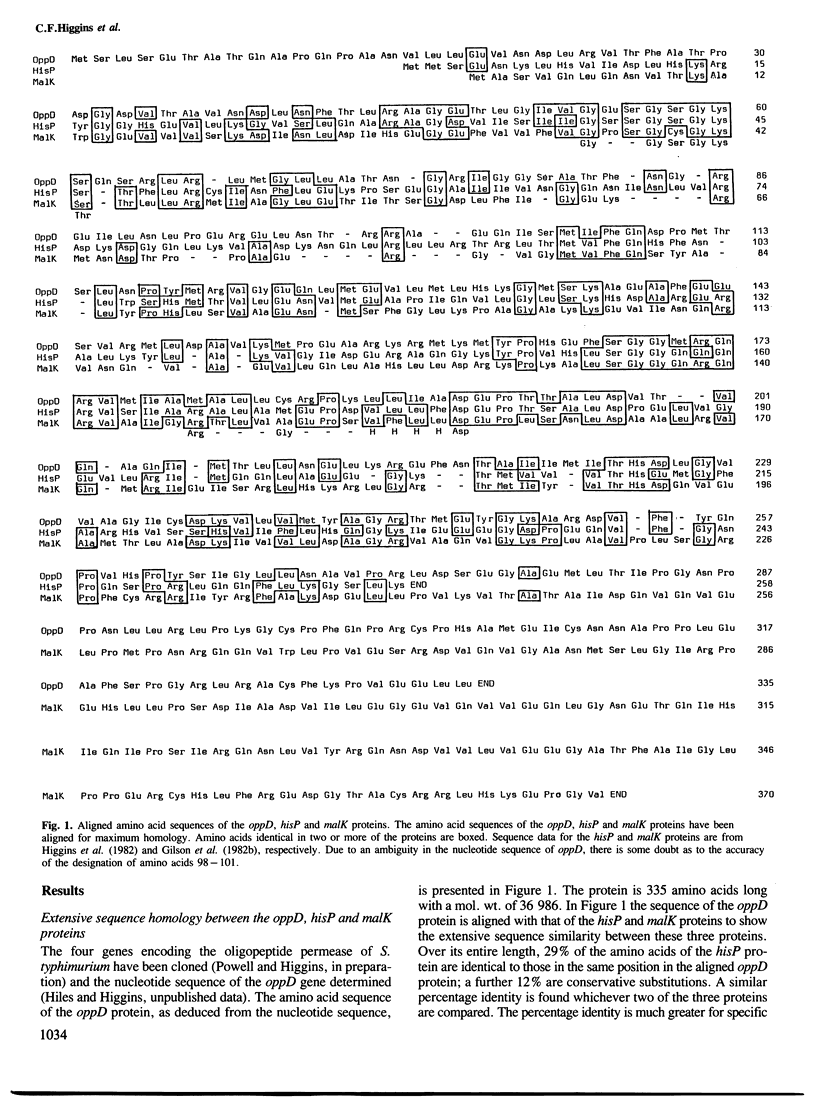
Abstract
Bacterial periplasmic binding protein-dependent transport systems require the function of a specific substrate-binding protein, located in the periplasm, and several membrane-bound components. We present evidence for a nucleotide-binding site on one of the membrane components from each of three independent transport systems, the hisP, malK and oppD proteins of the histidine, maltose and oligopeptide permeases, respectively. The amino acid sequence of the oppD protein has been determined and this protein is shown to share extensive homology with the hisP and malK proteins. Three lines of evidence lead us to propose the existence of a nucleotide-binding site on each of these proteins. A consensus nucleotide-binding sequence can be identified in the same relative position in each of the three proteins. The oppD protein binds to a Cibacron Blue affinity column and can be eluted by ATP but not by CTP or NADH. The oppD protein is labelled specifically by the nucleotide affinity analogue 5'-p-fluorosulphonylbenzoyladenosine. The identification of a nucleotide-binding site provides strong evidence that transport by periplasmic binding protein-dependent systems is energized directly by the hydrolysis of ATP or a closely related nucleotide. The hisP, malK and oppD proteins are thus responsible for energy-coupling to their respective transport systems.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Amzel L. M., Pedersen P. L. Proton atpases: structure and mechanism. Annu Rev Biochem. 1983;52:801–824. doi: 10.1146/annurev.bi.52.070183.004101. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Casadaban M. J., Chou J. In vivo formation of gene fusions encoding hybrid beta-galactosidase proteins in one step with a transposable Mu-lac transducing phage. Proc Natl Acad Sci U S A. 1984 Jan;81(2):535–539. doi: 10.1073/pnas.81.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R. F. Affinity labeling of purine nucleotide sites in proteins. Annu Rev Biochem. 1983;52:67–91. doi: 10.1146/annurev.bi.52.070183.000435. [DOI] [PubMed] [Google Scholar]
- Daruwalla K. R., Paxton A. T., Henderson P. J. Energization of the transport systems for arabinose and comparison with galactose transport in Escherichia coli. Biochem J. 1981 Dec 15;200(3):611–627. doi: 10.1042/bj2000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Boos W., Schwartz M., Szmelcman S. Energy-coupling of the transport system of Escherichia coli dependent on maltose-binding protein. Eur J Biochem. 1977 May 2;75(1):187–193. doi: 10.1111/j.1432-1033.1977.tb11516.x. [DOI] [PubMed] [Google Scholar]
- Ferro-Luzzi Ames G., Nikaido K. Phosphate-containing proteins of Salmonella typhimurium and Escherichia coli. Analysis by a new two-dimensional gel system. Eur J Biochem. 1981 Apr;115(3):525–531. [PubMed] [Google Scholar]
- Froshauer S., Beckwith J. The nucleotide sequence of the gene for malF protein, an inner membrane component of the maltose transport system of Escherichia coli. Repeated DNA sequences are found in the malE-malF intercistronic region. J Biol Chem. 1984 Sep 10;259(17):10896–10903. [PubMed] [Google Scholar]
- Gilson E., Higgins C. F., Hofnung M., Ferro-Luzzi Ames G., Nikaido H. Extensive homology between membrane-associated components of histidine and maltose transport systems of Salmonella typhimurium and Escherichia coli. J Biol Chem. 1982 Sep 10;257(17):9915–9918. [PubMed] [Google Scholar]
- Gilson E., Nikaido H., Hofnung M. Sequence of the malK gene in E.coli K12. Nucleic Acids Res. 1982 Nov 25;10(22):7449–7458. doi: 10.1093/nar/10.22.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., Giddens R. A., Jones-Mortimer M. C. Transport of galactose, glucose and their molecular analogues by Escherichia coli K12. Biochem J. 1977 Feb 15;162(2):309–320. doi: 10.1042/bj1620309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Haag P. D., Nikaido K., Ardeshir F., Garcia G., Ames G. F. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature. 1982 Aug 19;298(5876):723–727. doi: 10.1038/298723a0. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M., Jamieson D., Powell L. M. Genetic map of the opp (Oligopeptide permease) locus of Salmonella typhimurium. J Bacteriol. 1983 Feb;153(2):830–836. doi: 10.1128/jb.153.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M. Periplasmic protein associated with the oligopeptide permeases of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Sep;155(3):1434–1438. doi: 10.1128/jb.155.3.1434-1438.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson A. C., Weatherwax R., Ames G. F. ATP-binding sites in the membrane components of histidine permease, a periplasmic transport system. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7333–7337. doi: 10.1073/pnas.81.23.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth B. G., Higgins C. F. Genetic organization of the oligopeptide permease (opp) locus of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Mar;153(3):1548–1551. doi: 10.1128/jb.153.3.1548-1551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Hunt A. G., Masters P. S., Lieberman M. A. Requirements of acetyl phosphate for the binding protein-dependent transport systems in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1213–1217. doi: 10.1073/pnas.76.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt A. G., Hong J. Properties and characterization of binding protein dependent active transport of glutamine in isolated membrane vesicles of Escherichia coli. Biochemistry. 1983 Feb 15;22(4):844–850. doi: 10.1021/bi00273a021. [DOI] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J. Molecular considerations relevant to the mechanism of active transport. Nature. 1981 Jul 16;292(5820):201–204. doi: 10.1038/292201a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lieberman M. A., Hong J. S. Energization of osmotic shock-sensitive transport systems in Escherichia coli requires more than ATP. Arch Biochem Biophys. 1976 Jan;172(1):312–315. doi: 10.1016/0003-9861(76)90080-1. [DOI] [PubMed] [Google Scholar]
- Plate C. A. Requirement for membrane potential in active transport of glutamine by Escherichia coli. J Bacteriol. 1979 Jan;137(1):221–225. doi: 10.1128/jb.137.1.221-225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A., Suit J. L., Jetten A. M., Luria S. E. Effects of colicin K on a mutant of Escherichia coli deficient in Ca 2+, Mg 2+-activated adenosine triphosphatase. J Biol Chem. 1974 Oct 10;249(19):6138–6143. [PubMed] [Google Scholar]
- Shuman H. A. Active transport of maltose in Escherichia coli K12. Role of the periplasmic maltose-binding protein and evidence for a substrate recognition site in the cytoplasmic membrane. J Biol Chem. 1982 May 25;257(10):5455–5461. [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J. Identification of the malK gene product. A peripheral membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1981 Jan 25;256(2):560–562. [PubMed] [Google Scholar]
- Shuman H. A. The maltose-maltodextrin transport system of Escherichia coli. Ann Microbiol (Paris) 1982 Jan;133A(1):153–159. [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. The action of tributyltin chloride on the uptake of proline and glutamine by intact cells of Escherichia coli. Can J Biochem. 1979 Dec;57(12):1376–1383. doi: 10.1139/o79-183. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. T., Cass K. H., Stellwagen E. Blue dextran-sepharose: an affinity column for the dinucleotide fold in proteins. Proc Natl Acad Sci U S A. 1975 Feb;72(2):669–672. doi: 10.1073/pnas.72.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]