Abstract
Background
The feasibility and practicality of preoperative rehabilitation (PR) programs remains quite controversial in the treatment of lung cancer (LC). This study explored whether a short-term high-intensity rehabilitation program could improve postoperative outcomes compared to those achieved with conventional inspiratory muscle training (IMT).
Methods
A three-armed randomized controlled trial comparing the two training modalities and routine care was conducted in surgical LC patients. Patient groups received one of three treatment regimens: (I) high-intensity pulmonary rehabilitation (PR) that combined IMT with conventional resistance training (CRT) (combined PR group); (II) conventional PR (single IMT group); or (III) routine preoperative preparation (control group). The primary endpoint was a change in the occurrence of post-operative pulmonary complications (PPCs) that occurred within 30 days after surgery, while secondary endpoints included changes in length of hospital stay, quality of life (QoL) scores, 6-min walk distance (6-MWD) and peak expiratory flow (PEF).
Results
A total of 90 enrolled patients were randomized into three groups with a computer-based 1:1:1 ratio. The intention-to-treat analysis of the study revealed that, compared with the Control Group, the Combined PR Group had a significant increase in ∆6-MWD (by 32.67 m, P=0.002), ∆PEF (by 14.3 L/min, P=0.001), ∆global scores (by 3.7, P=0.035); and a reduced ∆average total hospital stay (by 3.2 d, P=0.001) and ∆postoperative stay (by 3.6 d, P=0.001). With regard to PPC rate, the Combined PR Group had a somewhat lower PPC severity (grade II–V) compared to the Control Group.
Conclusions
This hospital-based short-term program of PR combining high-intensity IMT with CRT was significantly superior to the conventional IMT program, indicating that this approach would be a feasible strategy for treating LC patients, especially those waiting operations with surgery-related risk factors.
Keywords: Short-term, high-intensity, preoperative rehabilitation (PR), lung cancer (LC)
Introduction
Globally, lung cancer (LC) is the one of the most frequent malignant tumor, with the leading cause of cancer-related deaths (1). For pre-malignant and early-to-mid stages of LC, surgery remains the optimal treatment, although multidisciplinary treatments are also prevalent (2,3). Postoperative pulmonary complications (PPCs) are primary and promoting factors of poor outcomes (4). To improve clinical outcomes for LC patients, there has been a growing interest in the role of preventive and therapeutic management strategies during the past decades, and mounting evidence reveals that 2–4 weeks long preoperative PR treatments can promote physical-psychological improvements in exercise capacity, functional status, and quality of life (QoL) (5,6). However, due to diversity in local adaptation, whether previously reported treatment regimen can be adaptively applied in other countries with developing medical systems, such as China, remains unclear. As such, the feasibility and practicability of these procedures in developing nations need further researches.
The choice of preoperative rehabilitation (PR) program for patients undergoing LC lobectomy is governed by potential patient risk factors for PPCs, including advanced age, smoking status, obesity, chronic obstructive pulmonary disease (COPD), poor lung function, and history of thoracic surgery. These risk factors are defined by national expert consensus and guidelines drafted by the Ministry of Health Clinical Pathway Audit Committee of the Thoracic Surgery Expert Panel [2012] and the definitions of the Society of Thoracic Surgeons [2012] (7). Hence, we hypothesis that a program combined IMT with CRT has better improvements in cardio-thoracic pulmonary function (6-MWD) and postoperative outcomes (PPC rate), compared with a single IMT program or routine preoperative preparations. To better explore a feasible PR pattern for Chinese LC patients with PPC risk factors, especially those waiting therapeutic surgeries, we designed this prospective randomized controlled trial using a selected study population treated with a short-term program.
Methods
Ethical review and approval
This prospective three-armed randomized controlled trial was adhered to the Declaration of Helsinki, and approved by the Clinical Trials and Biomedical Ethics Committee of Sichuan University West University Hospital and Chinese Ethics Committee of Registering Clinical Trials (ChiCTR-IOR-16008109). All enrolled patients signed informed consent and patient information was obtained.
Study subjects and grouping
A total of 90 preoperative LC volunteers were recruited from the Department of Thoracic Surgery and Department of Rehabilitation Medicine, West China Hospital, between November 1, 2015 and May 31, 2016. Accompanying risk factors were assigned according to the Ministry of Health Clinical Pathway Audit Committee of the Thoracic Surgery Expert Panel [2012]. Participants were divided into three groups and randomization was performed by the coordinating investigator with a computer-based 1:1:1 ratio.
Inclusion/exclusion criteria
Inclusion criteria were: (I) a definite diagnosis of primary non-small cell lung cancer (NSCLC) based on preoperative pathological examination and following NSCLC diagnosis and treatment guidelines; (II) presence of PPC risk factors, including age >70 years, body mass index (BMI) >30, COPD with a heavy smoking history (≥20 pack-year or a preoperative smoking control time ≤2 weeks), forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) (FEV1/FVC) ratio ≤70%, or prior history of thoracic surgery; (III) no surgical contraindication and willingness to undergo video-assistant thoracic surgery (VATS) or traditional open thoracotomy (open); and (IV) patient agreement to receive preoperative interventions.
Exclusion criteria were: patients who had contraindications to the PR regimen or risk of adverse events including myocardial infarction or cerebrovascular accident within one year, unstable angina pectoris, aneurysm, recent history (<90 days) of hemoptysis, severe arrhythmia, musculoskeletal or mental disorders.
Preoperative PR program
The patients in the combined PR group were treated for one week with high-intensity preoperative PR using IMT and aerobic endurance exercise. This one-week PR program was primarily a physical-based intervention that focused on exercise endurance and resistance training or a combination of methods, such as inspiratory muscle training (IMT), and CRT, coupled with psychological-educational guidance to cope with pre-operative anxiety and depression, or even perioperative activities. The entire PR process was performed during the in-hospital time, and all participants were assessed and recorded by a statistician who was blind to the study design. While the patients in the single IMT group received conventional single-mode IMT, and the control group patients underwent routine preoperative preparations, including preoperative education for in-hospital, preoperative preparation (relevant examinations arrangements e.g.,) and essential encouragement or psychological caring.
The preoperative PR procedure was:
On the first day of the PR program, 6-MWT, pulmonary function test (PFT) and health-related QoL (HRQoL) test were evaluated and recorded for all participants. The 6-MWT and PFTs were performed to assess the patients’ initial cardiopulmonary function based on the American Thoracic Society Pulmonary Function Standards Committee. During the 6-MWT, Borg dyspnea scores, index of fatigue, 6-MWD, PEF, and energy consumption (Kcal) values were calculated and recorded. The HRQoL test was evaluated by chart review and scored with EORTC QLQ-C30&LC13_CN (version 3), which has a self-evaluation score reflecting the related status, with higher scores associated with good status.
The daily exercises described below included IMT and CRT for the Combined PR group, and IMT for the IMT Group. IMT involved abdominal and thoracic breathing training that was performed in the patient ward under the supervision and guidance of trained nurses: (I) abdominal breathing training: the purpose of this training was to strengthen the diaphragm muscles. For this training, patients assumed a supine position, and inhaled slowly through the nose to their maximum lung capacity. Patients then briefly held their breath before exhaling slowly through the lips with their abdominal muscles tightened. This exercise was performed two to three times daily for 15–20 minutes per session; (II) thoracic breathing training: this training was undertaken to strengthen intercostal muscles and used a simple respiratory training device (Voldyne 5000, Sherwood Medical Supplies, St Louis, MO, USA). Patients were guided to exhale calmly at the beginning, and then deeply inhaled through the suction nozzle of the device, and after holding for several seconds, they then exhaled slowly. Patients performed these exercises for 20 minutes at least four times daily. Meanwhile, for CRT, a NuStep cross-training apparatus (NuStep, Inc., Ann Arbor, Michigan) was used at a rehabilitation training center under the guidance of physiologists. At the beginning of the CRT, the patients adjusted the resistance of the NuStep according to their own speed and power, and then progressively increased the resistance range; patients used the NuStep twice daily for 20 minutes per session. For all training exercises described above, the procedure was stopped if the patients showed any obvious discomfort, such as shortness of breath, dyspnea or exhaustion, and they were allowed to rest until their condition allowed them to withstand subsequent training.
At the end of the PR program period, the 6-MWT, PFT, and HRQoL tests were performed again and then received the arranged surgery.
Postoperative management
Antibiotics were used to prevent or treat infection, and intravenous patient-controlled analgesia (tramadol hydrochloride injection, 1–1.5 mg/h) was used during the initial three postoperative days, followed by oral non-steroidal analgesics (ibuprofen soft capsules, 200 mg, and twice a day). Chest CT scans and haematological examinations were performed routinely to determine whether pulmonary infection, aerothorax or pleural effusion occurred, and to decide sequential whether to treat these symptoms specially (8).
Outcome measures
Primary endpoints
PPCs were redefined and classified into five grades according to the Clavien-Dindo Complication Classification System (Table 1), and the PPCs were ultimately defined as Clavien-Dindo grade II to grade V. Common PPC criteria were: pneumonia [confirmed by new infiltrates by X-ray imaging, white cell count (WBC) >11×109/L, temperature >38.5 °C, and purulent sputum], atelectasis (too much sputum or the sputum was too thick to allow expulsion and thus required bronchoscopy and sputum suction to remove), bronchopleural fistula, positive pleural effusion, prolonged chest tubes (>7 days), prolonged mechanical ventilation (>24 hours) (6), or reoperation.
Table 1. Classifications of postoperative pulmonary complications of thoracic surgery*.
| Grade I |
| Cough, transient, not due to other causes |
| Pneumonia: new onset purulent sputum; fever >38.5 °C, imageological infiltrate, WBC count >11×109/L, negative blood cultures; no focus outside the lungs |
| Microatelectasis, microaerothorax or air leakage: persistent, leak duration <7 days |
| Dyspnea: symptom improves by oxygen inhalation |
| Grade II |
| Cough, productive, not due to other causes |
| Dyspnea: bronchospasm or sputum blockage needing pharmacological intervention |
| Pneumonia or wound infection: positive bacterial cultures, needing antibiotics change |
| Hypercarbia requiring treatment, such as mechanical ventilation >24 hours |
| Blood transfusions and total parenteral nutrition support |
| Grade III |
| Severe atelectasis needing bronchoscope and aspiration |
| Pleural effusion or persistent air leak needing tube relocation or thoracentesis |
| Prolonged duration of tube drainage: duration >7 days, due to persistent air leakage or pleural effusion |
| Re-operation: bronchopleural fistula, chylothorax, active thoracic hemorrhage |
| Grade IV |
| Postoperative mechanical ventilation >48 hours |
| Pulmonary embolism; ARDS |
| Single or multi organ failure |
| Return to ICU, due to other life-threatening complications or organ dysfunction |
| Grade V |
| Death |
*, according to the Clavien-Dindo Complication Classification System (9). Grade I: the abnormal course without the need for pharmacological, surgical, endoscopic or radiological interventions; Grade II: requiring pharmacological treatment other than such allowed for grade I complications; Grade III: requiring surgical, endoscopic or radiological intervention; Grade IV: life-threatening complication or requiring IC/ICU-management; Grade V: death. IC, intensive care; ICU, intensive care unit.
Secondary endpoints
Other outcomes including the length of in-hospital stay, 6-MWD, PEF, fatigue and dyspnea index, and QoL scores, which were set as secondary endpoints. 6-MWD, PEF, and fatigue and dyspnea resistance index were analyzed to assess differences before and after physical training/preparation. EORTC-QLQ-C30 and EORTC–LC13 scores were also used to analyze the subjective self-evaluation.
Statistical analysis
The primary endpoint of the study was to reduce the rate of PPCs that occurred in the 30 days after surgery. Type-I error (α) was set at 5% with 80% statistical power. We expected to produce a 30% difference in the PPC rate. This prediction was based on unpublished study data that revealed an 8.0% PPC rate in the PR group. Based on this finding, at least 27 patients were needed for each arm. In addition, we predicted that 10% of patients would drop out. Using a two-sided alternative, we thus needed to include 30 patients in each group.
All continuous variables were presented as mean ± standard deviation (SD). The continuous variables in the Combined PR Group and Single IMT Group were compared separately with the Control Group by a two-sided independent samples t-test. Frequency data were compared between the groups using the χ2 test. All results were considered significant at P<0.05. Statistical analyses were performed using SPSS software V.21.0.
Results
Study population and characteristics
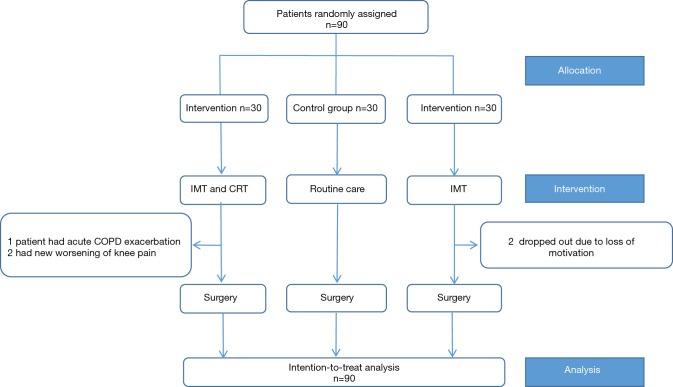
From November 2015 to May 2016, a total of 90 LC patients awaiting lobectomy at the Department of Thoracic Surgery of West China Hospital were included. Among those patients, 16 had an advanced age of >70 y, 12 had COPD, 8 had an ASA score >3, 9 had a BMI >30 and 1 had a prior history of VATS wedge resection. The majority of patients were male (62/90, 68.9%), and among these, most (46/62; 74%) were smokers. During the rehabilitation, 5 patients dropped out because of loss of motivation (n=2), acute COPD exacerbation (n=1), and a new worsening of knee pain (n=2) (Figure 1). In consideration of the intention to treat (ITT) principle, we included these patients in the final analysis. Of the enrolled patients, 66 (73.3%) and 24 (26.7%) underwent VATS lobectomy and open lobectomy, respectively. All groups were comparable in terms of demographic and surgical characteristics, with no significant differences seen between the groups. The details of the study population and characteristics are listed in Table 2.
Figure 1.
The study flow of the work. The intentional analysis was adopted in the study.
Table 2. Baseline characteristics of patients.
| Variables | Combined PR group (n=30) | Single IMT group (n=30) | Control group (n=30) | P1 | P2 |
|---|---|---|---|---|---|
| Age (years, mean ± SD) | 63.0±8.7 | 64.1±5.3 | 63.6±6.5 | 0.751 | 0.761 |
| Gender (male), n (%) | 20 (66.7) | 21 (70.0) | 21 (70.0) | 0.781 | 1.000 |
| PFT, mean ± SD | |||||
| FEV1 (L) | 2.3±0.6 | 2.3±0.8 | 2.2±0.7 | 0.103 | 0.635 |
| PPoFEV1% | 73.1±16.0 | 64.3±19.1 | 66.1±16.6 | 0.317 | 0.698 |
| FVC (L) | 3.2±0.7 | 3.3±0.8 | 3.2±0.6 | 0.640 | 0.586 |
| DLCO (mL/min/mmHg) | 22.8±4.8 | 21.8±4.8 | 22.5±4.7 | 0.836 | 0.584 |
| ppoDLCO% | 76.4±17.1 | 74.2±17.7 | 77.0±15.9 | 0.886 | 0.616 |
| Risk factors, n (%) | |||||
| ASA score>3 | 3 (10.0) | 3 (10.0) | 2 (6.7) | 1.000 | 1.000 |
| Current smoking status | 7 (23.3) | 6 (20.0) | 7 (23.3) | 1.000 | 0.767 |
| COPD | 5 (16.7) | 4 (13.3) | 6 (20.0) | 0.739 | 0.488 |
| BMI >30 | 3 (10.0) | 4 (13.3) | 2 (6.7) | 1.000 | 0.424 |
| Prior thoracic surgery | 1 (3.3) | 0 (0) | 0 (0) | 1.000 | 1.000 |
| Clinical stage, n (%) | 0.277 | 0.139 | |||
| Stage I | 16 (53.3) | 14 (46.7) | 17 (56.7) | ||
| Stage II | 10 (33.3) | 10 (33.3) | 11 (36.6) | ||
| Stage III | 4 (13.3) | 6 (20.0) | 2 (6.7) | ||
| Surgical approach, n (%) | 0.598 | 0.787 | |||
| VATS | 17 (56.7) | 20 (66.7) | 19 (63.3) | ||
| Open | 13 (43.3) | 10 (33.3) | 11 (36.7) | ||
| Total in-hospital stay (days), mean ± SD | 14.1±2.7 | 15.7±.3.0 | 17.3±4.3 | 0.001 | 0.114 |
| Preoperative | 8.3±1.1 | 7.6±2.6 | 7.9±2.0 | 0.316 | 0.660 |
| Postoperative | 5.8±3.0 | 8.1±2.1 | 9.4±4.6 | 0.001 | 0.170 |
P1: P value, baseline compared in combined PR group vs. control group; P2: P value, baseline compared in single IMT group vs. control group. IMT, inspiratory muscle training; FEV1, forced expiratory volume in one second; ppoFEV1%, postoperative predicted FEV1%; DLCO, diffusion capacity for carbon monoxide of the lung; ppoDLCO%, postoperative predicted DLCO%; COPD, chronic obstructive pulmonary disease; BMI, body mass index; VATS, video assisted thoracic surgery.
Endpoint outcomes
PPCs
Pneumonia was the most frequent PPC among the three treatment groups (17.8%, total; combined PR group: 13.3%, IMT group: 16.7%, and control group: 23.3%), and was followed by pleural effusion (5.56%), air leakage (5.56%), and atelectasis (4.4%) (Table 3). An analysis of the patients with PPC grade II–V according to the Clavien-Dindo Complication Classification System (Table 4) showed a significant difference between the Combined PR Group vs. the Control Group (P=0.045), but there were no significant differences between the IMT Group and Control Group (P=0.273).
Table 3. Person-time of postoperative pulmonary complications between groups.
| Complication criteria | Combined PR group (n=30) | Single PR group (n=30) | Control group (n=30) | P1 | P2 |
|---|---|---|---|---|---|
| Pneumonia | 4 (13.3) | 5 (23.3) | 7 (23.3) | 0.317 | 0.519 |
| Pleural effusion needing tube | 1 (3.3) | 2 (6.7) | 2 (6.7) | ||
| Atelectasis needing toilet bronchoscope | 2 (6.7) | 1 (3.3) | 1 (3.3) | ||
| Empyema | 1 (3.3) | 1 (3.3) | 1 (3.3) | ||
| Mechanical ventilation >48 h | 1 (3.3) | 1 (3.3) | 2 (6.7) | ||
| Bronchopleural fistula | 0 (0.0) | 0 (0.0) | 1 (3.3) | ||
| Chylothorax | 0 (0.0) | 1 (3.3) | 1 (3.3) | ||
| Back to ICU | 0 (0.0) | 1 (3.3) | 1 (3.3) | ||
| Air leak >7 days | 2 (6.7) | 1 (3.3) | 2 (6.7) | ||
| Pulmonary embolism | 0 (0.0) | 1 (3.3) | 1 (3.3) | ||
| ARDS or respiratory failure | 0 (0.0) | 0 (0.0) | 1 (3.3) | ||
| Death | 0 (0.0) | 0 (0.0) | 1 (3.3) |
P1: P value of combined PR group vs. control group; P2: P value of single IMT group vs. control group. ICU, intensive care unit; ARDS, acute respiratory distress syndrome.
Table 4. Differences in person-time of PPCs grades between-groups.
| Complication grade | Combined PR group (n=30) | IMT group (n=30) | Control group (n=30) | P1 | P2 |
|---|---|---|---|---|---|
| Grade I | 14 (46.7) | 14 (46.7) | 16 (53.3) | 0.606 | 0.606 |
| Grade II | 4 (13.3) | 5 (16.7) | 8 (26.7) | 0.197 | 0.347 |
| Grade III | 3 (10.0) | 3 (10.0) | 3 (10.0) | 1.000 | 1.000 |
| Grade IV | 1 (3.3) | 1 (3.3) | 2 (6.7) | 1.000 | 1.000 |
| Grade V | 0 (0) | 0 (0) | 1 (3.3) | 1.000 | 1.000 |
| PPC rate (grade II–V) | 5 (13.3) | 8 (26.7) | 12 (40.0) | 0.045 | 0.273 |
P1: P value of combined PR group vs. control group; P2: P value of single IMT group vs. control group. PPCs, postoperative pulmonary complications; IMT, inspiratory muscle training; PR, pulmonary rehabilitation.
Length of in-hospital stay
There was no statistically significant difference in preoperative length of hospital stay between the two PR groups and the Control Group, but the total and postoperative length of stay for the Combined PR Group was statistically shorter compared to the Control Group by a mean 3.2 days and 3.6 days (14.1±2.7 vs. 17.3±4.3 d, P=0.001; 5.8±3.0 vs. 9.4±4.6 d, P=0.001; respectively), while there was no difference between the IMT Group and Control Group.
6-MWT
No differences were found in ∆Fatigue score (Borg) and ∆Dyspnea score (Borg) between the two PR groups and the Control Group, while the ∆6-MWD (36.67±48.57 vs. 4.00±27.30 m, P=0.002) and ∆PEF (30.00±33.11 vs. −0.32±37.18 L/min, P=0.001) in the Combined PR Group were improved significantly compared to Control Group, and the ∆PEF for the Combined PR Group was significantly higher compared to the Single IMT Group (30.00±33.11 vs. 15.70±42.78 m, P=0.004). No difference in ∆PEF (P=0.127) and ∆6-MWD (P=0.740) was found between the Single IMT Group and Control Group.
QoL scores
The QoL scores were self-evaluated according to physical and psychological status. A significant difference (P=0.035) in the overall scores for ∆Global QoL was seen between the Combined PR Group and Control Group, while no statistically significant difference among the groups was seen for physical/emotional function and dyspnea score, although there were subjective improvements in treatment effects (Table 5).
Table 5. Between-group differences between before and after intervention for CRT, IMT and QoL.
| Outcome variables | Combined PR group (n=30) | Single IMT group (n=30) | Control group (n=30) | P1 | P2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |||||
| CRT & IMT | ||||||||||
| 6-MWD (m) | 477.2±102.8 | 513.8±98.0 | 470.3±89.0 | 476.5±86.5 | 496.8±86.0 | 500.8±82.3 | 0.002 | 0.740 | ||
| Fatigue score | 1.5±1.1 | 1.4±0.6 | 1.5±1.3 | 1.4±1.3 | 1.4±1.1 | 1.5±0.8 | 0.485 | 0.389 | ||
| Dyspnea score | 1.1±1.2 | 0.8±1.0 | 1.1±1.5 | 1.1±1.3 | 1.1±0.9 | 1.2±0.7 | 0.110 | 0.470 | ||
| PEF (L/min) | 390.3±115.8 | 420.3±113.2 | 386.0±95.2 | 401.7±85.9 | 383.0±105.7 | 382.7±106.3 | 0.001 | 0.127 | ||
| FEV1 (L) | 2.2±0.6 | 2.3±0.6 | 2.3±0.8 | 2.3±0.8 | 2.1±0.5 | 2.2±0.7 | 0.790 | 0.522 | ||
| FVC (L) | 3.2±0.7 | 3.2±0.7 | 3.2±0.8 | 3.3±0.9 | 3.0±0.6 | 3.1±0.7 | 0.373 | 0.414 | ||
| DlCO, mL/min/mmHg | 22.5±4.1 | 22.8±4.8 | 21.6±4.9 | 21.8±4.8 | 22.0±4.1 | 22.5±4.7 | 0.265 | 0.463 | ||
| QoL evaluation | ||||||||||
| Global QoL* | 71.9±13.8 | 74.2±12.1 | 68.1±14.0 | 70.0±13.9 | 68.9±11.8 | 67.5±11.9 | 0.035 | 0.144 | ||
| Physical function* | 88.9±6.4 | 90.0±6.3 | 89.1±7.7 | 89.3±7.9 | 88.0±6.4 | 87.6±6.9 | 0.229 | 0.571 | ||
| Emotional function* | 85.0±9.6 | 90.0±7.7 | 85.8±9.1 | 87.2±8.7 | 83.9±11.6 | 87.8±9.0 | 0.590 | 0.816 | ||
| Dyspnea score† | 15.6± 19.0 | 8.9±15.0 | 14.4±18.9 | 10.0±17.8 | 8.9±15.0 | 13.3±16.6 | 0.840 | 0.186 | ||
*, higher scores indicate better functioning (scaled from 0–100); †, lower scores indicate less dyspnea (scaled from 0–100). P1: P value of ∆after-before in combined PR group vs. control group; P2: P value of ∆after-before in single IMT group vs. control group. CRT, conventional resistance training; IMT, inspiratory muscle training; 6-MWD, 6-min walk distance; PEF, peak expiratory flow; FEV1, forced expiratory volume in one second; DLCO, diffusion capacity for carbon monoxide of the lung; QoL, quality of life.
Discussion
This exploratory experiment showed that a one-week high-intensity PR program had a better effect on the PPC rate compared with a conventional IMT PR program or in-hospital routine care and this modified program may thus be a feasible approach to treat LC patients who have potential PPC risk factors, and moreover, serve as a guide or reference for further research regarding the PR of LC patients.
According to our results, 90% of participants in the Combined PR Group and 91.7% in the IMT Group completed the seven-day rehabilitation period, and among the 3 in the Combined PR Group who did not complete the regimen, 1 patients had acute COPD exacerbation and 2 had new worsening of knee pain, while 2 in the IMT Group dropped out due to loss of motivation. These completion rates suggest that compliance with the programs is likely to be high and also supports the feasibility of the combined PR pattern.
The main intervention measurements we used in this study were PR training (IMT or a combination of IMT and CRT). Despite an increasing number of studies confirming that PR is an effective treatment for improving exercise tolerance, reducing dyspnea, and improving QoL (6,10-13), there remains a lack of a standard procedure or practical guidance for LC patients, and few studies have explored the effect of a combined short-term and high-intensity rehabilitation program for preoperative LC patients. These reasons provide the strongest evidence to support the use of IMT and CRT as a combined intervention for PR, and we hypothesize that the combined high intensity program would have a better effect in terms of reducing PPCs than traditional regimens with IMT alone. An IMT program (7 times a week, 20 minutes per session, for at least 2 weeks) is the most-widely used method that can be performed in the patient’s room or at home, during busy or idle time. IMT positively enhances respiratory muscle strength, which reflects the relative load for breathing, coughing, and huffing (14,15). Moreover, Sutton et al. showed that improved forced expiration maneuvers could be more effective than coughing for improving clearance of bronchoalveolar hyper-secretions (16). Hulzebos et al. also reported that preoperative IMT could reduce the PPC incidence and postoperative length of stay (17), as well as the postoperative QoL (18). Meanwhile, CRT requires that the patient take an active role in improving peak exercise tolerance (19), and strengthening quadriceps after CRT has been completed (20). As the evaluation index for aerobic endurance exercise, 6-MWD shows a close correlation to peak oxygen consumption (peak VO2), and are an excellent predictor of reduced peak VO2 (21). Furthermore, as was shown in a recent study, CRT as a treatment goal can impact disease severity and provide clinically relevant exercise-tolerance in pediatric pulmonary arterial hypertension (22), as well as help to preserve ejection fraction in heart failure patients (23). Licker et al. conducted a randomized trial and revealed that preoperative high intensity interval training resulted in significant improvement in aerobic performances but failed to reduce early complications after LC resection (24).
We set 7 days as preoperative PR duration, which would improve the efficiency of PR and also favorably balance patient compliance contraindications as well as patient economic support. Several studies investigated the correlation between rehabilitation efficiency and PR duration, with one showing that one day of preoperative IMT could significantly decrease postoperative atelectasis (25). Moreover, Hulzebos et al. and Benzo et al. recommend two and four week durations, respectively (6,17). Meanwhile, Sekine et al. showed that a 3 to 15 day rehabilitation period could significantly improve respiratory muscle strength and recovery of pulmonary function (5,26). Unfortunately, these suggested longer program durations are often not appropriate for Chinese patients, especially when the program duration exceeds 2 weeks. The reasons for the difficulties may lie in deficiencies in community health care and public health consciousness, particularly in underserved rural areas. Although patients in developed countries with more sophisticated health care delivery systems can receive preoperative PR training at home or at a personal clinic, China lacks relevant support facilities, and also has a shortage of hospital beds and patient economic capacity. For these reasons, LC patients in China may avoid delaying surgical treatment, and have a strong incentive to undergo tumor removal as quickly as possible. Here we investigated the appropriate preoperative waiting time, and found that LC patients usually spent 5–7 days finishing the necessary preoperative examinations in our crowded medical center, and thus a one-week rehabilitation period could be undertaken without delaying surgery. However, programs with more than one-week duration could significantly reduce patient compliance.
PPCs were the major evaluation index to assess the effect of one particular treatment, and were also used as the primary endpoint in this study. A recent meta-analysis showed that preoperative exercise-based PR could reduce the PPC incidence 12), which was thought to be a mixed outcome based on physical and psychological evidence. We used the Clavien-Dindo complication classification system to classify the PPCs into five grades, and the PPCs were ultimately defined as Clavien-Dindo grade II to grade V. When analyzing PPCs classified by grade I–V, there were significant differences in grade II–V PPCs between the combined PR group and control group (P=0.045).
In terms of QoL, interestingly, the EORTC QLQ-C30 scores were significantly improved in the PR groups, likely because of the education these patients received, and the global QoL scores were significantly higher for the Combined PR Group compared with the control group. Moreover, emotional function scores, which represent levels of preoperative anxiety and depression, were similar between the three groups. This outcome was consistent with previous studies that showed little effect of preoperative PR on emotional function scores (20,27). Nonetheless, anxiety and depression could actually be alleviated through the care support and education that patients receive in-hospital as they await surgery. With appropriate educational guidance, intensive physical intervention could also improve muscle strength and exercise tolerance, as was previously reported (20). Meanwhile, the increased intensity of the combined PR program could enhance self-confidence in the face of surgical stress, while patients in both PR groups who gained more knowledge about perioperative activities were more cooperative, which could be helpful during postoperative recovery.
The generalizability of our findings may be restricted because of a few limitations in the study. First, all study participants were enrolled from a single regional medical center in western China, such that the study design may have center-specific bias. Second, although the HR-QoL scores were significantly improved in all groups, there was no significant difference between the groups. As such, a more detailed assessment system may be needed to define the differences in further studies. Moreover, in our study, the incentive spirometer Voldyne was used, as it is popular used in China so far, however, the device is unfortunately not the best way to improve respiratory muscle performances (28). Meanwhile, we intended to increase the exercise duration combining IMT and CRT during the 7 days to achieve the effect of “high-intensity”, as in a relative short it is hardly to incrementally increase the workload. In the future study, the below PR model would be investigated in depth. To better evaluate program effectiveness in terms of improving/enhancing cardiopulmonary endurance, some instruments, for example, the cardiopulmonary exercise test (CPET), should be used in future studies.
In conclusion, compared with IMT program, by applying exercise regimens and increasing physical activity in LC patients with risk factors of PPCs, the combined program could better improve the exercise capacity, inspiratory muscle strength and QoL, which additionally contributed to alleviate the PPCs severity. This hospital-based short-term pattern of PR combining high-intensity IMT with CRT could may be a feasible strategy for treating LC patients, especially those with risk factors of PPCs awaiting surgeries. Furthermore, it provided a reference to encourage further research on LC patients of all stages.
Acknowledgements
The authors thank all the members of the Department of Rehabilitation Medicine for their generous technical assistance and guidance, and the nurse team led by Prof. Mei Yang for their nursing care support and health education on perioperative activities. We also thank all the study participants for their kind participation and cooperation. No person involved in this study received compensation for their contribution.
Funding: This study was funded by two project grants (No. 2014SZ0148 and No. 2015SZ0158) from the Foundation of Science and Technology support plan, Department of Sichuan Province, China.
Ethical Statement: This prospective three-armed randomized controlled trial was adhered to the Declaration of Helsinki, and approved by the Clinical Trials and Biomedical Ethics Committee of Sichuan University West University Hospital and Chinese Ethics Committee of Registering Clinical Trials (ChiCTR-IOR-16008109). All enrolled patients signed informed consent and patient information was obtained.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Mao Y, Yang D, He J, et al. Epidemiology of Lung Cancer. Surg Oncol Clin N Am 2016;25:439-45. [DOI] [PubMed] [Google Scholar]
- 2.Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [DOI] [PubMed] [Google Scholar]
- 3.Kim AW, Detterbeck FC, Boffa DJ, et al. Characteristics associated with the use of nonanatomic resections among Medicare patients undergoing resections of early-stage lung cancer. Ann Thorac Surg 2012;94:895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonsen DF, Sogaard M, Bozi I, et al. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med 2015;109:1340-6. [DOI] [PubMed] [Google Scholar]
- 5.Sekine Y, Chiyo M, Iwata T, et al. Perioperative rehabilitation and physiotherapy for lung cancer patients with chronic obstructive pulmonary disease. Jpn J Thorac Cardiovasc Surg 2005;53:237-43. [DOI] [PubMed] [Google Scholar]
- 6.Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer 2011;74:441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez FG, Kosinski AS, Burfeind W, et al. The Society of Thoracic Surgeons Lung Cancer Resection Risk Model: Higher Quality Data and Superior Outcomes. Ann Thorac Surg 2016;102:370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Lai Y, Gao K, et al. Surfactant Protein-D: A sensitive predictor for efficiency of preoperative pulmonary rehabilitation. Int J Surg 2017;41:136-42. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crandall K, Maguire R, Campbell A, et al. Exercise intervention for patients surgically treated for Non-Small Cell Lung Cancer (NSCLC): a systematic review. Surg Oncol 2014;23:17-30. [DOI] [PubMed] [Google Scholar]
- 11.Yamana I, Takeno S, Hashimoto T, et al. Randomized Controlled Study to Evaluate the Efficacy of a Preoperative Respiratory Rehabilitation Program to Prevent Postoperative Pulmonary Complications after Esophagectomy. Dig Surg 2015;32:331-7. [DOI] [PubMed] [Google Scholar]
- 12.Stefanelli F, Meoli I, Cobuccio R, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg 2013;44:e260-5. [DOI] [PubMed] [Google Scholar]
- 13.Sebio Garcia R, Yáñez Brage MI, Giménez Moolhuyzen E, et al. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2016;23:486-97. [DOI] [PubMed] [Google Scholar]
- 14.Refai M, Pompili C, Salati M, et al. Can maximal inspiratory and expiratory pressures during exercise predict complications in patients submitted to major lung resections? A prospective cohort study. Eur J Cardiothorac Surg 2014;45:665-69; discussion 9-70. [DOI] [PubMed] [Google Scholar]
- 15.Ishida H, Kobara K, Osaka H, et al. Correlation between Peak Expiratory Flow and Abdominal Muscle Thickness. J Phys Ther Sci 2014;26:1791-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton PP, Parker RA, Webber BA, et al. Assessment of the forced expiration technique, postural drainage and directed coughing in chest physiotherapy. Eur J Respir Dis 1983;64:62-8. [PubMed] [Google Scholar]
- 17.Hulzebos EH, Helders PJ, Favie NJ, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. Jama 2006;296:1851-7. [DOI] [PubMed] [Google Scholar]
- 18.Bissett BM, Leditschke IA, Neeman T, et al. Inspiratory muscle training to enhance recovery from mechanical ventilation: a randomised trial. Thorax 2016;71:812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cade WT, Reeds DN, Peterson LR, et al. Endurance Exercise Training in Young Adults with Barth Syndrome: A Pilot Study. JIMD Rep 2017;32:15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbane G, Tropman D, Jackson D, et al. Evaluation of an early exercise intervention after thoracotomy for non-small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: randomised controlled trial. Lung Cancer 2011;71:229-34. [DOI] [PubMed] [Google Scholar]
- 21.Kehmeier ES, Sommer MH, Galonska A, et al. Diagnostic value of the six-minute walk test (6MWT) in grown-up congenital heart disease (GUCH): Comparison with clinical status and functional exercise capacity. Int J Cardiol 2016;203:90-7. [DOI] [PubMed] [Google Scholar]
- 22.Douwes JM, Hegeman AK, van der Krieke MB, et al. Six-minute walking distance and decrease in oxygen saturation during the six-minute walk test in pediatric pulmonary arterial hypertension. Int J Cardiol 2016;202:34-9. [DOI] [PubMed] [Google Scholar]
- 23.Zotter-Tufaro C, Mascherbauer J, Duca F, et al. Prognostic Significance and Determinants of the 6-Min Walk Test in Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail 2015;3:459-66. [DOI] [PubMed] [Google Scholar]
- 24.Licker M, Karenovics W, Diaper J, et al. Short-Term Preoperative High-Intensity Interval Training in Patients Awaiting Lung Cancer Surgery: A Randomized Controlled Trial. J Thorac Oncol 2017;12:323-33. [DOI] [PubMed] [Google Scholar]
- 25.Yánez-Brage I, Pita-Fernández S, Juffé-Stein A, et al. Respiratory physiotherapy and incidence of pulmonary complications in off-pump coronary artery bypass graft surgery: an observational follow-up study. BMC Pulm Med 2009;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiono S, Abiko M, Sato T. Postoperative complications in elderly patients after lung cancer surgery. Interact Cardiovasc Thorac Surg 2013;16:819-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stigt JA, Uil SM, van Riesen SJ, et al. A randomized controlled trial of postthoracotomy pulmonary rehabilitation in patients with resectable lung cancer. J Thorac Oncol 2013;8:214-21. [DOI] [PubMed] [Google Scholar]
- 28.Paiva DN, Assmann LB, Bordin DF, et al. Inspiratory muscle training with threshold or incentive spirometry: Which is the most effective? Rev Port Pneumol (2006) 2015;21:76-81. [DOI] [PubMed] [Google Scholar]