Abstract
Transbronchial lung biopsy with a cryoprobe, or cryobiopsy, is a promising new bronchoscopic biopsy technique capable of obtaining larger and better-preserved samples than previously possible using traditional biopsy forceps. Over two dozen case series and several small randomized trials are now available describing experiences with this technique, largely for the diagnosis of diffuse parenchymal lung disease (DPLD), in which the reported diagnostic yield is typically 70% to 80%. Cryobiopsy technique varies widely between centers and this predominantly single center-based retrospective literature heterogeneously defines diagnostic yield and complications, limiting the degree to which this technique can be compared between centers or to surgical lung biopsy (SLB). This review explores the broad range of cryobiopsy techniques currently in use, their rationale, the current state of the literature, and suggestions for the direction of future study into this promising but unproven procedure.
Keywords: Bronchoscopy, bronchoscopic surgical procedures, lung diseases, interstitial
Introduction
Transbronchial biopsy (TBB) with a cryoprobe, or cryobiopsy, is a relatively new procedure for sampling lung parenchyma (1). This procedure entails using a flexible cryoprobe (Figure 1) to rapidly freeze an area of peripheral lung, which is subsequently extracted as a cryobiopsy. Cryobiopsy specimens are larger than those obtained using traditional forceps (Figures 2,3). As such, initial use of this technique has focused on the diagnosis of diffuse parenchymal lung diseases (DPLDs), a class of conditions for which traditional forceps TBB is of limited value due to small sample size and frequent crush artifact (2-4). Initial cryobiopsy experience in this setting has been favorable, with most reports suggesting this procedure establishes specific DPLD diagnoses in 70–80% of cases (5). However, existing cryobiopsy literature is significantly limited by lack of procedure standardization, variable diagnostic endpoints, non-uniform grading of complications, and reliance on generally weak study methodology. This practical state-of-the-art review focuses on current cryobiopsy techniques, potential risks and benefits of procedural variations in current use with regard to impact on diagnostic yield and complication rates, limitations of the current literature, and direction for future study.
Figure 1.
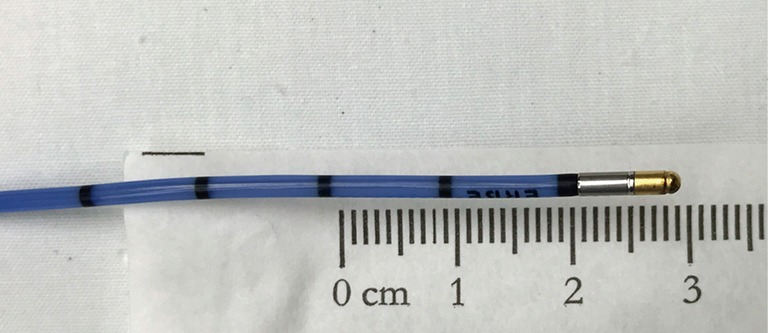
Flexible cryoprobe, with insulated catheter and blunt metal tip where rapid extreme cooling occurs when activated.
Figure 2.
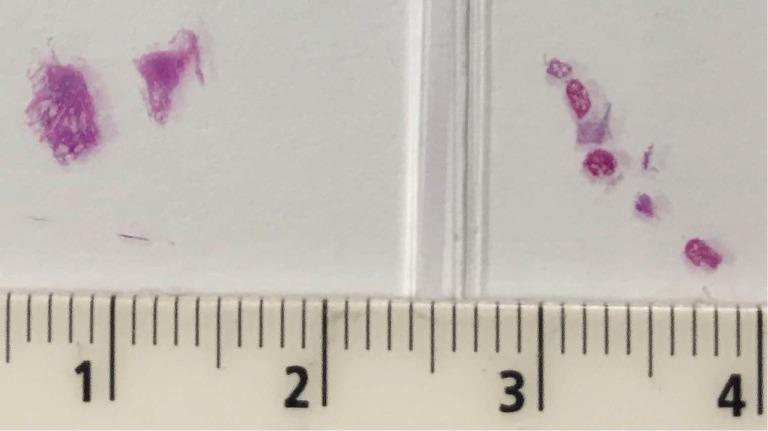
Fixed, stained, and cut cryobiopsy specimen (left) and several smaller traditional forceps transbronchial biopsies (right) on a slide.
Figure 3.
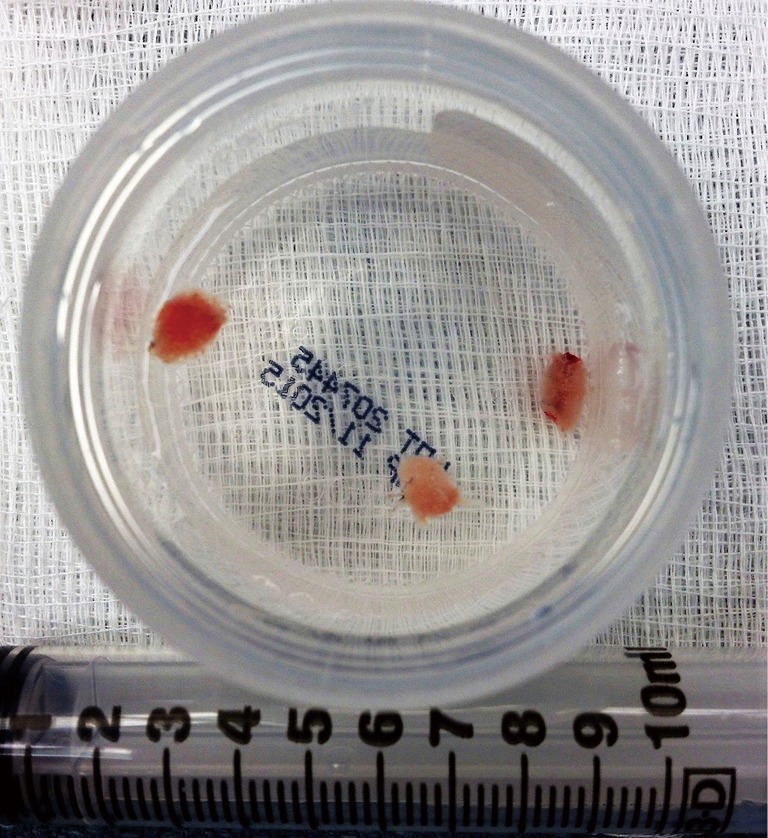
Freshly obtained cryobiopsy specimens floating in formalin.
DPLD: definitions and current diagnostic algorithm
Classifying and subsequently diagnosing those with DPLD has long been a challenge, in part due to epistemological limitations on the nature and clinical significance of these heterogeneous disease processes. Liebow developed the initial seminal interstitial pneumonia classification system on the basis of histopathological signatures of these idiopathic or inflammatory diseases predominantly affecting the interstitial component of the lung in specific and distinctive patterns (6). The idiopathic interstitial pneumonias (IIPs), as they are now codified after several revisions to this original classification, continued to be defined largely on the basis of histopathological findings (7-9).
Imaging characteristics were eventually incorporated into the diagnostic classification of some IIPs as more advanced cross-sectional techniques became available (8-10). This is most prominent in idiopathic pulmonary fibrosis (IPF), the diagnosis of which may be established according to current guidelines on the basis of characteristic findings on high-resolution computed tomography scan of the chest (HRCT) with compatible clinical history, as the positive predictive value of such typical clinicoradiologic features for IPF is 90–100% (11). However, IPF remains the only IIP for which a clinicoradiologic diagnosis can be considered definite; histologic examination of lung tissue is recommended for diagnostic certainty of all other IIPs. Furthermore, only approximately 50% of those with IPF will meet clinicoradiologic criteria for definitive diagnosis, with biopsy advised in those with compatible but not definite imaging findings (8,9,12,13). In this group, the histopathological pattern known as usual interstitial pneumonia (UIP) allows the definite diagnosis of IPF.
There also exists a broad range of diffuse non-neoplastic and non-infectious parenchymal lung diseases not classified as IIPs which are best grouped under the less specific umbrella term of DPLD, sometimes referred to as interstitial lung disease (ILD). This larger group includes diseases with histopathological findings identical to those in the IIP group but with known etiology, commonly connective tissue disease or respiratory exposures (8). Other entities with well-defined clinical, radiologic, and pathologic definitions are sometimes considered part of the broader DPLD class, including lymphangioleiomyomatosis, sarcoidosis, pulmonary Langerhans cell histiocytosis, and eosinophilic pneumonia. Finally, neoplastic or infectious processes masquerading as suspected DPLDs or less well-defined inflammatory syndromes characterized by diffuse abnormalities on lung imaging are also commonly encountered.
A consensus clinical diagnosis reached by a multidisciplinary committee comprised of expert pulmonologists, pathologists, and radiologists after review of available clinical, radiologic, and pathologic data is currently recommended (8,11,14). Histopathologic information remains critical, as it has been found to carry the most weight in these diagnostic discussions, underscoring the need for lung biopsy in many cases (14).
Lung biopsy in DPLD
Until recently, there were generally two modalities by which biopsies for DPLD could be obtained: surgical lung biopsy (SLB) and bronchoscopic transbronchial forceps lung biopsy. SLB obtains large biopsy samples (several centimeters in greatest dimension), usually via video-assisted thoracoscopic surgery. TBB using traditional forceps yields small biopsy samples, on the order of 1 to 3 mm in greatest dimension, often with significant crush artifact (4).
Most DPLDs affect the lung in a heterogeneous and patchy manner, both macroscopically and microscopically, necessitating large biopsy specimens to identify all histopathological features required for confident diagnosis. This is well-reflected in the observation that small biopsies obtained via bronchoscopic TBB using forceps successfully diagnose only 20–30% of DPLD cases and occasionally misrepresent the overall disease process (2,3,15). SLB, conversely, allows a histopathological diagnosis in 90% of cases or greater (16-18), and is thus considered the biopsy modality of choice.
Despite recommendations advocating for SLB in indeterminate DLPD, its utilization has notably declined since the early 2000s (19). Two likely contributors to this decline were the adoption of clinicoradiologic criteria allowing for noninvasive definitive diagnosis of IPF in some cases and adoption of multidisciplinary discussion as the preferred approach to diagnosis, replacing the traditional histologic gold standard (11,14). A third major barrier to pursuing SLB in this setting relates to its perceived risk-benefit ratio. SLB is associated with significant morbidity and mortality; the rate of in-hospital mortality following SLB for DPLD was recently found to be 1.7% in a large dataset, with a complication rate of 30% (including post-operative pneumothorax, pneumonia, respiratory failure) (19). Mortality was slightly lower at 1.5% for elective operations but markedly higher at 16% for operations labeled “non-elective,” presumably performed in the setting of acute disease exacerbations. This is consistent with the high mortality reported in other series, particularly in those ultimately diagnosed with IPF biopsied in the setting of acute respiratory decline (20).
In the absence of specific effective treatment for many of these diseases, some practitioners have understandably been reluctant to consider a diagnostic procedure fraught with complications. However, the appropriateness of this nihilistic attitude is questionable. Establishing the diagnosis of IPF, for example, still has significant prognostic value, especially considering that the average life expectancy at diagnosis is similar to that of many advanced cancers. Timely referral to lung transplant, inclusion in clinical trials, and discontinuation of ineffective and potentially harmful treatments are also unquestionably desirable potential benefits of definitive IPF diagnosis. In addition, the recent Food and Drug Administration-approval of pirfenidone and nintedanib for the treatment of IPF has reinvigorated interest in completing full evaluations for DPLDs and further highlighted the need for safer lung biopsy techniques (11,21-23).
Transbronchial lung cryobiopsy
Cryoprobe development and history of bronchoscopic use
Modern cryoprobes consist of long flexible insulated catheters with blunt metal tips that can be advanced through the working channel of the bronchoscope. The metal tip cools rapidly to extremely cold temperatures via the Joule-Thomson effect: as compressed gas is released at a high flow rate into the tip of the probe, it rapidly expands and cools to a temperature of –79°C (using carbon dioxide) or –89°C (using nitrous oxide) within seconds (1,24-26). The gas then escapes down the catheter via an efferent channel and is vented to the atmosphere.
The cryoprobe was initially devised for neurosurgical applications; rapidly freezing a region of interest allowed surgeons to anticipate potential neurological consequences before irreversibly lesioning brain tissue (24). Cryoprobes have been used bronchoscopically since the late 1960s for cryodestruction of abnormal tissue in the visualized large airways. Repeated freeze-thaw cycles cause necrosis of treated tissues, which slough and are ultimately cleared during a subsequent bronchoscopy (27,28). In the early 2000s, more robust flexible cryoprobes allowed for the development of cryoadhesion or cryorecanalization, in which frozen tissue adherent to the tip of the cryoprobe is mechanically avulsed, allowing for immediate clearance of unwanted endobronchial tissue (28). Transbronchial cryobiopsy, which employs this same basic concept, was developed in the late 2000s based on this experience.
Basic transbronchial cryobiopsy technique
Transbronchial cryobiopsy, hereafter referred to simply as cryobiopsy, refers to the use of a cryoprobe to obtain samples of peripheral lung tissue. The basic technique, initially described in 2009, bears superficial resemblance to TBB using traditional forceps (1). The cryoprobe is advanced through the working channel of the bronchoscope into the peripheral lung and then activated for several seconds, causing surrounding parenchyma to rapidly freeze and adhere to the cryoprobe tip. The bronchoscope and cryoprobe with attached frozen biopsy are then removed en-bloc from the airway; this is necessary because the cryobiopsy is significantly larger than the working channel of the bronchoscope and thus cannot be extracted through this channel (Figure 4). Cryoprobe tip with frozen biopsy are then submerged in saline to rapidly thaw and release the biopsy from the cryoprobe, which is then removed from the working channel as the bronchoscope is re-introduced into the airway. Resulting biopsies tend to be 7–10 mm in greatest dimension, significantly larger than traditional forceps biopsies, and notably lack crush artifact (4,5,29). Significant variations on this basic technique are employed at various cryobiopsy centers worldwide and are discussed later in this review.
Figure 4.

Cryoprobe advanced through the flexible bronchoscope’s working channel, which has been activated for 5 seconds in saline causing an ice ball to form around its tip. Samples frozen by the cryoprobe are much too large to be withdrawn through the working channel, requiring the bronchoscope, cryoprobe, and frozen adherent specimen to be removed from the airway en-bloc.
Safety considerations: competing risk of hemorrhage and pneumothorax
The principle risks of cryobiopsy, airway hemorrhage and pneumothorax, are familiar to practitioners of traditional forceps TBB. Pneumothorax results from a biopsy obtained so distally that the visceral pleura is violated, while the risk of major hemorrhage is thought to be greater in the more proximal region of the peripheral lung, where vessels have branched less extensively and are larger but still too distal to be protected by the cartilaginous rings of the central airway. Because cryobiopsy disrupts a much greater volume of lung tissue than traditional small forceps TBB, a malpositioned cryoprobe has the capacity to cause greater pleural or vascular disruption. Optimal cryoprobe placement with respect to the pleural line is therefore of paramount importance in balancing these two competing risks.
Efficacy and safety of transbronchial lung cryobiopsy for DPLD
Several single-center studies have been published, mostly to report on cryobiopsy diagnostic yield and complication rate and safety. The majority, 19/26 (73.1%), are retrospective. Results of currently published studies are summarized in Tables 1–4. As noted in the tables, most centers with published data use a freeze time of 3–5 seconds performed in the most affected area of the lungs per computed tomography scan. Otherwise, there is little consistency between studies, making it difficult to ascertain clear information about safety, diagnostic yield and procedural technique.
Table 1. Methodology of published cryobiopsy studies.
| First author | Year | na | Design | Inclusion | Notable exclusions | Outcome |
|---|---|---|---|---|---|---|
| Babiak (1) | 2009 | 41 | Retro (+TBB) | DPLD | PaO2 ≤60 on 2LNC O2, RVSP ≥40 | Safety, yield |
| Griff (4) | 2011 | 15 | Pro | DPLD | – | Morphometric analysis (cryobx v. TBB) |
| Kropski (30) | 2013 | 25 | Retro | DPLD | – | Yield |
| Fruchter (31) | 2013 | 15 | Retro | Immunocompromised | HIV, recent neutropenia, SpO2 <90% despite non-rebreather mask | Yield, safety |
| Fruchter (32) | 2013 | 40 | Retro (v. TBB) | Lung transplant | – | Yield, safety |
| Yarmus (33) | 2013 | 17 | Pro (+TBB) | Lung transplant | FEV1 <0.8L, diffuse bullous disease, PaO2 <55 or SpO2 <92% on room air | Safety, feasibility |
| Casoni (25) | 2014 | 69 | Pro | Fibrotic DPLD | FEV1 <0.8L, FVC <50%, DLCO <35%, PaO2 ≤55 on RA, PASP >40 | Yield |
| Griff (34) | 2014 | 52 | Retro | DPLD | Neoplastic disease | Yield |
| Pajares (29) | 2014 | 39 | RCT (v. TBB) | DPLD | PaO2 ≤60, honeycombing on HRCT | Yield (cryobx v. TBB) |
| Fruchter (35) | 2014 | 75 | Retro | DPLD | – | Yield |
| Pourabdollah (36) | 2014 | 41 | Pro (+TBB) | DPLD | – | Yield (cryobx v. TBB) |
| Hernandez-Gonzales (37) | 2015 | 33 | Retro | DPLD | – | Yield, safety, cost |
| Hagmeyer (38) | 2015 | 32 | Retro | DPLD, not SLB candidate | – | Yield |
| Gershman (39) | 2015 | 300 | Retro (v. TBB) | DPLD, lung tx, immunocompromised | – | Yield, safety (cryobx v. TBB) |
| Ravaglia (5) | 2016 | 297 | Retro | DPLD | FEV1 <0.8L, FVC <50%, DLCO <35% PaO2 ≤55 on RA, PASP >40 |
Yield, safety |
| Ramaswamy (40) | 2016 | 56 | Retro (+TBB) | DPLD | – | Yield (cryobx v. TBB) |
| Tomassetti (41) | 2016 | 58 | Pro | DPLD | – | Impact on MDC diagnosis |
| Hagmeyer (42) | 2016 | 19 | Pro/Retrob | DPLD ref. for SLB | – | Yield, safety |
| Echevarria (43) | 2016 | 100 | Retro | DPLD ref. for SLB | – | Yield, safety |
| Sriprasart (44) | 2017 | 74 | Retro | DPLD | – | Yield, safety |
| DiBardino (45) | 2017 | 25 | Retro | DPLD, lung tx, nodules | – | safety |
| Bango-Alvarez (46) | 2017 | 106 | Pro | DPLD | PH, FEV1 <40%, FVC <50%, DLCO <40% | Yield, safety |
| Kronborg (47) | 2017 | 38 | Pro | DPLD | FVC <50%, DLCO <35%, BMI ≥35, honeycombing on HRCT | Yield, safety |
| Berim (48) | 2017 | 10 | Retro | DPLD | – | Feasibility (REBUS-guided) |
| Ravaglia (49) | 2017 | 46 | Pro | DPLD | FEV1 <0.8L, FVC <50%, DLCO <30% PaO2 <50 on RA, PASP >50 |
Yield, single v. multiple segments |
| Ussavarungsi (50) | 2017 | 74 | Retro | DPLD | – | Yield, safety |
All studies are single-center. Almost all including DLPD patients specifically excluded those meeting clinicoradiological criteria for idiopathic pulmonary fibrosis (IPF). All pulmonary function testing percentages indicate percent of predicted value. a, in studies with prospective or historical comparator arm, only the number of cryobiopsy cases is listed; b, reported results of both a retrospective series of cases and interim results from an ongoing prospective study. Retro, retrospective; +TBB, all subjects underwent traditional forceps transbronchial lung biopsy in addition to cryobiopsy; Pro, prospective; RCT, randomized controlled trial; v., versus; DPLD, diffuse parenchymal lung disease; SLB, surgical lung biopsy; lung tx, lung transplant; ref., referred; PaO2, partial pressure of oxygen in arterial blood; LNC, liters of nasal cannula oxygen; RVSP, right ventricular systolic pressure; HIV, human immunodeficiency virus; SpO2, peripheral blood oxygen saturation by pulse oximeter; FEV1, forced expired volume in one second; FVC, forced vital capacity; DLCO, diffusing capacity of lungs for carbon monoxide; HRCT, high resolution computed tomography scan of the chest; RA, room air; PASP, pulmonary arterial systolic pressure; PH, pulmonary hypertension; BMI, body mass index; cryobx, cryobiopsy; TBB, traditional forceps transbronchial biopsy; MDC, multidisciplinary committee; REBUS, radial endobronchial ultrasound.
Table 2. Variability in cryobiopsy procedure technique.
| First author | Year | Anes. | Airway | Vent. | BB | Pleural distance | Fluoro | Probe | Gas | Freeze time (s) | Bx # | Segments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Babiak (1) | 2009 | DS | ETT | Spont | No | 1 | Yes | 2.4 | N2O | 4 | 2 | S |
| Griff (4) | 2011 | ? | ? | ? | ? | 1–2 | Yes | ? | ? | 5 | 1 | S |
| Kropski (30) | 2013 | DS | ETT | Spont | No | 2 | Yes | 1.9 | CO2 | 4 | 2–3 | M |
| Fruchter (31) | 2013 | CS | Trans-oral | Spont | No | ? | Yes | 2.4 | N2O | 4 | 2–3 | S |
| Fruchter (32) | 2013 | CS | Trans-oral | Spont | No | 1–2 | Yes | 2.4 | N2O | 4 | 2–3 | S |
| Yarmus (33) | 2013 | GA/DS | RB/LMA | Jet/spont | Yesa | ? | Yes | 1.9 | ? | 3 | 5 | ? |
| Casoni (25) | 2014 | DS | RB | Spont | Yes | 1 | Yes | 2.4 | CO2 | 5–6 | 3 | S |
| Griff (34) | 2014 | CS/DS | ? | ? | No | 1–2 | Yes | 1.9 | CO2 | 3–5 | 1–2 | ? |
| Pajares (29) | 2014 | DS | ETT | Spont | Yes | ? | Yes | 2.4 | ? | 3–4 | 4 | M |
| Fruchter (35) | 2014 | CS | Trans-oral | Spont | No | 1–2 | Yes | 2.4 | N2O | 4 | 3 | M |
| Pourabdollah (36) | 2014 | DS | ? | ? | ? | ? | ? | 2.4 | N2O | 3 | 1 | S |
| Hernandez-Gonzales (37) | 2015 | GA | ETT | ? | Yes | ? | Yes | 1.9 | ? | 3–4 | 3 | M |
| Hagmeyer (38) | 2015 | DS/GA | ETT/RB | ? / jet | No | 1–1.5 | Yes | ? | N2O | 4–5 | 2–4 | M |
| Gershman (39) | 2015 | CS | Trans-oral | Spont | No | ? | Yes | 2.4 | N2O | 4 | 2–4 | S |
| Ravaglia (5) | 2016 | DS | RB | Spont | Yes | <1 | Yes | 2.4 | CO2 | 5 | 3 | S |
| Ramaswamy (40) | 2016 | CS | Trans-oral | Spont | No | ? | Yes | 2.4 | ? | 4–5 | 2 | M |
| Tomassetti (41) | 2016 | DS | RB | Spont | Yes | 1 | Yes | 2.4 | CO2 | 5–6 | 3 | S |
| Hagmeyer (42) | 2016 | DS | ETT | ? | No | 1–1.5 | Yes | 1.9 | N2O | 4–5 | 3–5 | M |
| Echevarria (43) | 2016 | GA | ETT | ? | Yesb | 1–2 | Yes | 2.4 | N2O | 4 | 4 | S |
| Sriprasart (44) | 2017 | DS | LMA, ETT | Spont | Noc | 1 | Yes | 1.9/2.4 | ? | 5–8 | 5 | M |
| DiBardino (45) | 2017 | ? | LMA, ETT | ? | No | variable | in 40% | 1.9/2.4 | N2O | 2–4 | 4–6 | ? |
| Bango-Alvarez (46) | 2017 | CS | Trans-oral | Spont | Noc | 1–2 | No | 1.9 | ? | 5 | 2–5 | M |
| Kronborg (47) | 2017 | GA | ETT | ? | Yes | 1 | Yes | 1.9/2.4 | ? | 5–7 | 4 | S |
| Berim (48) | 2017 | GA | ETT | ? | No | 1–2d | Yes | 2.4 | ? | 3 | 3 | M |
| Ravaglia (49) | 2017 | DS | RB | Spont | Yes | ? | Yes | 2.4 | CO2 | 5 | 4 | S/Me |
| Ussavarungsi (50) | 2017 | DS | ETT | Spont | Yes | ? | Yes | 1.9 | ? | 3–5 | 3 | M |
Probe-to-pleura distance in centimeter; probe size in millimeter; freeze times in seconds. a, BB used with rigid; no BB used with LMA; b, PTA balloon used; c, two scope method used in lieu of bronchial blocker; d, radial endobronchial ultrasound (REBUS) utilized to further inform probe placement; e, randomized to biopsy in single segment vs. multiple segments. Anes., anesthesia; DS, deep sedation; CS, conscious sedation; GA, general anesthesia; ETT, endotracheal tube; RB, rigid bronchoscope or tracheoscope; LMA, laryngeal mask airway; Vent., mechanism of ventilation; Spont., spontaneous; BB, bronchial blocker; Bx #, number of biopsies per case sought or obtained on average; S, single segment biopsied; M, multiple segments biopsied.
Table 3. Biopsy characteristics and diagnostic yields.
| First author | Year | Mean diameter | Mean area | Histpath. yield | Clinical yield | Clinical dx determination | % IPF/UIP |
|---|---|---|---|---|---|---|---|
| Babiak (1) | 2009 | – | 15.1 | – | 94% | Clin-Rad-Path | 37% |
| Griff (4) | 2011 | – | 17.1 | n/a (morphometric analysis) | |||
| Kropski (30) | 2013 | 8.7 | 64.2 | – | 80% | Clin-Rad-Path | 28% |
| Fruchter (31) | 2013 | – | 9 | 100% | n/a (immunocompromise patients) | ||
| Fruchter (32) | 2013 | – | 10 | 100% | n/a (lung transplant patients) | ||
| Yarmus (33) | 2013 | – | 50 | n/a (lung transplant patients) | |||
| Casoni (25) | 2014 | – | 43 | 76% | 88% | MDC | 68% |
| Griff (34) | 2014 | 6.9 | – | 79%a | – | MDC | 25% |
| Pajares (29) | 2014 | 4.1 | 14.7 | 74% | 51% | MDC | 0/18%b |
| Fruchter (35) | 2014 | – | 9.0 | 70% | 98% | MDC | 9% |
| Pourabdollah (36) | 2014 | – | 22 | 52.5% | 77.5% | MDC | – |
| Hernandez-Gonzales (37) | 2015 | 4 | – | 79% | 84% | MDC | 15% |
| Hagmeyer (38) | 2015 | – | – | 72% | 72% | MDC | 9% |
| Gershman (39) | 2015 | – | – | n/a (retrospective safety analysis) | |||
| Ravaglia (5) | 2016 | – | – | 83% | – | MDC | 31% |
| Ramaswamy (40) | 2016 | 4-26 | – | 66% | 89%c | MDC | |
| Tomassetti (41) | 2016 | – | – | 69%d | 91% | MDC | 69% |
| Hagmeyer (42) | 2016 | – | – | – | 75% | MDC | 14% |
| Echevarria (43) | 2016 | – | – | – | 97% | MDC | 11% |
| Sriprasart (44) | 2017 | 14.7 | 63.5 | 88% | – | – | 15% |
| DiBardino (45) | 2017 | – | – | 56% | 82%e | MDC | 6%e |
| Bango-Alvarez (46) | 2017 | 5.1 | 15.7 | – | 86% | MDC | 21% |
| Kronborg (47) | 2017 | 6.4 | – | 74% | 97% | MDC | 26% |
| Berim (48) | 2017 | – | – | n/a (feasibility study of REBUS-guided cryobiopsy) | |||
| Ravaglia (49) | 2017 | – | 30 | 78-96%f | – | – | 33% |
| Ussavarungsi (50) | 2016 | 9.2 | – | 51% | 78% | MDC | 0% |
Mean diameter reported in millimeters; mean area reported in mm2. Variable definitions of definite or confident clinical diagnosis used across these studies; some only counted diagnoses of the idiopathic interstitial pneumonias (IIPs) or specific well-defined non-IIP entities; others considered cryobiopsy yielding nonspecific findings such as “interstitial fibrosis” as a positive diagnostic study (35) in yield calculations. a, reported as concordance rate with clinical diagnosis; b, 18% of cryobiopsies demonstrated UIP-pattern, but no MDC diagnoses of IPF were reported; c, overall clinical diagnostic rate reported as 96%, but in 7% of cases traditional forceps transbronchial biopsy established the diagnosis; d, this is specifically the rate of definite UIP-pattern histopathological diagnosis in this series. e Including only the diffuse parenchymal lung disease (DPLD) subgroup in this series; f, lower yield when all cryobiopsies obtained in one segment; higher when a second segment was sampled. Histopath., histopathological; dx, diagnosis; IPF, idiopathic pulmonary fibrosis; UIP, usual interstitial pneumonia; Clin-Rad-Path, clinical-radiological-pathological diagnosis without mention of formal multidisciplinary committee diagnosis; n/a, not applicable; MDC, multidisciplinary committee; REBUS, radial endobronchial ultrasound.
Table 4. Major complications of cryobiopsy.
| First author | Year | PTX: alla | PTX: chest tubeb | Serious bleedc | Escalation of cared | AE | Death | Overall CR |
|---|---|---|---|---|---|---|---|---|
| Babiak (1) | 2009 | 2 (4.8%) | 2 (4.8%) | 0 | – | – | – | 2 (4.8%) |
| Griff (4) | 2011 | 0 | 0 | 0 | – | – | – | 0 |
| Kropski (30) | 2013 | 0 | 0 | 0 | 2 (8%) | – | – | 2 (8%) |
| Fruchter (31) | 2013 | 0 | 0 | 0 | – | – | – | 0 |
| Fruchter (32) | 2013 | 0 | 0 | 0 | – | – | – | 1 (2.5%) |
| Yarmus (33) | 2013 | 1 (4.8%) | 1 (4.8%) | 0 | 0 | 0 | 0 | 1 (4.8%) |
| Casoni (25) | 2014 | 19 (28%) | 14 (20%) | 1 (4%) | – | 1 (4%) | 1 (4%–AE) | 20 (29%) |
| Griff (34) | 2014 | 0 | 0 | 0 | – | – | – | 0 (0%) |
| Pajares (29) | 2014 | 3 (8%) | ? | 0 | 3 (8%) | – | 0 | 3 (8%) |
| Fruchter (35) | 2014 | 2 (2.6%) | ? | 3 (4%) | – | 0 | 0 | 5 (7%) |
| Pourabdollah (36) | 2014 | (No safety data reported) | ||||||
| Hernandez-Gonzales (37) | 2015 | 4 (12%) | 1 (3%) | 0 | 1 (3%) | 0 | 0 | 4 (12%) |
| Hagmeyer (38) | 2015 | 6 (19%)e | 6 (19%) | 2 (6%)f | – | – | – | 8 (26%) |
| Gershman (39) | 2015 | 15 (5%) | 6 (2%) | 16 (5%)g | 10 (3%) | – | 0 | 47 (15.5%) |
| Ravaglia (5) | 2016 | 60 (20%) | 46 (15.5%) | 0 | ?h | 1 (0.3%) | 1 (0.3%–AE)i | 66 (22%) |
| Ramaswamy (40) | 2016 | 11 (20%) | ? | 1 (2%) | – | 0 | 0 | 12 (22%) |
| Tomassetti (41) | 2016 | 19 (33%) | 15 (25%) | 0 (0%) | 7 (12%) | 1 (1.7%) | 1 (1.7%–AE)i | 19 (33%) |
| Hagmeyer (42) | 2016 | 5 (26%) | 4 (21%) | 8 (42%) | – | 1 (2%) | 1 (2%–AE + MI) | – |
| Echevarria (43) | 2016 | 3 (3%) | 3 (3%) | 10 (10%) | – | – | 1 (1%–PTX, cancer) | 16 (16%) |
| Sriprasart (44) | 2017 | 5 (7%) | 5 (7%) | 1 (1%) | 8 (11%) | – | 3 (4%–AS, OP, PE) | 8 (11%) |
| DiBardino (45) | 2017 | 2 (8%) | 1 (4%) | 3 (12%) | 6 (24%) | – | 0 | 6 (24%) |
| Bango-Alvarez (46) | 2017 | 5 (4.7%) | ? | 0 | – | 0 | 0 | 5 (5%) |
| Kronborg (47) | 2017 | 10 (26%) | 8 (21%) | 3 (8%) | – | 0 | 0 | 18 (47%) |
| Berim (48) | 2017 | – | – | 2 (20%) | – | – | – | 2 (20%) |
| Ravaglia (49) | 2017 | 7 (16%) | ? | 0 | – | – | 0 | 7 (16%) |
| Ussavarungsi (50) | 2017 | 1 (1.4%) | 1 (1.4%) | 9 (12%) | 2 (3%) | – | 0 | 12 (16%) |
Complication rates expressed as: incident number (percent of all cases). a, all reported pneumothoraces; b, pneumothoraces requiring chest tube thoracostomy drainage; c, serious bleeding enumerated here if author defined it as serious according to society guideline, additional airway intervention or other additional procedure was performed (i.e., bronchial blockade, rigid bronchoscopy, tranexamic acid administration), or respiratory failure or death resulted; d, outpatient to inpatient; inpatient floor to intensive care unit; if specified by author; e, in small subgroup utilizing rigid bronchoscopy with jet ventilation, PTX rate was 43%; f, two subjects required rigid bronchoscopy to control bleeding; authors reported 17 of 31 cases had “severe” bleeding by BTS criteria (51) but state it was controlled by bronchoscopic tamponade in all but the two rigid cases; g, no significant difference in serious bleeding rates between cryobiopsy and traditional forceps transbronchial biopsy (TBB) in this analysis; h, sixteen of 297 discharged day-of; unclear how many were planned admissions or already inpatient; i, not clear whether this is a new post-procedure death related to acute exacerbation of underlying diffuse parenchymal lung disease (DPLD) or the same death reported by Casoni (25). PTX, pneumothorax; AE, acute exacerbation of underlying diffuse parenchymal lung disease; CR, complication rate; MI, myocardial infarction; AS, aortic stenosis; OP, organizing pneumonia; PE, pulmonary embolism.
Included in the tables is a single randomized control trial by Pajares et al. that compares diagnostic yield and safety of traditional forceps TBB to cryobiopsy (29). Cryobiopsy provided a diagnostic yield of 74% compared to TBB 34.1%. Larger sample size and fewer artifacts were seen in the cryobiopsy group. Complications were similar between groups with the exception of moderate bleeding, which was 56.4% in the cryobiopsy group compared to 34.2% in the TBB group. The most common histological diagnosis in this study was non-specific interstitial pneumonia (NSIP) and the higher yield of cryobiopsy was attributed to a larger sample with less artifact and more alveolar tissue. The authors also made a note that subsequent fixation of the samples for immunohistochemistry provided high quality detection of both cytoplasmic and nuclear antigens.
An interesting report by Tomassetti et al. provides the most robust evidence for the utility of cryobiopsy in the clinical diagnosis of DPLD. This study evaluated and compared the impact of cryobiopsy and SLB on the diagnostic impression and confidence of IPF in the context of a multidisciplinary discussion (41). This was a cross-sectional study whereby expert clinicians, radiologists and pathologists were asked to evaluate clinical and radiological data followed by the addition of biopsy results both individually and then as a group for patients with fibrotic lung disease on HRCT without a typical UIP pattern. Not surprisingly, they observed a significant increase in diagnostic confidence when biopsy data were provided but, importantly, this improvement was not significantly different between the two biopsy techniques (29% to 63% with cryobiopsy and from 30% to 65% with SLB). This study supports the role of cryobiopsies for the diagnosis of DPLD in a multidisciplinary group setting.
Several systematic reviews and meta-analyses have been performed, though conclusions reached have been limited by heterogeneity between studies. Two groups were interested in evaluating cryobiopsy diagnostic yield and complications. Johannson et al. included 11 studies (seven full text and four conference abstracts) with unique populations, reporting on 731 patients (52). This review reported a diagnostic yield of 78% (range, 74–98%) when cryobiopsies were used in isolation for diagnosis and 86% (range, 51–98%) when a multidisciplinary consensus was used. Pneumothorax frequency had a mean value of 8.8% (range, 0–25.9%) and the mean bleeding rate was 26.6% (range, 0–78%). Dhooria et al. included 14 studies reporting on 1,183 patients and found diagnostic yield to be 76.9–85.9% (53). Complications included pneumothorax (6.8%), severe bleeding (0.3%) and death (0.1%). This analysis adjusted for quality and validity of the studies using the QualSyst tool; significant publication bias was determined to be present.
Two meta-analyses compared the diagnostic yield and safety of cryobiopsy and SLB. Ravaglia et al. retrospectively analyzed 447 cases with ILD undergoing cryobiopsy or SLB and also systematically reviewed the literature (5). They noted decreased length of hospital stay and mortality for patients who underwent cryobiopsy (2.6 days, 0.3%) versus SLB (6.9 days, 2.7%). Diagnostic yield from their retrospective review was higher for SLB at 98.7% compared to cryobiopsy at 82.8%. They also noted that adverse events such as persistent fever (4.7%), prolonged air leak (3.3%) and acute exacerbation of ILD (3.3%) were higher in SLB compared to cryobiopsy cases. The second, by Iftikhar et al. also compared the diagnostic accuracy and safety of cryobiopsy to SLB. The pooled diagnostic yields for cryobiopsy and SLB were 83.7% (76.9–88.8%) and 92.7% (87.6–95.8%), respectively (54). This group went on to compare pooled sensitivity as 87% vs. 91%, pooled specificity 57% vs. 58%, and summary receiver operating curve of 0.85 vs. 0.74 for cryobiopsy and SLB, respectively. This analysis also noted a decreased hospital length of stay from 6.1 days to 2.6 days as well as a decreased 30–60 days mortality of 0.7% vs. 1.8% with cryobiopsy compared to SLB.
Current cryobiopsy technique
Cryobiopsy technique has evolved since its original description and is currently performed with significant variation in key aspects of the procedure across centers. These variations, their rationale, and potential drawbacks are reviewed below. Much of this evolution has been in pursuit of enhancing safety, particularly from major hemorrhage. A safe and technically successful cryobiopsy procedure entails the following: (I) adequate oxygenation and ventilation in the setting of significant lung disease; (II) retrieval of biopsies of sufficient size from representative regions of abnormality to permit high-quality histopathological assessment; (III) optimal positioning of the cryoprobe with respect to the pleura to minimize the risk of pneumothorax and major bleeding; (IV) bleeding and pneumothorax preparedness and management. Procedural techniques employed in series published to date are summarized in Table 2.
It should be clearly stated that there is unlikely to ever be consensus on a single “ideal” cryobiopsy technique. Just as any operation can be accomplished using variations in technique built upon a common technical foundation that promotes safety and procedural success, cryobiopsy can be performed safely and effectively using a variety of specific procedural elements. However, the operator must be keenly aware of the risks and benefits of specific procedural decisions, and some procedure elements are probably necessary at a minimum to promote patient safety.
Patient selection
The most common indication for cryobiopsy is indeterminate DPLD after thoughtful clinical evaluation including HRCT (5), although small series have investigated its use in other settings, including lung transplant surveillance, lung nodules, suspected small airway disease, and infiltrates in immunocompromised patients (31-33,55,56). A well-defined pre-procedural evaluation protocol has not been established. Nonetheless, anticoagulation and antiplatelet agents should be held for an appropriate period of time (57) and basic laboratory studies obtained to assess for renal dysfunction, coagulopathy, and thrombocytopenia. Pulmonary function testing should be available before cryobiopsy; FEV1 <0.8 L or <50% predicted, forced vital capacity (FVC) <50% predicted, and diffusing capacity of lungs for carbon monoxide (DLCO) <35% or <50% predicted have excluded biopsy candidates in some series, though not in all (5,25,29). Transthoracic echocardiography has been used in some series to exclude those with estimated pulmonary arterial systolic pressure greater than 40 mmHg, though routine pre-procedural TTE is not universally obtained (1,5,25,30). Significant hypoxemia, defined as PaO2 <55–60 mmHg on room air or while receiving 2 liters per minute of nasal oxygen has also been considered a contraindication by some but not others (5,25,29,30).
Interestingly, a meta-analysis of 994 cryobiopsy cases did not find a relationship between lower pulmonary function testing values and complication rate, unlike SLB in which the analogous relationship is well-established (5). This finding should, however, be considered preliminary until prospective investigations stratifying procedure outcomes by pre-operative pulmonary status are conducted. In the interim, it seems most reasonable to consider bleeding diathesis due to disease or inability to appropriately hold anticoagulant or antiplatelet agents an absolute contraindication and FVC <50% predicted, DLCO <35% predicted, or presence of significant pulmonary hypertension as relative contraindications to cryobiopsy.
Airway management, anesthesia, and ventilation
Cryobiopsy has been performed via trans-oral bronchoscopy without advanced airway, laryngeal mask airway, endotracheal tube, and rigid bronchoscope (1,5,25,30,33,39). Most series have utilized intubation with an endotracheal tube (advanced over flexible bronchoscope, after topical anesthesia is provided to the vocal cords and trachea) or rigid bronchoscopy to facilitate rapid re-entry into the airway after each biopsy is obtained.
Deep sedation or general anesthesia is generally employed to facilitate patient tolerance of these advanced airway interventions and prevent cough which can complicate efforts to control airway hemorrhage (5). Propofol and fentanyl or remifentanil are commonly used (5). Topical airway anesthesia should also be performed to limit cough. Choice of deep sedation versus general anesthesia also tends to dictate ventilation strategy; deeply sedated patients can maintain adequate spontaneous respirations while general anesthesia typically necessitates mechanical ventilation. Proponents of deep sedation suggest avoidance of conventional positive pressure or jet ventilation during biopsy may reduce the risk of pneumothorax, though no studies have directly compared these anesthetic and ventilation strategies.
Cryoprobe size, cryogen, and activation-freeze time
The volume of lung parenchyma frozen and subsequently extractable as a cryobiopsy is dependent on the surface area of the probe tip, how cold the tip becomes during activation (dependent on the cryogen gas utilized), and how long the probe is activated. Of these, the latter factor probably most determines biopsy size. In animal studies, there is significant correlation between cryobiopsy size and probe activation time, with biopsy area of 4–5 mm2 after two seconds of 1.9 mm cryoprobe activation and up to 7–9 mm2 after a 5 second activation (58,59).
No studies have systematically investigated the effect of probe size or driver gas on subsequent transbronchial cryobiopsy size. Currently available flexible cryoprobes (ERBE Elektromedizin GmBH, Tübingen, Germany) are 90 cm in length and feature tips 1.9 mm or 2.4 mm in diameter. In an in vivo animal study of large airway mucosal biopsy using both cryoprobes, mean biopsy diameter was only slightly larger using the 2.4 mm probe after 1, 2, and 3 second freeze times and biopsy weight was not significantly different after 3 second freezes (60); how well this reflects cryoprobe behavior in the peripheral lung is not clear. Neither probe size is predominant in published cases to date and some series report using both probes in an interchangeable fashion (5,44,45,47). Likewise, both carbon dioxide and nitrous oxide have seen wide use as the cryogen gas for cryobiopsy. Mean biopsy diameter and viewable biopsy surface area vary widely in extant reports without clear association between probe or cryogen used, even when comparing between similar freeze times (5,58). This might be due in part to inconsistent reporting of these measures and variable methods used to calculate viewable surface area.
There is weak evidence suggesting the 2.4 mm cryoprobe may be more likely to cause pneumothorax. One meta-analysis identified a trend toward increased pneumothorax rates using the 2.4 mm rather than the 1.9 mm cryoprobe (54). In addition, several series from the same group utilizing the 2.4 mm probe have reported pneumothorax rates inordinately higher than most other reports at 20–28%, though this could also be attributed to biopsies being targeted within 1 centimeter of the pleura (5,25). Future prospective randomized study might clarify whether there is any significant difference in complication rates (or diagnostic yield) when using the 2.4 mm versus the 1.9 mm probe.
Probe to pleura distance
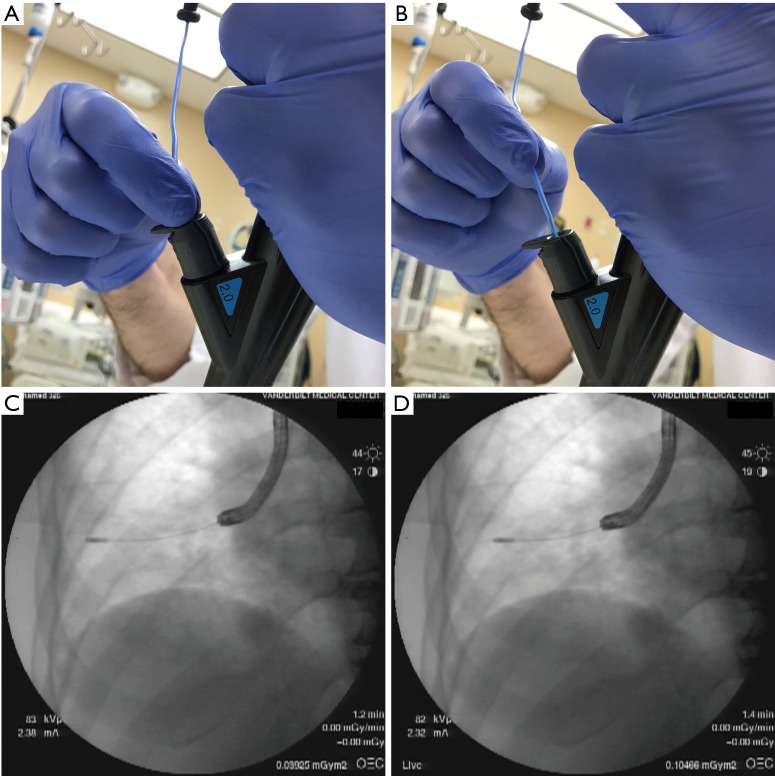
The distance between activated cryoprobe and pleura has major potential implications for both yield and safety. The probe-to-pleura distance can be established in several ways. Almost all cases to date have been performed under fluoroscopic guidance. Lateral airways are preferable, such that the advancing cryoprobe intersects the pleura in perpendicular fashion. When this is the case, the approximately 1 cm long cryoprobe tip visible on fluoroscopy (Figure 1) can be used to estimate the distance the probe is withdrawn before activation. Lightly pinching the cryoprobe catheter as it exits the working channel while the tip is in contact with the pleura and measuring the length of catheter withdrawn can be utilized when the probe is in anteriorly or posteriorly-directed airways (Figures 5,6). It is noteworthy that the tactile feedback from flexible cryoprobes is significantly dampened compared to traditional TBB forceps, which can sometimes make it difficult to determine when the pleura has been reached. This is particularly true for the larger 2.4 mm probe. Fluoroscopically observing the cryoprobe “catching” on the internal aspect of the ribs as the patient breathes can reassure the operator that the pleura has been reached when engaging anteriorly or posteriorly-directed airways (Figure 7).
Figure 5.
Methods to estimate probe-to-pleura distance. In panels A and C, the cryoprobe is in contact with the pleura; the cryoprobe is pinched lightly as it exits from the bronchoscope. Keeping fingers pinched at the same location, the probe catheter is withdrawn the desired length, illustrated in panel B with fluoroscopic result in panel D. When engaging a lateral airway that intersects the pleura perpendicularly, the approximately 1 cm long radiopaque cryoprobe tip can also help inform the distance the probe has been withdrawn. Note that this is not the case in this series of images, in which an anterior or posterior segment has been engaged; despite withdrawing the cryoprobe nearly 2.5 cm, the cryoprobe tip remains close to where it appeared when in contact with the pleura on fluoroscopy. Probe-to-pleura estimation by pinched finger method is more reliable in these cases.
Figure 6.

Fluoroscopic sequence showing detection of the pleural line and performance of a cryobiopsy (61). Available online: http://www.asvide.com/articles/1610
Figure 7.

Fluoroscopic illustration of the cryoprobe “catching” or “bouncing” on the internal aspect of the ribs as the patient breaths (62). Available online: http://www.asvide.com/articles/1611
Sampling immediately subpleural parenchyma by activating the cryoprobe within 1 cm of the pleural line has been advocated by some, particularly in the setting of fibrotic disease concerning for possible UIP/IPF (25,63). The trade-off appears to be higher pneumothorax rate. Casoni et al., in a series continued and reported by Ravaglia et al., reported pneumothorax rates of 20–28% utilizing rigid bronchoscopy under deep sedation with maintenance of spontaneous respiration when biopsies were taken within 1 cm of the pleura using the 2.4 mm cryoprobe (5,25). The overall diagnostic yield in the larger series was 82.8% with UIP/IPF being the most common diagnosis, identified in 31% of cases (5). Similar diagnostic yields, however, have also been reported by authors utilizing a probe-to-pleura distance of up to 2 cm using both cryoprobes; the rate of UIP diagnosis in these series is variable (30,34,35,43). One series had no UIP diagnoses in 74 cases; unfortunately, the probe-to-pleura distance was not reported (50).
Biopsies further from the pleura might be more likely to sample bronchioles and therefore be more valuable in the setting of suspected small airway disease, and one small series has reported preliminary success diagnosing constrictive bronchiolitis with the 1.9 mm probe activated approximately 2 cm from the pleura (56). When specifically reported, high rates of bronchiole representation in cryobiopsy samples have been found with probe-to-pleura distances of 1–2 cm (32,64) and, in an animal model, at 1 cm (58).
The major risk of more proximal sampling is major hemorrhage. No studies have directly examined bleeding risk at various probe-to-pleura distances and existing data is complicated by lack of a standardized post-biopsy hemorrhage grading system and non-uniform reporting of the probe-to-pleura distance used (5,29,37). Further study is required to clarify the optimal probe-to-pleura distance with respect to balancing yield and complications, and whether the distance should vary based on suspected disease.
Biopsy target selection and biopsy number
The optimal location for biopsy for the diagnosis of DPLD is unclear at this time; almost all series simply report targeting areas of abnormality on pre-procedure CT scan or sometimes the “most affected” lung parenchyma. Avoidance of dense honeycombing or preference for regions of ground glass abnormality have been specified by a minority of authors (37,50).
There is significant variability between series in the number of biopsies sought and whether they are performed in a single segment, multiple segments within the same lobe, or multiple lobes of the same lung. Number of biopsies sought or obtained per case has varied from 1 to 6, with three biopsies per case being most common. There does appear to be a trend of increasing biopsy number per case in recent years. The lower lobes are most commonly biopsied, with current studies evenly split between sampling a single segment or multiple segments. Biopsy of multiple lobes has been less commonly reported (29,30,35,37,44-46,50,64) but is of potential interest given SLB data suggesting inter-lobar histopathological discordance is common (65).
Ravaglia et al. recently published the first direct comparison involving biopsy number and targeting (49). Forty-six subjects with suspected DPLD were randomized to have cryobiopsies obtained from a single lower lobe segment or two different lower lobe segments. The diagnostic yield after the first biopsy was 69%. A second biopsy in the same segment increased the diagnostic yield to 78%, which failed to meet statistical significance. However, a second biopsy from a second segment increased yield significantly to 96%. This yield is higher than that reported in other series in which multiple segments (or lobes) were sampled and needs further confirmatory study (29,35,37,38,40,44,46,50,64).
Bleeding preparedness and management
Much of the technical evolution of cryobiopsy since its original description has centered on improving safety, particularly in protecting against major airway bleeding. Cryobiopsy is clearly capable of producing massive hemorrhage, as several authors have reported (45,48,64). As such, this procedure should only be performed by operators prepared for and adept at the management of major airway bleeding.
Several alterations to the original procedure aimed at limiting airway bleeding have seen widespread adoption. The most notable of these is routine placement of a bronchial blocker proximal to the biopsied segment, which is inflated after each biopsy to control emergent hemorrhage while the bronchoscope is out of the airway. After the bronchoscope has been reintroduced, the blocker is deflated (Figure 8). Mild or moderate bleeding can typically be managed by standard flexible bronchoscopic techniques (e.g., scope tamponade, iced saline). In the event of significant bleeding, the blocker can be reinflated to tamponade the bleed while protecting the rest of the lungs. Routine bronchial blocker use was initially reported by authors utilizing rigid bronchoscopy (25,33). Subsequently, a technique allowing placement of an endobronchial blocker external to an endotracheal tube was described, such that the blocker does not encumber the bronchoscope moving in or out of the endotracheal tube (67). Blockers have also been used with laryngeal mask airways (68). Some use their blocker to test-occlude the lung before the first biopsy to ensure adequate pulmonary reserve should prolonged single-lung ventilation be required in the event of major bleeding (50).
Figure 8.

Routine use of an endobronchial blocker to protect against post-biopsy hemorrhage (66). Available online: http://www.asvide.com/articles/1612
Use of an endotracheal tube or rigid bronchoscope is felt by most to be an important part of bleeding preparedness. An in situ endotracheal tube allows for rapid mainstem intubation of the non-biopsied lung in the setting of an uncontrolled bleed, which can happen even with a bronchial blocker in place in the setting of balloon malfunction or untimely cough. Some authors prefer silicone wire-spiral reinforced endotracheal tubes, as their enhanced flexibility improves ease of intubation over the bronchoscope and, if necessary, mainstem intubation (1,30). The rigid bronchoscope or tracheoscope provides a large, mobile conduit through which multiple adjuncts to manage hemorrhage can be placed, including flexible bronchoscopes and bronchial blockers.
Almost all reported cases to date have used fluoroscopy to inform desired probe-to-pleura distance. One notable exception is the series of 25 cases reported by DiBardino et al., in which only 40% of procedures utilized fluoroscopy. Three major hemorrhages occurred and a third of non-fluoroscopy procedures incurred a serious complication (45). It is also noteworthy that 84% of cases were conducted through a laryngeal mask airway, bronchial blockers were not employed in a prophylactic manner in any cases, and no procedure protocol was in place to locally standardize cryobiopsy technique (69). This experience is valuable as a cautionary tale; it highlights how important it is to carefully consider the risks and benefits of all procedural technique decisions. This process should ideally culminate in the development of an institutional procedure protocol, particularly at start-up cryobiopsy programs.
New innovations aimed at further reducing the risk of major bleeding are frequently reported. Echevarria-Uraga et al., reported on their experience using an angioplasty balloon catheter (4 cm long, 6 mm diameter) placed distally (near or overlying the location of each cryobiopsy) using an angled hydrophilic guidewire. Post-biopsy bleeding was controlled with a 3-minute inflation of this balloon following each biopsy in 84 of 100 procedures (43). Berim et al., reported on their use of radial endobronchial ultrasound to detect significant vascular structures in prospective biopsy target regions in a limited series of six patients, without any major bleeds in this small cohort (48). Two series have piloted the use of two bronchoscopes to allow more rapid re-entry into the airway after each biopsy in lieu of endobronchial blocker use, with low rate of serious hemorrhage reported (44,46). Kronborg-White et al., administered intravenous tranexamic acid at the start of all cryobiopsy procedures, with moderate bleeding in 16% of cases, some of whom received additional tranexamic acid (47). Finally, a smaller 1.1 mm cryoprobe used in conjunction with an oversheath is in development. This system permits the bronchoscope to remain wedged in the biopsied segment as the smaller cryobiopsy is extracted through the working channel, which is protected by the oversheath. To date, this probe has only been tested in two in vivo animal studies, and it is unclear whether biopsies will be of sufficient size to diagnose DPLDs (59,70).
Issues for the pathologist
Cryobiopsy specimens are processed for histologic interpretation in the same way as other pathology specimens. There are some caveats. The tissue is so delicate that the pathologist should not attempt to section the specimens grossly. There should be minimal manipulation of the tissue from the time it is removed from the cryoprobe to the time that it is embedded in paraffin. The tissue should be embedded in the paraffin block oriented to maximize the cut surface area (to optimize pattern recognition).
Pathologic interpretation of cryobiopsy specimens is similar to other lung biopsies done for DPLD (71,72). The pathologist should first try to make a specific diagnosis (e.g., carcinoma, pulmonary Langerhans cell histiocytosis) or identify distinctive features that greatly narrow the differential diagnosis (such as granulomas). If a definitive diagnosis cannot be made, the pathologist should assess for a histologic pattern similar to those recognized in surgical lung biopsies and which can be correlated with clinical and radiologic findings at the time of multidisciplinary discussion. Cryobiopsies that are 5 mm or greater in diameter provide sufficient tissue to fill the 4°¡ microscopic field in most microscopes, and at this magnification a pattern may be recognizable, such as UIP or nonspecific interstitial pneumonia.
Since cryobiopsies represent a new technique, the pathologist may have a pretest bias that they should be interpreted like traditional forceps transbronchial biopsies and that they are too small for pattern recognition. The pathologist should try to overcome this bias and view these specimens as one would view a SLB given that cryobiopsies are often large enough to appreciate a pattern and lack the crush artifact that may obfuscate histology in forceps biopsies. There is also almost certainly a learning curve when starting to process and interpret cryobiopsy samples.
Finally, the histopathological limitations of cryobiopsies need to be acknowledged. Since they are significantly smaller than surgical lung biopsies, it is not surprising that the pathologic diagnostic rate is lower. Since there is less total tissue to evaluate in comparison to surgical lung biopsies, there is the potential to miss focal lesions or structures that are only present in a scattered distribution, e.g., granulomas in hypersensitivity pneumonitis, aspirated food material, or isolated tumor cells.
Challenges, limitations, and future directions
While the results of more than two dozen series of cryobiopsy for DLPD are now available, including over 1,500 cases, issues surrounding its diagnostic efficacy and safety relative to SLB remain. The body of work currently available speaks to the promise of this procedure, but methodological concerns and wide variation in procedure technique limit the generalizability, potentially the validity, and the degree to which these results (dominated by retrospective single-center data) can be compared to the SLB literature. Unresolved issues include: lack of procedure technique standardization; lack of uniform reporting on yield, complications, and technical aspects; lack of direct comparison to SLB; lack of estimation of procedure risk stratified by pre-operative status; and lack of controlled studies of inter-rater reliability for pathological interpretation of cryobiopsy specimens. Addressing these issues may better delineate where cryobiopsy should be considered in the diagnostic algorithm for DPLD.
Large, multicenter trials directly comparing cryobiopsy to its appropriate comparator of SLB would be ideal, and several such studies are enrolling (73,74). Expense, lack of technique standardization, and limited appetite for SLB among providers and prospective subjects may hinder such trials, however. Meanwhile, academic interest in cryobiopsy is accelerating judging by the increasing number of publications per year since its introduction. Improving the quality of reports using less-robust methodologies while working toward randomized controlled trial (RCT)-level evidence is paramount in advancing our understanding of ideal cryobiopsy application, outcomes, and risk.
Procedure technique varies widely between current cryobiopsy operators, hindering efforts to pool results of available studies into single estimations of yield and risk. Highly variable definitions of what constitutes a positive diagnostic result or a serious complication and inconsistent descriptions of technique used only compound this issue. In essence, the current cryobiopsy literature describes many techniques instead of one, each being judged for success and complication by different criteria.
Several steps might be simultaneously undertaken to improve understanding on technical issues. A consensus opinion of the most experienced cryobiopsy operators regarding optimal or acceptable cryobiopsy technique is urgently needed as this procedure begins to be incorporated into new centers. Recommendations regarding airway management options, use of bronchial blockers and fluoroscopy, and procedure training, for example, might mitigate the risk of future poor outcomes in start-up cryobiopsy programs like that reported recently by DiBardino (45). Additionally, adoption of standardized grading systems for airway hemorrhage and other complications would enhance our ability to compare complication rates across technical variations. Finally, more uniform reporting of procedure-related variables and outcome measures (including those common in the surgical but not cryobiopsy literature, such as 30-day mortality) would improve the degree to which cryobiopsy data can be compared across centers and to SLB.
The degree to which pre-procedure physiologic status affects yield and complication rates is another area in which little data currently exist. Risk of morbidity and mortality in SLB is greater in the setting of diminished pulmonary reserve (as assessed by pulmonary function testing) or in procedures performed urgently due to acute exacerbation of the underlying DPLD (5,19). Ravaglia et al., in a recent meta-analysis, did not find evidence of an analogous increase in risk when cryobiopsy was performed in patients with less reserve, though the strength of this statement is limited by the overall quality of the body of cryobiopsy data (5). Detailed reporting of pre-procedure status in future cryobiopsy reports and pre-specified stratification of procedure risk by pre-procedure status, when sufficient number of cases are available, would help clarify this issue.
Finally, while awaiting RCT-level evidence directly comparing cryobiopsy to SLB, the establishment of a cryobiopsy registry capturing all of the aforementioned details (pre-procedure physiologic status, technical aspects employed, standardized complication and yield reporting) in a systematic prospective manner would be a major step forward. Such a registry would allow more reliable comparison of various technical variations on yield and complication rate and a more valid comparison to existing SLB data using the same endpoints.
Conclusions
Transbronchial cryobiopsy is a relatively new procedure in which large biopsies of peripheral lung tissue are obtained bronchoscopically; initial studies suggest this technique can establish a diagnosis in 70–80% of DPLD cases with a much better safety profile than SLB. However, the overall quality of currently available evidence, largely limited to retrospective, single-center case series or meta-analyses thereof, is poor and subject to significant bias. Furthermore, there is wide variation in procedure technique and methodologic issues limit the degree to which results of these technical variations can be accurately compared. Standardization of procedure elements and training, pursuit of randomized multicenter trials comparing directly to SLB, and establishment of an international cryobiopsy registry to monitor the effect of technical variations on outcomes are all urgently needed. Nonetheless, given the current available evidence, cryobiopsies seem ideally positioned to replace surgical lung biopsies as the first biopsy modality in the diagnostic algorithm of DPLDs.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration 2009;78:203-8. 10.1159/000203987 [DOI] [PubMed] [Google Scholar]
- 2.Berbescu EA, Katzenstein A-LA, Snow JL, et al. Transbronchial biopsy in usual interstitial pneumonia. Chest 2006;129:1126-31. 10.1378/chest.129.5.1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheth JS, Belperio JA, Fishbein MC, et al. Utility of Transbronchial versus Surgical Lung Biopsy in the Diagnosis of Suspected Fibrotic Interstitial Lung Disease. Chest 2017;151:389-99. 10.1016/j.chest.2016.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griff S, Ammenwerth W, Schönfeld N, et al. Morphometrical analysis of transbronchial cryobiopsies. Diagn Pathol 2011;6:53. 10.1186/1746-1596-6-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravaglia C, Bonifazi M, Wells AU, et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Comparative Study versus Video-Assisted Thoracoscopic Lung Biopsy and a Systematic Review of the Literature. Respiration 2016;91:215-27. 10.1159/000444089 [DOI] [PubMed] [Google Scholar]
- 6.Liebow AA. Definition and classification of interstitial pneumonias in human pathology. Prog Respir Res 1975;8:1-33. 10.1159/000398285 [DOI] [Google Scholar]
- 7.Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol 1994;18:136-47. 10.1097/00000478-199402000-00003 [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Costabel U, Hansell DM, et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2013;188:733-48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Thoracic Society. European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [DOI] [PubMed] [Google Scholar]
- 10.Müller NL, Colby TV. Idiopathic interstitial pneumonias: high-resolution CT and histologic findings. Radiographics 1997;17:1016-22. 10.1148/radiographics.17.4.9225401 [DOI] [PubMed] [Google Scholar]
- 11.Raghu G, Collard HR, Egan JJ, et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med 2011;183:788-824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164:193-6. 10.1164/ajrccm.164.2.2101090 [DOI] [PubMed] [Google Scholar]
- 13.Raghu G, Mageto YN, Lockhart D, et al. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: A prospective study. Chest 1999;116:1168-74. 10.1378/chest.116.5.1168 [DOI] [PubMed] [Google Scholar]
- 14.Flaherty KR, King TE, Raghu G, et al. Idiopathic Interstitial Pneumonia: What Is the Effect of a Multidisciplinary Approach to Diagnosis? Am J Respir Crit Care Med 2004;170:904-10. 10.1164/rccm.200402-147OC [DOI] [PubMed] [Google Scholar]
- 15.Wall CP, Gaensler EA, Carrington CB, et al. Comparison of transbronchial and open biopsies in chronic infiltrative lung diseases. Am Rev Respir Dis 1981;123:280-5. [DOI] [PubMed] [Google Scholar]
- 16.Bensard DD, McIntyre RC, Waring BJ, et al. Comparison of video thoracoscopic lung biopsy to open lung biopsy in the diagnosis of interstitial lung disease. Chest 1993;103:765-70. 10.1378/chest.103.3.765 [DOI] [PubMed] [Google Scholar]
- 17.Miller JD, Urschel JD, Cox G, et al. A randomized, controlled trial comparing thoracoscopy and limited thoracotomy for lung biopsy in interstitial lung disease. Ann Thorac Surg 2000;70:1647-50. 10.1016/S0003-4975(00)01913-5 [DOI] [PubMed] [Google Scholar]
- 18.Carnochan FM, Walker WS, Cameron EW. Efficacy of video assisted thoracoscopic lung biopsy: an historical comparison with open lung biopsy. Thorax 1994;49:361-3. 10.1136/thx.49.4.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchinson JP, Fogarty AW, McKeever TM, et al. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016;193:1161-7. 10.1164/rccm.201508-1632OC [DOI] [PubMed] [Google Scholar]
- 20.Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J 2001;17:175-9. 10.1183/09031936.01.17201750 [DOI] [PubMed] [Google Scholar]
- 21.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431-40. 10.1164/rccm.201006-0894CI [DOI] [PubMed] [Google Scholar]
- 22.Nathan SD, Shlobin OA, Weir N, et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 2011;140:221-9. 10.1378/chest.10-2572 [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646-64. 10.1164/ajrccm.161.2.ats3-00 [DOI] [PubMed] [Google Scholar]
- 24.Cooper IS, Lee AS. Cryostatic congelation: a system for producing a limited, controlled region of cooling or freezing of biologic tissues. J Nerv Ment Dis 1961;133:259-63. 10.1097/00005053-196109000-00013 [DOI] [PubMed] [Google Scholar]
- 25.Casoni GL, Tomassetti S, Cavazza A, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One 2014;9:e86716. 10.1371/journal.pone.0086716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiwand O. Cryosurgery in Pulmonology. In: Korpan NN. editor. Basics of Cryosurgery. Vienna: Springer Vienna, 2001:171-92. [Google Scholar]
- 27.Sheski FD, Mathur PN. Endoscopic treatment of early-stage lung cancer. Cancer Control 2000;7:35-44. [DOI] [PubMed] [Google Scholar]
- 28.Hetzel M, Hetzel J, Schumann C, et al. Cryorecanalization: a new approach for the immediate management of acute airway obstruction. J Thorac Cardiovasc Surg 2004;127:1427-31. 10.1016/j.jtcvs.2003.12.032 [DOI] [PubMed] [Google Scholar]
- 29.Pajares V, Puzo C, Castillo D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: A randomized trial: Transbronchial cryobiopsy. Respirology 2014;19:900-6. 10.1111/resp.12322 [DOI] [PubMed] [Google Scholar]
- 30.Kropski JA, Pritchett JM, Mason WR, et al. Bronchoscopic Cryobiopsy for the Diagnosis of Diffuse Parenchymal Lung Disease. PLoS ONE 2013;8:e78674. 10.1371/journal.pone.0078674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fruchter O, Fridel L, Rosengarten D, et al. Transbronchial Cryobiopsy in Immunocompromised Patients with Pulmonary Infiltrates: A Pilot Study. Lung 2013;191:619-24. 10.1007/s00408-013-9507-z [DOI] [PubMed] [Google Scholar]
- 32.Fruchter O, Fridel L, Rosengarten D, et al. Transbronchial cryo-biopsy in lung transplantation patients: first report. Respirology 2013;18:669-73. 10.1111/resp.12037 [DOI] [PubMed] [Google Scholar]
- 33.Yarmus L, Akulian J, Gilbert C, et al. Cryoprobe transbronchial lung biopsy in patients after lung transplantation: a pilot safety study. Chest 2013;143:621-6. 10.1378/chest.12-2290 [DOI] [PubMed] [Google Scholar]
- 34.Griff S, Schönfeld N, Ammenwerth W, et al. Diagnostic yield of transbronchial cryobiopsy in non-neoplastic lung disease: a retrospective case series. BMC Pulm Med 2014;14:171. 10.1186/1471-2466-14-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fruchter O, Fridel L, El Raouf BA, et al. Histological diagnosis of interstitial lung diseases by cryo-transbronchial biopsy: Cryobiopsy in ILD. Respirology 2014;19:683-8. 10.1111/resp.12296 [DOI] [PubMed] [Google Scholar]
- 36.Pourabdollah M, Shamaei M, Karimi S, et al. Transbronchial lung biopsy: the pathologist’s point of view. Clin Respir J 2016;10:211-6. 10.1111/crj.12207 [DOI] [PubMed] [Google Scholar]
- 37.Hernández-González F, Lucena CM, Ramírez J, et al. Cryobiopsy in the Diagnosis of Diffuse Interstitial Lung Disease: Yield and Cost-Effectiveness Analysis. Arch Bronconeumol 2015;51:261-7. 10.1016/j.arbr.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 38.Hagmeyer L, Theegarten D, Wohlschläger J, et al. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin Respir J 2016;10:589-95. 10.1111/crj.12261 [DOI] [PubMed] [Google Scholar]
- 39.Gershman E, Fruchter O, Benjamin F, et al. Safety of Cryo-Transbronchial Biopsy in Diffuse Lung Diseases: Analysis of Three Hundred Cases. Respiration 2015;90:40-6. 10.1159/000381921 [DOI] [PubMed] [Google Scholar]
- 40.Ramaswamy A, Homer R, Killam J, et al. Comparison of Transbronchial and Cryobiopsies in Evaluation of Diffuse Parenchymal Lung Disease. J Bronchology Interv Pulmonol 2016;23:14-21. 10.1097/LBR.0000000000000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic Lung Cryobiopsy Increases Diagnostic Confidence in the Multidisciplinary Diagnosis of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2016;193:745-52. 10.1164/rccm.201504-0711OC [DOI] [PubMed] [Google Scholar]
- 42.Hagmeyer L, Theegarten D, Treml M, et al. Validation of transbronchial cryobiopsy in interstitial lung disease - interim analysis of a prospective trial and critical review of the literature. Sarcoidosis Vasc Diffuse Lung Dis 2016;33:2-9. [PubMed] [Google Scholar]
- 43.Echevarria-Uraga JJ, Pérez-Izquierdo J, García-Garai N, et al. Usefulness of an angioplasty balloon as selective bronchial blockade device after transbronchial cryobiopsy: Balloon for airway blocking after TBCB. Respirology 2016;21:1094-9. 10.1111/resp.12827 [DOI] [PubMed] [Google Scholar]
- 44.Sriprasart T, Aragaki A, Baughman R, et al. A Single US Center Experience of Transbronchial Lung Cryobiopsy for Diagnosing Interstitial Lung Disease With a 2-Scope Technique. J Bronchology Interv Pulmonol 2017;24:131-5. 10.1097/LBR.0000000000000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiBardino DM, Haas AR, Lanfranco AR, et al. High Complication Rate after Introduction of Transbronchial Cryobiopsy into Clinical Practice at an Academic Medical Center. Ann Am Thorac Soc 2017;14:851-7. 10.1513/AnnalsATS.201610-829OC [DOI] [PubMed] [Google Scholar]
- 46.Bango-Álvarez A, Ariza-Prota M, Torres-Rivas H, et al. Transbronchial cryobiopsy in interstitial lung disease: experience in 106 cases - how to do it. ERJ Open Res 2017;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kronborg-White S, Folkersen B, Rasmussen TR, et al. Introduction of cryobiopsies in the diagnostics of interstitial lung diseases - experiences in a referral center. Eur Clin Respir J 2017;4:1274099. 10.1080/20018525.2016.1274099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berim IG, Saeed AI, Awab A, et al. Radial Probe Ultrasound-Guided Cryobiopsy. J Bronchology Interv Pulmonol 2017;24:170-3. 10.1097/LBR.0000000000000368 [DOI] [PubMed] [Google Scholar]
- 49.Ravaglia C, Wells AU, Tomassetti S, et al. Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Disease: Comparison between Biopsy from 1 Segment and Biopsy from 2 Segments - Diagnostic Yield and Complications. Respiration 2017;93:285-92. 10.1159/000456671 [DOI] [PubMed] [Google Scholar]
- 50.Ussavarungsi K, Kern RM, Roden AC, et al. Transbronchial Cryobiopsy in Diffuse Parenchymal Lung Disease: Retrospective Analysis of 74 Cases. Chest 2017;151:400-8. 10.1016/j.chest.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 51.Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. 10.1136/thoraxjnl-2013-203618 [DOI] [PubMed] [Google Scholar]
- 52.Johannson KA, Marcoux VS, Ronksley PE, et al. Diagnostic Yield and Complications of Transbronchial Lung Cryobiopsy for Interstitial Lung Disease. A Systematic Review and Metaanalysis. Ann Am Thorac Soc 2016;13:1828-38. [DOI] [PubMed] [Google Scholar]
- 53.Dhooria S, Sehgal IS, Aggarwal AN, et al. Diagnostic Yield and Safety of Cryoprobe Transbronchial Lung Biopsy in Diffuse Parenchymal Lung Diseases: Systematic Review and Meta-Analysis. Respir Care 2016;61:700-12. 10.4187/respcare.04488 [DOI] [PubMed] [Google Scholar]
- 54.Iftikhar IH, Alghothani L, Sardi A, et al. Transbronchial Lung Cryobiopsy and Video-Assisted Thoracoscopic Lung Biopsy in the Diagnosis of Diffuse Parenchymal Lung Disease: A Meta-analysis of Diagnostic Test Accuracy. Ann Am Thorac Soc 2017. [Epub ahead of print]. 10.1513/AnnalsATS.201701-086SR [DOI] [PubMed] [Google Scholar]
- 55.Schuhmann M, Bostanci K, Bugalho A, et al. Endobronchial ultrasound-guided cryobiopsies in peripheral pulmonary lesions: a feasibility study. Eur Respir J 2014;43:233-9. 10.1183/09031936.00011313 [DOI] [PubMed] [Google Scholar]
- 56.Lentz RJ, Fessel JP, Johnson JE, et al. Transbronchial Cryobiopsy for the Diagnosis of Constrictive Bronchiolitis in Veterans Returning from Service in Iraq and Afghanistan: A Proof-of-Concept Case Series. Am J Respir Crit Care Med 2015;191:A3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baron TH, Kamath PS, McBane RD. Management of antithrombotic therapy in patients undergoing invasive procedures. N Engl J Med 2013;368:2113-24. 10.1056/NEJMra1206531 [DOI] [PubMed] [Google Scholar]
- 58.Ing M, Oliver RA, Oliver BGG, et al. Evaluation of Transbronchial Lung Cryobiopsy Size and Freezing Time: A Prognostic Animal Study. Respiration 2016;92:34-9. 10.1159/000447329 [DOI] [PubMed] [Google Scholar]
- 59.Yarmus LB, Semaan RW, Arias SA, et al. A Randomized Controlled Trial of a Novel Sheath Cryoprobe for Bronchoscopic Lung Biopsy in a Porcine Model. Chest 2016;150:329-36. 10.1016/j.chest.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 60.Franke K-J, Theegarten D, Hann von Weyhern C, et al. Prospective Controlled Animal Study on Biopsy Sampling with New Flexible Cryoprobes versus Forceps: Evaluation of Biopsy Size, Histological Quality and Bleeding Risk. Respiration 2010;80:127-32. 10.1159/000287251 [DOI] [PubMed] [Google Scholar]
- 61.Lentz RJ, Argento AC, Colby TV, et al. Fluoroscopic sequence showing detection of the pleural line and performance of a cryobiopsy. Asvide 2017;4:298. Available online: http://www.asvide.com/articles/1610
- 62.Lentz RJ, Argento AC, Colby TV, et al. Fluoroscopic illustration of the cryoprobe “catching” or “bouncing” on the internal aspect of the ribs as the patient breaths. Asvide 2017;4:299. Available online: http://www.asvide.com/articles/1611
- 63.Poletti V, Hetzel J. Transbronchial Cryobiopsy in Diffuse Parenchymal Lung Disease: Need for Procedural Standardization. Respiration 2015;90:275-8. 10.1159/000439313 [DOI] [PubMed] [Google Scholar]
- 64.Lentz RJ, Taylor TM, Kropski JA, et al. Utility of Flexible Bronchoscopic Cryobiopsy for Diagnosis of Diffuse Parenchymal Lung Diseases. J Bronchol Interv Pulmonol 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med 2001;164:1722-7. 10.1164/ajrccm.164.9.2103074 [DOI] [PubMed] [Google Scholar]
- 66.Lentz RJ, Argento AC, Colby TV, et al. Routine use of an endobronchial blocker to protect against post-biopsy hemorrhage. Asvide 2017;4:300. Available online: http://www.asvide.com/articles/1612
- 67.Hohberger LA, DePew ZS, Utz JP, et al. Utilizing an Endobronchial Blocker and a Flexible Bronchoscope for Transbronchial Cryobiopsies in Diffuse Parenchymal Lung Disease. Respiration 2014;88:521-2. 10.1159/000368616 [DOI] [PubMed] [Google Scholar]
- 68.Sastre JA, Cordovilla R, Jiménez MF, et al. Management of a transbronchial cryobiopsy using the i-gel® airway and the Arndt endobronchial blocker. Can J Anaesth 2014;61:886-8. 10.1007/s12630-014-0191-0 [DOI] [PubMed] [Google Scholar]
- 69.Lentz RJ, Argento AC, Rickman OB, et al. Transbronchial Cryobiopsy: A Cautionary Tale and Opportunities for Improvement. Ann Am Thorac Soc 2017;14:1230-1. [DOI] [PubMed] [Google Scholar]
- 70.Franke K-J, Linzenbold W, Nuessle D, et al. A New Tool for Transbronchial Cryobiopsies in the Lung: An Experimental Feasibility ex vivo Study. Respiration 2016;91:228-34. 10.1159/000443990 [DOI] [PubMed] [Google Scholar]
- 71.Colby TV, Tomassetti S, Cavazza A, et al. Transbronchial Cryobiopsy in Diffuse Lung Disease: Update for the Pathologist. Arch Pathol Lab Med 2017;141:891-900. 10.5858/arpa.2016-0233-RA [DOI] [PubMed] [Google Scholar]
- 72.Raparia K, Aisner DL, Allen TC, et al. Transbronchial Lung Cryobiopsy for Interstitial Lung Disease Diagnosis: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2016;140:1281-4. 10.5858/arpa.2016-0258-SA [DOI] [PubMed] [Google Scholar]
- 73.Wahidi MM. Comparison of Transbronchial, Cryoprobe and VATS Biopsy For the Diagnosis of Interstitial Lung Disease (ILD). ClinicalTrials.gov 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT01972685?term=cryobiopsy&rank=14
- 74.Bourdin A. Pathological Comparisons of Surgical Open Lung Biopsies and Cryobiopsies in Non-IPF ILD (CryoPID). ClinicalTrials.gov 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT02763540?term=cryobiopsy&rank=10