Introduction
Lymph node involvement is the most important factor affecting the prognosis and treatment of patients with potentially resectable lung cancer. N2 disease consists of patients with mediastinal disease ipsilateral side to the primary tumor, categorized as Stage IIIA for T1-3 patients. More than 1.5 million new cases of lung cancer are diagnosed annually worldwide. About 20% of all patients with non-small cell lung cancer (NSCLC) present with stage IIIA N2 disease. These patients have a higher failure rate both loco-regionally and distantly, with an overall 5-year survival rate of 10–40% (1,2). N2 disease presents with a wide range of severity. This has also been schematized as occult nodal disease, discrete nodal disease, and infiltrative nodal disease (1). The first two categories are amenable to surgery, the latter is not, as it is not possible to obtain R0 resection with bulky nodal disease.
Several imaging techniques have been employed as noninvasive staging tools. Computed tomography (CT) scan of the chest is useful in providing anatomic detail, but the accuracy of chest CT scanning in differentiating benign from malignant lymph nodes in the mediastinum is poor. Positron emission tomography (PET) scanning has a better sensitivity and specificity than chest CT scan for staging lung cancer in the mediastinum, and distant metastatic disease can be detected by PET scanning as well. Integrated PET/CT shows substantially higher accuracy in overall tumor staging over CT and PET interpreted separately. However, the sensitivity of integrated PET/CT to detect malignant nodal involvement is only 32.4% in nodes <10 mm, and 85.3% in nodes > or = 10 mm (3).
Therefore, non-invasive staging lacks sensitivity. Based on this, the American College of Chest Physicians recommends that with either chest CT or PET, abnormal findings must be confirmed by tissue biopsy to ensure accurate staging. Unfortunately, this is still not a common practice and many patients with “positive” PET/CT scans are erroneously labeled as having advanced disease and treated as such. This is particularly relevant when nodal status will impact treatment decisions, such as rendering patients ineligible for surgery after induction therapy. A recent review of over 80,000 stage IIIA N2 patients in the National Cancer Database indicated that only 23% of patients treated without surgery had histologic confirmation of N2 disease, and in surgical patients only 56% of treatment naïve patients had confirmed N2 disease (4).
Since the 1980’s, cervical mediastinoscopy has been extensively used in the staging of mediastinal lymph nodes. In a review of more than 6,500 patients undergoing mediastinoscopy between 1985 and 2003, the average sensitivity to detect mediastinal lymph node involvement was approximately 80% and the average false-negative rate was approximately 10% (5). False-negative results mainly occur in lymph node stations that are not reachable by mediastinoscopy. The yield of the technique is also surgeon dependent. In a study of practice patterns in the USA, Little et al. found that mediastinoscopy is infrequently performed, and even when it is performed, lymph nodes are biopsied in less than 50% of patients (6)! Recently, endobronchial ultrasound guided transbronchial biopsies (EBUS) and trans-oesophageal ultrasound-guided needle biopsy (EUS) techniques have provided a valuable adjunct for the evaluation of mediastinoscopy “blind spots” such as stations 5 and 8. The yield of EBUS seems to be equal if not superior to mediastinoscopy, especially when used to prove clinically suspected N2 involvement by size or PET scan findings, rather than to confirm a negative mediastinum (7). The sensitivity of these techniques improve using immunohistochemistry antibodies to cytokeratins, or other molecular detector with reverse transcriptase polymerase chain reaction (RT-PCR) methodology but this has not been proven to definitively impact survival or recurrence rates (8,9).
The role of mediastinal down staging
The rate of down staging of mediastinal nodes after induction chemotherapy alone is variable, on average 20–40% (10-12). One question is whether there are different induction strategies that would lead to a higher rate of nodal downstaging, and secondly, does this impact long term outcomes? Another question raised by many: should we resect only the patients who achieve nodal downstaging?
Four randomized trials have compared chemotherapy and chemo-radiotherapy as induction regimen in stage III NSCLC (13-16). Radiation doses range from 45 to 60+ Gy, and have been given both concurrently and sequentially. These trials suggest that induction chemo-radiotherapy results in better rates of resectability, pathological downstaging and pathological complete remissions than chemotherapy alone; this is not surprising, since concurrent chemoradiation represents more aggressive locoregional therapy prior to surgery. Mediastinal nodal downstaging when radiation is given preoperatively with chemotherapy is in the range of 40–65% (11,14,16,17). Unfortunately, “hitting the tumor hard” with aggressive induction therapy has not yielded a significant change in overall or progression-free survival. Shah et al. reported a systematic review and meta-analysis looking at the role of radiation therapy as induction therapy with chemotherapy and found no evidence to support radiation therapy in addition to chemotherapy as an induction regimen (18).
Based on randomized trials and meta-analysis demonstrating a survival benefit of induction chemotherapy plus surgery versus surgery alone (19-22), induction chemotherapy for stage IIIA-N2 NSCLC has become a reasonable option for the management of potentially resectable stage IIIA-N2 NSCLC. Several studies have suggested that nodal response after induction chemotherapy is an important prognostic factor. In one trial, induction chemotherapy produced a disease free survival of 48 months for patients downstaged to N0, compared to 8 months for patients with persistent pN1-3 disease (10). In the Intergroup 0139 trial, conversion to ypN0 status yielded a 5-year survival of 41% in surgical patients vs. 24% for ypN2 (17). A study by the Swiss SAKK Group concluded that patients with nodal down staging to N0-1 after induction therapy had improved disease-free survival and OS compared with patients with persistent mediastinal lymph node involvement. The median time to local relapse comparing persistent N2 versus N0-1 was 14.4 versus 43.8 months. In patients with pathologic response to chemotherapy, there was a significant reduction in the rate of distant metastasis as well (16).
However, this has not borne out in every study. A recent report of 100 consecutive biopsy proven N2 patients treated at Memorial Sloan Kettering Cancer Center and treated subsequently with induction chemotherapy, indicates that nodal downstaging occurred in 39% of patients to ypN0-1 (12). Comparison of ypN2 patients with ypN0-1 who underwent resection indicated nearly identical 5-year survival of approximately 40%, when complete resection was undertaken. About 70% had some form of adjuvant therapy when persistent nodal disease, which likely had a positive impact. About 2/3 of patients in this study would have been turned down for surgery (because of persistent N2 disease), yet instead had an operation and achieved the same survival rates as those who were downstaged. The authors indicate that they no longer perform invasive restaging of the mediastinum, since it will not dissuade them from operating, and they offer surgery to patients with persistent N2 disease based on clinical and radiographic findings as long as R0 resection appears to be possible. Another study by Stefani et al. (23), found in patients with N2 disease who had induction chemotherapy and then resection, that patients who demonstrated a clinical response, but not nodal downstaging, experienced a 5-year survival of 30%. Not unexpectedly, their outcomes were better than the group of nonresponders. They concluded that surgery is helpful regardless of N2 status after induction chemotherapy.
In a randomized trial, the German Lung Cancer Cooperative Group (14), Thomas et al. found in patients with stage III NSCLC amenable to surgery that preoperative chemotherapy with hyperfractionated radiation compared to chemotherapy alone preoperatively lead to increased pathological response and mediastinal down staging, but did not improve survival. Similarly, in a retrospective study, Higgins et al. showed that pre-operative chemotherapy and radiation therapy was associated with higher mediastinal complete pathologic response (pCR) rates but did not improve overall survival (11). The mediastinal pCR rates were significantly greater in those patients undergoing preoperative chemo-radiation therapy than in those undergoing chemotherapy alone (65% vs. 35%, P=0.02). This was especially notable in the subgroup of patients with multistation mediastinal involvement (68% vs. 11%, P=0.01). In contrast, the pCR rates at the primary site did not differ between the two subgroups (19% vs. 9%, P=0.2).
Thus, the importance of nodal downstaging remains uncertain. There are contradictory results from reputable studies; there are differences in the rate and impact of downstaging depending on the induction strategy (chemotherapy compared to chemoradiotherapy). Furthermore, there is general agreement that nodal downstaging portends a better prognosis, but patients without nodal downstaging, who do not have progression of disease on induction, seem to have better outcomes with surgery rather than no further therapy (i.e., stopping at definitive chemoradiation).
The diagnosis of residual N2 disease
The concept of invasive re-staging after induction therapy is important if the treating clinicians will only go on to resection if there has been downstaging of mediastinal disease (24,25). CT, PET or re-mediastinoscopy carry a false negative rate of 20–30% (26). Repeat mediastinoscopy can be technically difficult and the yield of EBUS after induction therapy can be too low. Hence, presently for those who believe in the concept of surgery only after down staging, the best approach seems to be an EBUS on initial evaluation followed by mediastinoscopy after induction therapy. An alternative strategy, if mediastinoscopy was done before neoadjuvant therapy, is VATS restaging of the mediastinum before committing to resection.
The study by Kamel et al. proposed the use of PET scan as a tool to determine the down staging of mediastinal nodal disease (27). Although as stated earlier, PET suffers from a lack of sensitivity to detect mediastinal nodal disease, the authors suggest in their study that PET can guide the surgeon as to the operability of the patient after induction therapy. Their study was designed as retrospective review of their data base of patients who had undergone surgical resection after induction therapy for clinical stage IIIA N2 NSCLC. They included 203 patients, and noted that 48% of them had a pathologic down staging to pN0 or pN1. This down staging was associated with a significant survival benefit from a 5-year survival of 35% to 56%. They also found on a multivariable analysis that upper or middle lobe location and less than 60% reduction of N2 SUV max on the PET scan were independent predicators of persistent N2 disease.
The study by Kamel et al. is not the first to report on the role of serial PET scanning to establish persistence of nodal disease (24). In a utopic world an imaging study would have a significant impact on the determination of operability of patients, but the paper has several limitations, the most important of which is the sensitivity of PET. The sensitivity, using a cutoff of 60% reduction in the SUV max was still poor, with 43% of patients who achieved this cut off having persistent N2 disease. The other issue with the study is that most patients had mediastinoscopy up front and they did not use EBUS in staging process and this was due to the fact that their study occurred across an era when EBUS was simply not available. However, the patients who were not downstaged in the mediastinum still achieved a survival rate (35%) that is substantially better than the survival typically seen with definitive chemoradiotherapy (10–20%), which argues for operating regardless of nodal status. If outcomes are acceptable with resection regardless of persistent N2 or downstaged N2, then why be concerned about downstaging of the mediastinum? Many centers have questioned this and have evolved their approach.
What to do with patients with persistent N2 disease identified at the time of resection
The question remains, how to treat the majority of patients who do not achieve downstaging? Obviously there is great need for better chemotherapy and targeted agents and the vacuum is still not filled. For now, some advocate definitive chemoradiation if this was used as an induction strategy; for those who had chemotherapy alone and have persistent N2 disease (proven by biopsy), then they would go on to chemoradiation concurrently and not have surgery. For those who believe surgery improves survival in the face of persistent N2 disease, for which there is substantial evidence, the addition of postoperative radiation therapy (PORT), for those who did not have it during induction, aims at decreasing ever further the rate of locoregional recurrence. Presently, locoregional recurrence is about 40% after chemotherapy and surgery, and it may be reduced further another 25–35% by PORT (28).
Nine randomized trials examined the impact of PORT on disease-free and overall survival in patients with resected stage I, II, and III NSCLC. These data from more than 2,000 patients were individually analyzed by the PORT Meta-analysis Trialists Group (29). Doses range from 30–60 Gy historically; presently PORT is dosed in the range of 50.4–54 Gy (30). Although the methods of delivery of radiation therapy were very different than now, PORT was shown to have a detrimental effect on overall survival in stage I–II. On the other hand, for N2 patients there was a small reduction in the rate of local recurrence. The combination of PORT and chemotherapy given concurrently has also been suggested to be beneficial in the Radiation Therapy Oncology Group-9705 (31). Using the surveillance, epidemiology, and end results database (SEER), Lally et al. investigated the association between survival and postoperative radiotherapy (PORT) in patients with resected NSCLC. In a population-based cohort, PORT use was associated with an increase in survival in patients with N2 nodal disease but not in patients with N1 and N0 nodal disease (32). These data were verified by Douillard et al. who studied the impact of PORT on survival of patients recruited in the Adjuvant Navelbine International Trialist Association (ANITA) randomized study of adjuvant chemotherapy (33). ANITA is a randomized trial of adjuvant cisplatin and vinorelbine chemotherapy vs. observation in completely resected NSCLC Stages IA to IIIB. Use of PORT was recommended for patients with nodal disease but was not randomized or mandatory. This retrospective evaluation suggested again a positive effect of PORT in N2 disease especially when patients received adjuvant chemotherapy (median survival 47.4 vs. 23.8 months).
Finally, in a retrospective study evaluating the impact of PORT and chemotherapy in patients who received induction chemotherapy followed by surgical resection, Amini et al. found that aggressive consolidative therapies may improve outcomes for patients with persistent N2 disease after induction chemotherapy and surgery (34). In their study group the rate of loco-regional failure rate was only 12%. However, the rate of distant recurrence was still high at 54%. The overall survival of patients who had PORT and chemotherapy was twice as high at 5 years than those who received only PORT (40% vs. 20%).
Summary
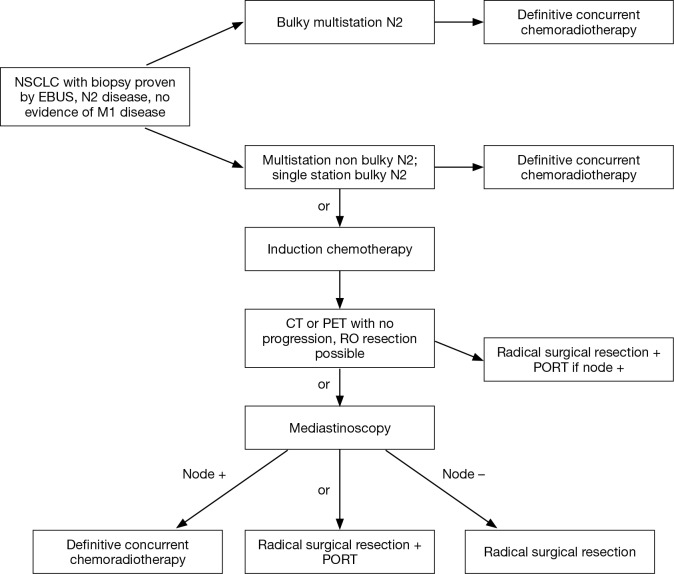
The implications of nodal downstaging in stage IIIA NSCLC remain controversial. Our strategy in this group of patients is summarized in Figure 1. Patients with bulky multistation N2 disease are considered non operable. Those with single station bulky or non-bulky disease have the option of being treated with definitive systemic and radiation therapy upfront or with a trimodality treatment involving induction systemic therapy followed by surgery and consolidative radiation post operatively, or induction chemoradiation followed by surgery. The best prognosis can be expected in those patients who had a pathologic complete response, or a downstaging of the N2 disease to N0 or N1 disease. But patients without progression, but with persistent N2 disease, have better outcomes than patients not having resection in most series. They deserve a chance! The optimal methods to determine the N2 status is a combination of imaging and EBUS on initial assessment, followed by mediastinoscopy after induction therapy. PET scanning can be used as a guide to determine those who have progressed on induction therapy and help in guiding the clinician prior to the EBUS as to the status of the nodes. If the multidisciplinary team believes N2 status after induction therapy will determine resection plans, then the nodes must be rebiopsied regardless of the FDG uptake in the nodes. If the team follows the philosophy that surgery is beneficial whether or not the N2 nodal disease has cleared, and an R0 resection can be accomplished, then CT scan alone post induction (to rule out disease progression) is sufficient prior to surgery.
Figure 1.
Suggested treatment schemes for patients with Stage IIIA, NSLC with N2 disease based on best evidence in 2017. NSCLC, non-small cell lung cancer; EBUS, endobronchial ultrasound guided transbronchial biopsy; PORT, postoperative radiation therapy; CT, computed tomography; PET, positron emission tomography.
Acknowledgements
None.
Provenance: This is an invited Editorial commissioned by Section Editor Dr. Claudio Caviezel (Division of Thoracic Surgery, University Hospital Zurich, Zurich, Switzerland).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S-40S. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:706-14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 3.Billé A, Pelosi E, Skanjeti A, et al. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg 2009;36:440-5. 10.1016/j.ejcts.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 4.Hancock J, Rosen J, Moreno A, et al. Management of clinical stage IIIA primary lung cancers in the National Cancer Database. Ann Thorac Surg 2014;98:424-32; discussion 432. 10.1016/j.athoracsur.2014.04.067 [DOI] [PubMed] [Google Scholar]
- 5.Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-20S. [DOI] [PubMed] [Google Scholar]
- 6.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6. 10.1016/j.athoracsur.2005.06.071 [DOI] [PubMed] [Google Scholar]
- 7.Almeida FA. Bronchoscopy and endobronchial ultrasound for diagnosis and staging of lung cancer. Cleve Clin J Med 2012;79 Electronic Suppl 1:eS11-6. [DOI] [PubMed]
- 8.Martin LW, D'cunha J, Wang X, et al. Detection of occult micrometastases in patients with clinical stage I Non-Small-Cell lung cancer: a prospective analysis of mature results of CALGB 9761 (alliance). J Clin Oncol 2016;34:1484-91. 10.1200/JCO.2015.63.4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rusch VW, Hawes D, Decker PA, et al. Occult metastases in lymph nodes predict survival in resectable non-small-cell lung cancer: report of the ACOSOG Z0040 trial. J Clin Oncol 2011;29:4313-9. 10.1200/JCO.2011.35.2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaklitsch MT, Herndon JE, Decamp MM, et al. Nodal downstaging predicts survival following induction chemotherapy for stage IIIA (N2) non-small cell lung cancer in CALGB protocol 8935. J Surg Oncol 2006;94:599-606. 10.1002/jso.20644 [DOI] [PubMed] [Google Scholar]
- 11.Higgins K, Chino JP, Marks LB, et al. Preoperative chemotherapy versus preoperative chemoradiotherapy for stage III (N2) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009;75:1462-7. 10.1016/j.ijrobp.2009.01.069 [DOI] [PubMed] [Google Scholar]
- 12.Ripley RT, Suzuki K, Tan KS, et al. Postinduction positron emission tomography assessment of N2 nodes is not associated with ypN2 disease or overall survival in stage IIIA non-small cell lung cancer. J Thorac Cardiovasc Surg 2016;151:969-77, 979.e1-3. [DOI] [PMC free article] [PubMed]
- 13.Sauvaget J, Rebischung J, Vannetzel J, et al. Phase III study of neo-adjuvant MVP versus MVP plus chemo radiation in stage III NSCLC. Proc Am Soc Clin Oncol 2000;19: 495A. [Google Scholar]
- 14.Thomas M, Rübe C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008;9:636-48. 10.1016/S1470-2045(08)70156-6 [DOI] [PubMed] [Google Scholar]
- 15.Fleck J, Camarzo J, Godoy D, et al. Chemoradiation therapy versus chemotherapy alone as neoadjuvant treatment for stage III non-small cell lung cancer: preliminary report of a phase III prospective randomized trial. Proc Am Soc Clin Oncol 1993;12:333. [Google Scholar]
- 16.Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049-56. 10.1016/S0140-6736(15)60294-X [DOI] [PubMed] [Google Scholar]
- 17.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. 10.1016/S0140-6736(09)60737-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg 2012;93:1807-12. 10.1016/j.athoracsur.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 19.Dai Y, Han B, Shen J, et al. Preoperative induction chemotherapy for resectable stage IIIA non-small-cell lung cancer: a meta-analysis of 13 double-blind, randomized clinical trials. Zhongguo Fei Ai Za Zhi 2008;11:398-405. [DOI] [PubMed] [Google Scholar]
- 20.Garrido P, González-Larriba JL, Insa A, et al. Long-term survival associated with complete resection after induction chemotherapy in stage IIIA (N2) and IIIB (T4N0-1) non small-cell lung cancer patients: the Spanish Lung Cancer Group Trial 9901. J Clin Oncol 2007;25:4736-42. 10.1200/JCO.2007.12.0014 [DOI] [PubMed] [Google Scholar]
- 21.Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. 10.1093/jnci/86.9.673 [DOI] [PubMed] [Google Scholar]
- 22.Rosell R, Gómez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. 10.1056/NEJM199401203300301 [DOI] [PubMed] [Google Scholar]
- 23.Stefani A, Alifano M, Bobbio A, et al. Which patients should be operated on after induction chemotherapy for N2 non-small cell lung cancer? Analysis of a 7-year experience in 175 patients. J Thorac Cardiovasc Surg 2010;140:356-63. 10.1016/j.jtcvs.2010.02.018 [DOI] [PubMed] [Google Scholar]
- 24.Cerfolio RJ, Bryant AS, Ojha B. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: a prospective study. J Thorac Cardiovasc Surg 2006;131:1229-35. 10.1016/j.jtcvs.2005.08.070 [DOI] [PubMed] [Google Scholar]
- 25.Elias AD, Skarin AT, Leong T, et al. Neoadjuvant therapy for surgically staged IIIA N2 non-small cell lung cancer (NSCLC) Lung Cancer 1997;17:147-61. 10.1016/S0169-5002(97)00658-2 [DOI] [PubMed] [Google Scholar]
- 26.de Cabanyes Candela S, Detterbeck FC. A systematic review of restaging after induction therapy for stage IIIa lung cancer: prediction of pathologic stage. J Thorac Oncol 2010;5:389-98. 10.1097/JTO.0b013e3181ce3e5e [DOI] [PubMed] [Google Scholar]
- 27.Kamel MK, Rahouma M, Ghaly G, et al. Clinical predictors of persistent mediastinal nodal disease after induction therapy for stage IIIA N2 Non-Small cell lung cancer. Ann Thorac Surg 2017;103:281-6. 10.1016/j.athoracsur.2016.06.061 [DOI] [PubMed] [Google Scholar]
- 28.Billiet C, Decaluwé H, Peeters S, et al. Modern post-operative radiotherapy for stage III non-small cell lung cancer May improve local control and survival: a meta-analysis. Radiother Oncol 2014;110:3-8. 10.1016/j.radonc.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 29.PORT Meta-analysis Trialists Group Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2005;(2):CD002142. [DOI] [PubMed] [Google Scholar]
- 30.Le Péchoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: a reassessment based on new data. Oncologist 2011;16:672-81. 10.1634/theoncologist.2010-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley JD, Paulus R, Graham MV, et al. Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIA non-small-cell lung cancer: promising long-term results of the Radiation Therapy Oncology Group--RTOG 9705. J Clin Oncol 2005;23:3480-7. 10.1200/JCO.2005.12.120 [DOI] [PubMed] [Google Scholar]
- 32.Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol 2006;24:2998-3006. 10.1200/JCO.2005.04.6110 [DOI] [PubMed] [Google Scholar]
- 33.Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized. Int J Radiat Oncol Biol Phys 2008;72:695-701. 10.1016/j.ijrobp.2008.01.044 [DOI] [PubMed] [Google Scholar]
- 34.Amini A, Correa AM, Komaki R, et al. The role of consolidation therapy for stage III non-small cell lung cancer with persistent N2 disease after induction chemotherapy. Ann Thorac Surg 2012;94:914-20. 10.1016/j.athoracsur.2012.04.088 [DOI] [PMC free article] [PubMed] [Google Scholar]