Abstract
Background
The diagnostic yield of peripheral pulmonary lesions (PPLs) by flexible bronchoscopy (FB) is still insufficient. To improve the diagnostic yield of bronchoscopy, several techniques such as endobronchial ultrasound (EBUS), virtual bronchoscopic navigation (VBN), and rapid on-site evaluation (ROSE) have been examined. The primary purpose of the present study was to evaluate the usefulness of combining EBUS, VBN, and ROSE for diagnosing small PPLs.
Methods
Patients with PPLs 30 mm or less on chest computed tomography (CT) were prospectively enrolled. We determined the responsible bronchus for the target lesions using VBN before bronchoscopy was performed. EBUS and ROSE were performed during the examination to determine whether the bronchus and specimen were adequate. On the basis of previous studies, we assumed that the diagnostic yield of 85% among eligible patients would indicate potential usefulness, whereas, the diagnostic yield of 75% would indicate the lower limit of interest. The required number of patients was estimated as 45 for a one-sided α value of 0.2 and a β value of 0.8. The primary study endpoint was the diagnostic yield.
Results
Between June 2014 and July 2015, we enrolled 50 patients in the present study, and we excluded 5 patients. The total diagnostic yield of 45 PPLs was 77.7%. In cases of lung cancer, the diagnostic yield was 84.2%. The sensitivity, specificity, positive predictive value, and negative predictive value of ROSE were 90.6%, 92.3%, 96.7%, and 80.0%, respectively. The diagnostic yield of PPLs from 20 to 30 mm was 87.5%, and the diagnostic yield of PPLs less than 20 mm was 66.7%. PPLs for which the probe was located within the lesion had the highest diagnostic yield.
Conclusions
We could not demonstrate usefulness for diagnosing small PPLs by combining EBUS, VBN, and ROSE. However, combining these techniques may be useful for diagnosing lung cancer.
Keywords: Endobronchial ultrasound (EBUS), peripheral pulmonary lesion (PPL), rapid on-site evaluation (ROSE), virtual bronchoscopic navigation (VBN), bronchoscopy
Introduction
Small peripheral pulmonary lesions (PPLs) found by computed tomography (CT) examination are usually evaluated by percutaneous needle biopsy (PNB), flexible bronchoscopy (FB), or surgical resection. The diagnostic yield of PNB has been reported at about 90% (1). CT-guided PNB (CT-PNB), especially, has a higher diagnostic yield than fluoroscopy-guided PNB (2). However, the diagnostic yield of PPLs by FB is still insufficient. Rivera et al. reviewed 10 studies on the diagnostic yields of small PPLs (3). They reported that the diagnostic yield of PPLs less than 20 mm was 34.2% and that of PPLs 20 mm or more was 63.2%. To improve these diagnostic yields, several techniques such as endobronchial ultrasonography with a guide sheath (EBUS-GS) or virtual bronchoscopic navigation (VBN) have been examined, and the usefulness of these techniques has been demonstrated in previous studies. Kurimoto et al. reported that the diagnostic yield of PPLs 30 mm or less was 74% with FB and EBUS-GS (4). Asahina et al. reported that the diagnostic yield of PPLs 30 mm or less was 63.3% with FB, EBUS-GS and VBN (5). Tamiya et al. reported that the diagnostic yield of PPLs 30 mm or less was 77.9% with FB, EBUS-GS, and VBN (6). Although EBUS-GS and VBN are useful techniques for determining the responsible bronchus for the target lesions, we have sometimes received negative results even if we performed specimen collection with these techniques. One reason for this must be a sampling error, which can occur if insufficient specimens are obtained. The rapid on-site evaluation (ROSE) system involves the immediate assessment of cytology during the examination. Thus, we hypothesized that the ROSE system would reduce the sampling error and improve the diagnostic yield of small PPLs.
Therefore, in the present study, we evaluated the usefulness of combining techniques such as EBUS, VBN, and ROSE to diagnose small PPLs.
Methods
Patients
Patients with PPLs 30 mm or less on chest CT who visited the National Hospital Organization Kinki-chuo Chest Medical Center were enrolled in this study. The inclusion criterion was patients with PPLs 30 mm or less on chest CT whose lesions were observed on a chest radiograph. Exclusion criteria were as follows: evidence of visible endobronchial lesions revealed by chest CT; severe arrhythmia or heart failure; severe respiratory failure or chronic respiratory failure; lidocaine allergy; pneumothorax; bleeding tendency. We collected patients’ data including sex, age, smoking history, medical history, site of the lesion, size of the lesion, the responsible bronchus for the target lesions according to results of VBN, signs on EBUS, the number of specimens collected until a positive ROSE, complications, the duration of the examination, and operator’s years of experience.
The present study was approved by our hospital’s institutional review board (approval number 462). All patients who met the study eligibility requirements and signed the informed consent form were included.
VBN
All patients underwent a chest CT scan so we could create a virtual bronchial image. We used LungPoint®, version 3.1.0 (Bronchus Medical, Inc., San Jose, CA) as the VBN system. We defined the target lesion by placing a three-dimensional spherical marker on the CT image, and we created virtual bronchoscopy navigation to the lesion. These processes were performed by each operator. The responsible bronchus for the target lesions was determined at least until the fourth generation of airways.
FB and EBUS-GS
We used a thin, flexible bronchoscope (P260F, 4.0-mm outer diameter; Olympus, Tokyo, Japan). EBUS was performed using an endoscope ultrasonography system, which was equipped with a 20-MHz mechanical radial-type probe (UM-S20-17S, Olympus). The EBUS probe was inserted into the GS and introduced into a target lesion. The images of EBUS were categorized as within, adjacent to, or outside of the target lesion. Then we removed the EBUS probe and performed a biopsy and/or brushing with the GS. We allowed the operator to use the forceps and/or curette without the GS if necessary. Radiographic fluoroscopy was used when the operators performed EBUS and obtained the specimen. The duration of the examination was defined as the time until removal of the FB after it passed through the vocal cord.
ROSE
The obtained specimen with biopsy, brushing or curettage was immediately placed on two slides. One slide was fixed in 95% ethanol for a conventional Papanicolaous stain, and another slide was used for ROSE. The steps of ROSE performed at our hospital are as follows: (I) rapid fixative (10 s); (II) water washing (a few seconds); (III) Gill Hematoxylin 5 (40 s); (VI) water washing (a few seconds); (V) 1% hydrochloric acid/70% ethyl alcohol (a few seconds); (VI) hot water (a few seconds); (VII) OG-6 (5 s); (VIII) EA-50 (40 s); (XI) 100% ethyl alcohol (a few seconds); (X) 100% ethyl alcohol (a few seconds); (XI) xylene (a few seconds); and (XII) xylene (a few seconds). As aforementioned, we used the ultrafast Papanicolaou staining, so we were able to diagnosis the specimen within 3 min after sample collection. This procedure and cytology evaluation were performed by cytotechnologists. The specimens with malignant cells were categorized as positive, and the specimens without malignant cells were categorized as negative. The number of repeat for ROSE was entrusted to the operator considering the burden on patients.
Algorithm of this study
The algorithm of this study is shown in Figure 1. The enrolled patients underwent VBN assessment within 1 month before bronchoscopy, and the operator determined the responsible bronchus for the target lesions. On bronchoscopic examination, we inserted the EBUS-GS into the responsible bronchus and categorized the echo image. Then we obtained a specimen and performed ROSE immediately. Final diagnosis was estimated by the pathological diagnosis. Lesions that could not be diagnosed were examined further (surgical resection, second bronchoscopy, CT-PNB, or follow-up). Invariant lesions of 6 months were diagnosed as benign lesions.
Figure 1.
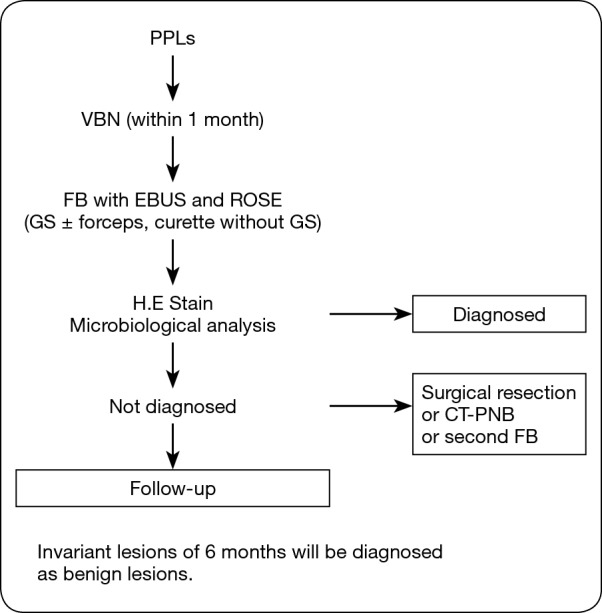
Algorithm of this study. PPLs, peripheral pulmonary lesions; VBN, virtual bronchoscopic navigation; EBUS, endobronchial ultrasound; ROSE, rapid on-site evaluation; GS, guide sheath; HE, hematoxylin-eosin; CT-PNB, computed tomography-guided percutaneous needle biopsy; FB, flexible bronchoscopy.
Analysis
This was a prospective phase II study with a historical control. The sample size was calculated using the Southwest Oncology Group one arm binomial tool. On the basis of previous studies (4-6), we assumed that the diagnostic yield of 85% among eligible patients would indicate potential usefulness, whereas, the diagnostic yield of 75% would indicate the lower limit of interest. The required number of patients was estimated as 45 for a one-sided α value of 0.2 and a β value of 0.8. The primary study endpoint was the diagnostic yield. The secondary study endpoints were the duration of the examination, the number of specimens collected until a positive ROSE, and complications.
Results
Between June 2014 and July 2015, we enrolled 50 patients in the present study, and we excluded 5 patients. One patient had a visible endobronchial lesion. One patient did not undergo EBUS examination. One patient’s PPL was consolidation, and two patients’ PPLs were not observed on a chest radiograph. Table 1 shows the characteristics of the 45 patients evaluated. The median age was 71 years (range, 49–85 years). The number of men was about three times that of women. The median diameter of the PPLs was 22 mm (range, 10–29 mm). The median duration of the examination was 34.2 min (range, 12.9–76 min). The generations of airway to reach PPLs were more than forth generation except for one PPL which had only third generation to reach. We diagnosed 35 patients by using FB. Two patients were diagnosed by surgical resection; 2 were diagnosed by second bronchoscopy; 2 were diagnosed by CT-PNB; and 4 were diagnosed as having a benign lesion, as it was invariant for 6 months. The number of specimens collected until a positive ROSE of each procedure was as shown. Complications included only two minor bleeds that required treatment with saline and adrenaline, and there were no severe events.
Table 1. Patient’s characteristics.
| Characteristics | Data |
|---|---|
| Age | |
| Median (range*) | 71 [49–85] |
| Sex, n (%) | |
| Male | 34 (75.6) |
| Female | 11 (24.4) |
| Smoking history, n (%) | |
| Yes | 38 (84.4) |
| No | 7 (15.6) |
| Medical history, n (%) | |
| Cardiac disease | 5 (11.1) |
| Malignancy | 8 (17.8) |
| Hyper tension | 13 (28.9) |
| Diabetes | 3 (6.7) |
| Lesion location, n (%) | |
| Rt S1 | 5 (11.1) |
| Rt S2 | 11 (24.4) |
| Rt S3 | 3 (6.7) |
| Rt S4 | 1 (2.2) |
| Rt S5 | 1 (2.2) |
| Rt S6 | 4 (8.9) |
| Rt S7 | 0 (0) |
| Rt S8 | 6 (13.3) |
| Rt S9 | 0 (0) |
| Rt S10 | 1 (2.2) |
| Lt S1+2 | 5 (11.1) |
| Lt S3 | 5 (11.1) |
| Lt S4 | 0 (0) |
| Lt S5 | 0 (0) |
| Lt S6 | 0 (0) |
| Lt S8 | 1 (2.2) |
| Lt S9 | 2 (4.4) |
| Lt S10 | 0 (0) |
| Lesion size (mm), n (%) | |
| 0–10 | 1 (2.2) |
| 11–20 | 20 (44.4) |
| 21–30 | 24 (53.3) |
| Time of examination (min) | |
| Median (range*) | 34.2 (12.9–76) |
| The number of specimens collected until a positive ROSE | |
| Median (range*) | |
| Brushing with GS (n=9) | 1 [1] |
| TBB with GS (n=15) | 2 [1-8] |
| Curettage without GS (n=3) | 1 [1-2] |
| TBB without GS (n=3) | 4 [3-6] |
| Complications, n (%) | |
| Bleeding | 2 (4.4) |
| Further examination, n (%) | |
| Surgical resection | 2 (4.4) |
| Second bronchoscopy | 2 (4.4) |
| CT guided biopsy | 2 (4.4) |
| Follow up | 6 (13.3) |
| VBN (diagnosed 35 lesions), n (%) | |
| Matched with assessment before bronchoscopy | 31 (88.6) |
| Not matched with assessment before bronchoscopy | 4 (11.4) |
| Operator’s experience (year) | |
| Median (range*) | 5 [2–12] |
*, Range (minimum value to maximum value). TBB, transbronchial biopsy; GS, guide sheath; CT, computed tomography; VBN, virtual bronchoscopic navigation.
Table 2 shows the diagnosis of PPLs and their diagnostic yields. The diagnostic yield of 45 PPLs was 77.7%. In cases of lung cancer, the diagnostic yield was 84.2%. Table 3 shows the difference in diagnostic yield according to the size of the target lesion and location of the probe. The diagnostic yield of PPLs from 20 to 30 mm was 87.5%, and the diagnostic yield of PPLs less than 20 mm was 66.7%. PPLs for which the probe was located within the lesion had the highest diagnostic yield. Table 4 shows the sensitivity, specificity, positive predictive value, and negative predictive value of ROSE.
Table 2. Diagnostic yield.
| Diagnosis | Yield |
|---|---|
| Lung cancer | 32/38 (84.2) |
| Tuberculosis | 1/2 (50.0) |
| Mycobacterium infection | 1/1 (100.0) |
| Inflammatory lesion | 1/1 (100.0) |
| Benign lesions | 0/3 (0) |
| Total | 35/45 (77.7) |
Data are presented as the number of lesions/the total lesions (%).
Table 3. Effect of lesion size and probe position on diagnostic yield.
| Parameter | Yield |
|---|---|
| Lesion size (mm) | |
| <20 | 14/21 (66.7) |
| ≥20 to <30 | 21/24 (87.5) |
| Probe position | |
| Within | 26/28 (92.9) |
| Adjacent | 5/10 (50) |
| outside | 4/7 (57.1) |
Data are presented as the number of lesions/the total lesions (%).
Table 4. Usefulness of ROSE.
| ROSE | Final pathological diagnosis | |
|---|---|---|
| Malignancy | Not malignancy | |
| Positive, n | 29 | 1 |
| Negative, n | 3 | 12 |
Sensitivity 90.6%, Positive predictive value 96.7%; specificity 92.3%, Negative predictive value 80.0%. ROSE, rapid on-site evaluation.
Discussion
This is the first prospective phase II study to evaluate the usefulness of combining EBUS, VBN, and ROSE to diagnosis small PPLs. It is clear that the diagnostic yield of CT-PNB is high; however, the most significant disadvantage of this examination is complications. Steinfort et al. assessed the comparative effectiveness of EBUS and CT-PNB in a randomized trial. In their study, pneumothorax occurred in 27% of patients who underwent CT-PNB, whereas pneumothorax occurred in only 3% of patients who underwent EBUS (7). This means that FB is a less invasive examination than CT-PNB. Therefore, we need to improve the diagnostic yield of FB for small PPLs with several techniques to reach the diagnostic yield of CT-PNB to reduce the invasiveness for patients.
VBN is a noninvasive method for evaluating the tracheobronchial tree. Several studies have reported the usefulness of this technique in terms of the diagnostic yield (8-10). Of 35 diagnosed patients, 31 were diagnosed according to the responsible bronchus for the target lesion using VBN before the examination. This result shows the usefulness of VBN, and we consider that this assessment may help reduce the number of bronchi that are mistaken to be responsible for the target lesions, which may lead to an increase in the diagnostic yield.
EBUS is used to assess whether the GS can reach the lesion. The location of the probe was important for improving the diagnostic yield. A location of the probe within the lesion had the highest diagnostic yield in several studies (4,6,11,12). Similar to that found in previous studies, the diagnostic yield of PPLs with the probe location within the lesion was highest compared to that with the probe location adjacent to and outside the lesion in the present study. The usefulness of EBUS was also reported in PPLs that were not visible on a radiograph. In retrospective analysis of the diagnostic efficacy of EBUS-GS and tomosynthesis images for ground-glass opacity pulmonary lesions, Izumo et al. reported that there was no significant difference whether the target lesion was visible on a radiograph (13). Yoshikawa et al. reported that 76 of 123 PPLs (61.8%) were diagnosed by EBUS-GS without fluoroscopy (14). Otherwise, several studies have reported the effectiveness of visibility on a radiograph for making the diagnosis (15,16). Thus, we unified the group of patients who had a lesion observed on a chest radiograph to exclude this factor.
ROSE is also a useful technique for improving the diagnostic yield. Bandoh et al. reported that using helical CT with multi-planar reconstruction and an ultrafast Papanicolaou stain for diagnosing PPLs by FB improved the diagnostic yield to 93% for malignant lesions compared to 60% in a historical control group (17). Before starting the study, we often experienced that changing biopsy or brushing with GS to biopsy or curetting without GS results in a positive ROSE. We infer two reasons for this result. The first reason is the difference in size among the specimens. Forceps without a GS are bigger than forceps with a GS, as shown in Figure 2. The second reason is the usefulness of changing the device. Tamiya et al. reported improvement in the diagnostic yield with the addition of other devices for diagnosing PPLs (18). Therefore, we allowed operators to change procedures like forceps and curettage without a GS if they thought it was necessary. Additionally, they made this decision depending on the results of ROSE. As a result, six patients were diagnosed by specimens collected without a GS in the present study. This result suggests that the ROSE system is useful even when the procedures change during the examination. To evaluate the effect of ROSE on the diagnostic yield of EBUS for PPLs, Chen et al. reported the usefulness of combining EBUS and ROSE. This retrospective study showed that ROSE increased the diagnostic yield for PPLs less than 30 mm or those more than 70 mm. They presumed that if the tumor became large, it had a central necrotic part, which could cause high false-negative results (19). This study may suggest that our combination of techniques is useful for large tumors considered necrotic lesions according to CT findings.
Figure 2.
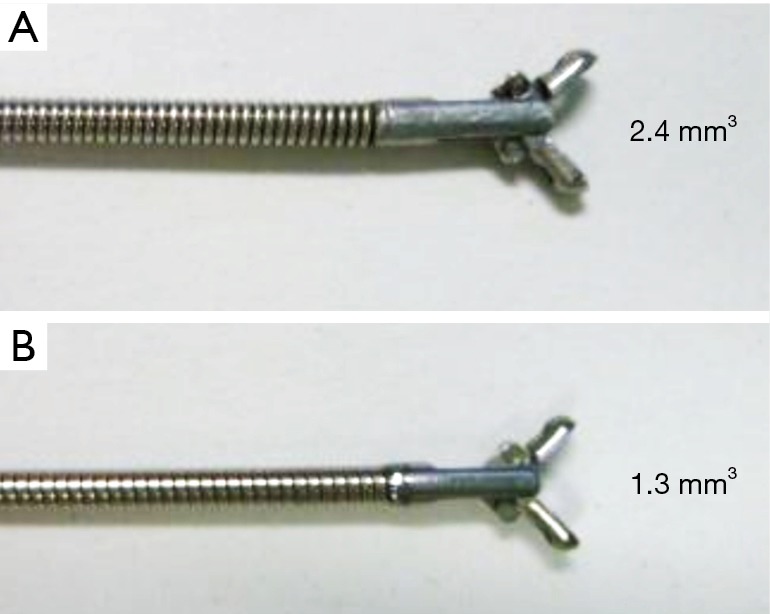
Difference in the biopsy size. (A) Forceps without the GS (1.8-mm outer diameter, 4.0-mm open width diameter, and 2.4 mm3 cup capacity); (B) forceps with the GS (1.5-mm outer diameter, 4.3-mm open width diameter, and 1.3 mm3 cup capacity).
In the present study, the diagnostic yield of lung cancer was high compared to overall diagnostic yield. We think this finding is due to the lack of specific histologic characterization of a benign lesion. For benign lesions, it is too difficult to determine whether we can obtain an adequate specimen even if we use the ROSE system. We assessed three benign lesions that were not diagnosed by FB. Two cases only had bronchial epithelial tissue, and one case had pulmonary artery and normal alveolar tissue.
There are several limitations to the current study. First, we allowed the operators to use forceps without a GS if necessary. Although we chose the same bronchus that we inserted the GS into, we could not see more distal than the sub-segmental bronchus. Therefore, we may have chosen a different bronchus when using EBUS-GS. Second, there was a difference in each operator’s proficiency with the use of EBUS-GS, VBN, and FB. Although we used the same operators, they became accustomed to a certain technique over time. Lastly, our study was performed at a single institution, and we compared our findings to historical data of other institutions; additionally, the sample size was small.
In conclusion, we could not demonstrate usefulness for diagnosing small PPLs by using a combination of EBUS, VBN, and ROSE. However, this combination of techniques may be useful for diagnosing lung cancer, and the ROSE system may be useful when the operator determines changing the procedures of specimen collection. We encourage further prospective studies of this combination which contains larger sample size.
Acknowledgements
We would like to thank all the participants of this study. We also thank the cytotechnologists of the National Hospital Organization Kinki-chuo Chest Medical Center.
Ethical Statement: The present study was approved by our hospital’s institutional review board (approval number 462). All patients who met the study eligibility requirements and signed the informed consent form were included.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123:115s-28s. 10.1378/chest.123.1_suppl.115S [DOI] [PubMed] [Google Scholar]
- 3.Rivera MP, Mehta AC. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131s-48s. [DOI] [PubMed] [Google Scholar]
- 4.Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. 10.1378/chest.126.3.959 [DOI] [PubMed] [Google Scholar]
- 5.Asahina H, Yamazaki K, Onodera Y, et al. Transbronchial biopsy using endobronchial ultrasonography with a guide sheath and virtual bronchoscopic navigation. Chest 2005;128:1761-5. 10.1378/chest.128.3.1761 [DOI] [PubMed] [Google Scholar]
- 6.Tamiya M, Okamoto N, Sasada S, et al. Diagnostic yield of combined bronchoscopy and endobronchial ultrasonography, under LungPoint guidance for small peripheral pulmonary lesions. Respirology 2013;18:834-9. 10.1111/resp.12095 [DOI] [PubMed] [Google Scholar]
- 7.Steinfort DP, Liew D, Irving LB. Radial probe EBUS versus CT-guided needle biopsy for evaluation of peripheral pulmonary lesions: an economic analysis. Eur Respir J 2013;41:539-47. 10.1183/09031936.00044612 [DOI] [PubMed] [Google Scholar]
- 8.Asano F, Matsuno Y, Shinagawa N, et al. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest 2006;130:559-66. 10.1378/chest.130.2.559 [DOI] [PubMed] [Google Scholar]
- 9.Tachihara M, Ishida T, Kanazawa K, et al. A virtual bronchoscopic navigation system under X-ray fluoroscopy for transbronchial diagnosis of small peripheral pulmonary lesions. Lung Cancer 2007;57:322-7. 10.1016/j.lungcan.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 10.Ishida T, Asano F, Yamazaki K, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax 2011;66:1072-7. 10.1136/thx.2010.145490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirakawa T, Imamura F, Hamamoto J, et al. Usefulness of endobronchial ultrasonography for transbronchial lung biopsies of peripheral lung lesions. Respiration 2004;71:260-8. 10.1159/000077424 [DOI] [PubMed] [Google Scholar]
- 12.Yamada N, Yamazaki K, Kurimoto N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest 2007;132:603-8. 10.1378/chest.07-0637 [DOI] [PubMed] [Google Scholar]
- 13.Izumo T, Sasada S, Chavez C, et al. The diagnostic utility of endobronchial ultrasonography with a guide sheath and tomosynthesis images for ground glass opacity pulmonary lesions. J Thorac Dis 2013;5:745-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshikawa M, Sukoh N, Yamazaki K, et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest 2007;131:1788-93. 10.1378/chest.06-2506 [DOI] [PubMed] [Google Scholar]
- 15.Ikezawa Y, Sukoh N, Shinagawa N, et al. Endobronchial ultrasonography with a guide sheath for pure or mixed ground-glass opacity lesions. Respiration 2014;88:137-43. 10.1159/000362885 [DOI] [PubMed] [Google Scholar]
- 16.Minezawa T, Okamura T, Yatsuya H, et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging 2015;15:21. 10.1186/s12880-015-0060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandoh S, Fujita J, Tojo Y, et al. Diagnostic accuracy and safety of flexible bronchoscopy with multiplanar reconstruction images and ultrafast Papanicolaou stain: evaluating solitary pulmonary nodules. Chest 2003;124:1985-92. 10.1378/chest.124.5.1985 [DOI] [PubMed] [Google Scholar]
- 18.Tamiya M, Sasada S, Uehara N, et al. The contribution of widely used devices in the diagnosis of peripheral pulmonary lesions in patients presenting with respiratory distress. Nihon Kokyuki Gakkai Zasshi 2009;47:663-8. [PubMed] [Google Scholar]
- 19.Chen CH, Cheng WC, Wu BR, et al. Improved diagnostic yield of bronchoscopy in peripheral pulmonary lesions: combination of radial probe endobronchial ultrasound and rapid on-site evaluation. J Thorac Dis 2015;7:S418-25. [DOI] [PMC free article] [PubMed] [Google Scholar]


