360: 333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chignalia AZ, Yetimakman F, Christiaans SC, et al The glycocalyx and trauma: a review. Shock. 2016; 45: 338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009; 6: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ebong EE, Lopez‐Quintero SV, Rizzo V, et al Shear‐induced endothelial NOS activation and remodeling via heparan sulfate, glypican‐1, and syndecan‐1. Integr Biol (Camb). 2014; 6: 338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamorte S, Ferrero S, Aschero S, et al Syndecan‐1 promotes the angiogenic phenotype of multiple myeloma endothelial cells. Leukemia. 2012; 26: 1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Rossi G, Evans AR, Kay E, et al Shed syndecan‐2 inhibits angiogenesis. J Cell Sci. 2014; 127: 4788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baeyens N, Mulligan‐Kehoe MJ, Corti F, et al Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci USA. 2014; 111: 17308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yan Z, Liu J, Xie L, et al Role of heparan sulfate in mediating CXCL8‐induced endothelial cell migration. PeerJ. 2016; 4: e1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dyer DP, Salanga CL, Volkman BF, et al The dependence of chemokine‐glycosaminoglycan interactions on chemokine oligomerization. Glycobiology. 2016; 26: 312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yurdagul A Jr, Finney AC, Woolard MD, et al The arterial microenvironment: the where and why of atherosclerosis. Biochem J. 2016; 473: 1281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis. 2016; 109: 708–15. [DOI] [PubMed] [Google Scholar]
- 37. McAlpine CS, Swirski FK. Circadian influence on metabolism and inflammation in atherosclerosis. Circ Res. 2016; 119: 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sorci‐Thomas MG, Thomas MJ. Microdomains, inflammation, and atherosclerosis. Circ Res. 2016; 118: 679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou J, Li YS, Chien S. Shear stress‐initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014; 34: 2191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dumont O, Loufrani L, Henrion D. Key role of the NO‐pathway and matrix metalloprotease‐9 in high blood flow‐induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol. 2007; 27: 317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeng Y, Liu J. Role of glypican‐1 in endothelial NOS activation under various steady shear stress magnitudes. Exp Cell Res. 2016; 348: 184–9. [DOI] [PubMed] [Google Scholar]
- 42. Weinbaum S, Zhang X, Han Y, et al Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA. 2003; 100: 7988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thi MM, Tarbell JM, Weinbaum S, et al The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper‐car” model. Proc Natl Acad Sci USA. 2004; 101: 16483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Florian JA, Kosky JR, Ainslie K, et al Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003; 93: e136–42. [DOI] [PubMed] [Google Scholar]
- 45. Yen WY, Cai B, Zeng M, et al Quantification of the endothelial surface glycocalyx on rat and mouse blood vessels. Microvasc Res. 2012; 83: 337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yao Y, Rabodzey A, Dewey CF Jr. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol. 2007; 293: H1023–30. [DOI] [PubMed] [Google Scholar]
- 47. Lipowsky HH. Protease activity and the role of the endothelial glycocalyx in inflammation. Drug Discov Today Dis Models. 2011; 8: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007; 1768: 923–40. [DOI] [PubMed] [Google Scholar]
- 49. Kimura T, Watanabe T, Sato K, et al Sphingosine 1‐phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg‐1 and Edg‐3. Biochem J. 2000; 348: 71–6. [PMC free article] [PubMed] [Google Scholar]
- 50. Kono M, Mi Y, Liu Y, et al The sphingosine‐1‐phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004; 279: 29367–73. [DOI] [PubMed] [Google Scholar]
- 51. Curry FR, Adamson RH. Tonic regulation of vascular permeability. Acta physiologica (Oxford, England). 2013; 207: 628–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Poti F, Gualtieri F, Sacchi S, et al KRP‐203, sphingosine 1‐phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL‐R‐/‐ mice. Arterioscler Thromb Vasc Biol. 2013; 33: 1505–12. [DOI] [PubMed] [Google Scholar]
- 53. Jung B, Obinata H, Galvani S, et al Flow‐regulated endothelial S1P receptor‐1 signaling sustains vascular development. Dev Cell. 2012; 23: 600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang L, Zeng M, Fan J, et al Sphingosine‐1‐phosphate maintains normal vascular permeability by preserving endothelial surface glycocalyx in intact microvessels. Microcirculation. 2016; 23: 301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


