Abstract
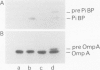
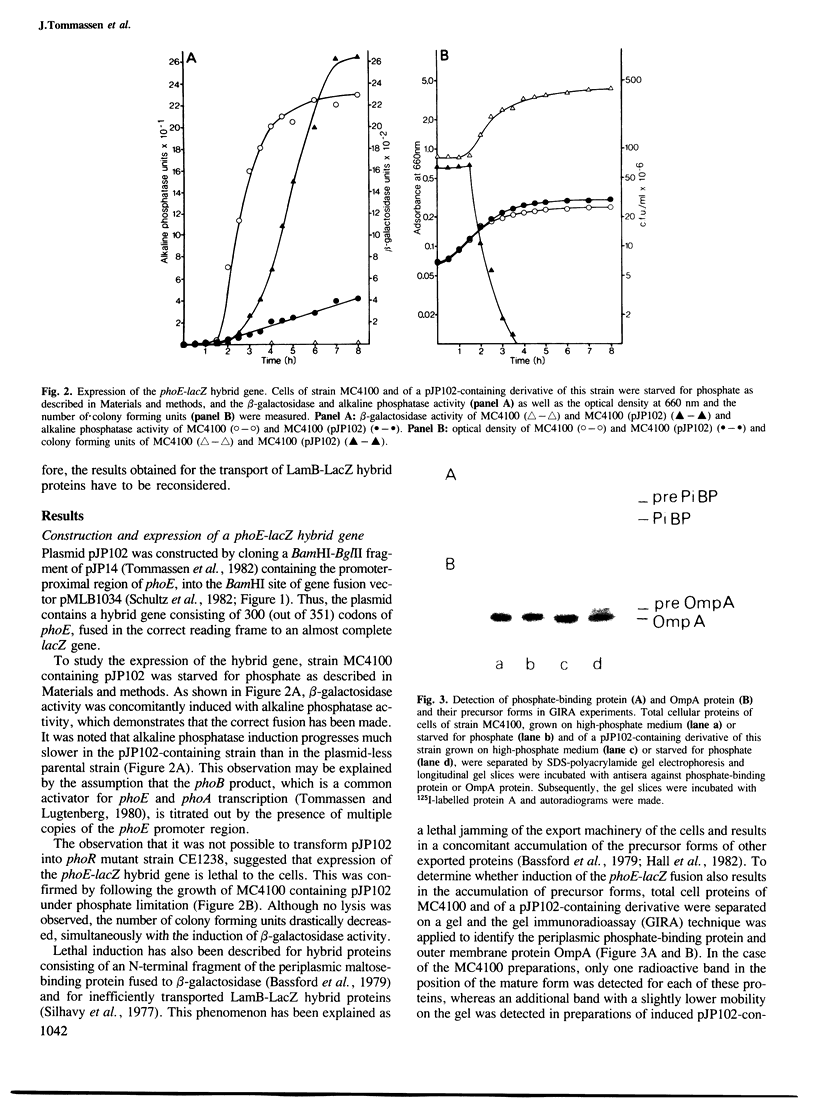
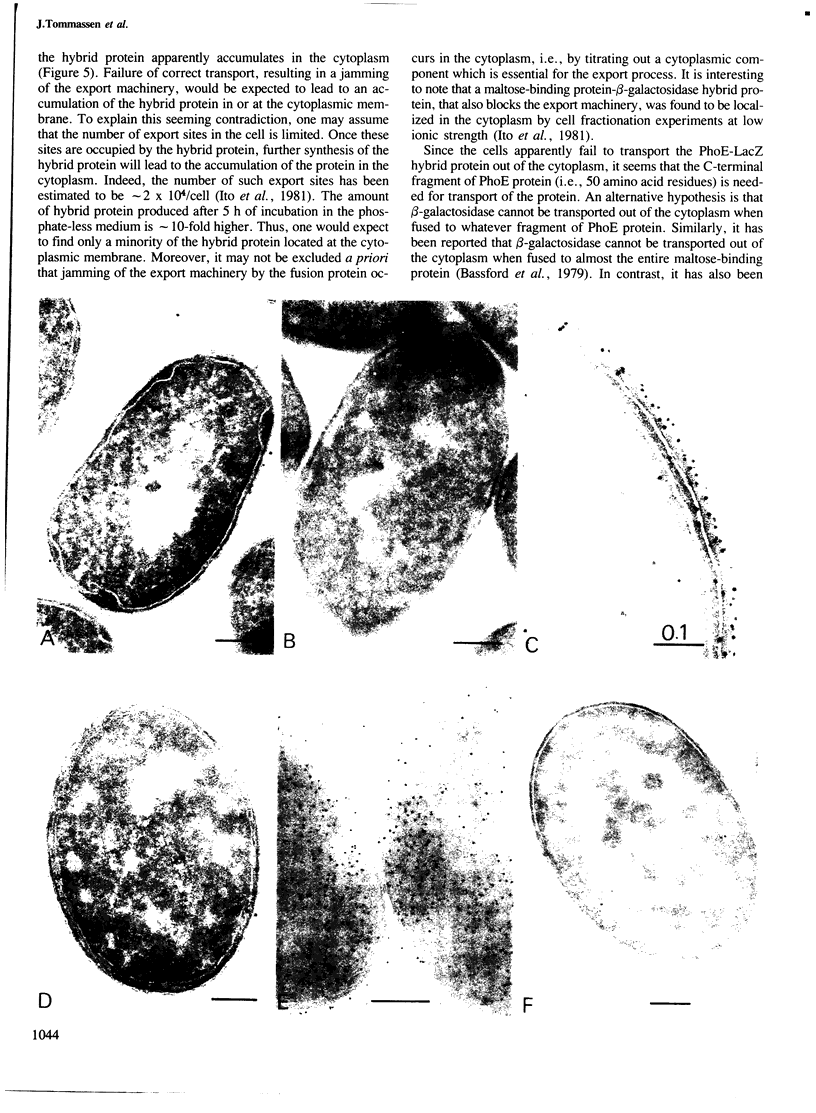
A phoE-lacZ hybrid gene encoding the N-terminal 300 amino acid residues of pre-PhoE protein, fused to an almost complete beta-galactosidase molecule was constructed in vitro. Cell fractionation experiments suggested that the hybrid gene product is transported to the outer membrane. However, by using immuno-cytochemical labelling on ultra-thin cryosections it was shown that the hybrid protein accumulated in the cytoplasm. Thus, it appears that: (i) data on the localization of hybrid proteins merely based on cell fractionation experiments are not reliable, and (ii) either the C-terminal 15% of PhoE protein contain information which is essential for transport, or PhoE-LacZ hybrid proteins can never be transported out of the cytoplasm. The implications of these results for current models on the translocation of outer membrane proteins are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedouelle H., Bassford P. J., Jr, Fowler A. V., Zabin I., Beckwith J., Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980 May 8;285(5760):78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hedgpeth J., Clément J. M., Silhavy T. J., Hofnung M. Sequence analysis of mutations that prevent export of lambda receptor, an Escherichia coli outer membrane protein. Nature. 1980 May 8;285(5760):82–85. doi: 10.1038/285082a0. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Schwartz M., Silhavy T. J. Sequence information within the lamB genes in required for proper routing of the bacteriophage lambda receptor protein to the outer membrane of Escherichia coli K-12. J Mol Biol. 1982 Mar 25;156(1):93–112. doi: 10.1016/0022-2836(82)90461-2. [DOI] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Korteland J., Tommassen J., Lugtenberg B. PhoE protein pore of the outer membrane of Escherichia coli K12 is a particularly efficient channel for organic and inorganic phosphate. Biochim Biophys Acta. 1982 Sep 9;690(2):282–289. doi: 10.1016/0005-2736(82)90332-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., van Boxtel R., Verhoef C., van Alphen W. Pore protein e of the outer membrane of Escherichia coli K12. FEBS Lett. 1978 Dec 1;96(1):99–105. doi: 10.1016/0014-5793(78)81071-0. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Moreno F., Fowler A. V., Hall M., Silhavy T. J., Zabin I., Schwartz M. A signal sequence is not sufficient to lead beta-galactosidase out of the cytoplasm. Nature. 1980 Jul 24;286(5771):356–359. doi: 10.1038/286356a0. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Overbeeke N., Bergmans H., van Mansfeld F., Lugtenberg B. Complete nucleotide sequence of phoE, the structural gene for the phosphate limitation inducible outer membrane pore protein of Escherichia coli K12. J Mol Biol. 1983 Feb 5;163(4):513–532. doi: 10.1016/0022-2836(83)90110-9. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Expression of outer membrane protein e of Escherichia coli K12 by phosphate limitation. FEBS Lett. 1980 Apr 7;112(2):229–232. doi: 10.1016/0014-5793(80)80186-4. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Van Scharrenburg G., Lugtenberg B. Antigenic relationships between pore proteins of Escherichia coli K12. Eur J Biochem. 1980 Sep;110(1):247–254. doi: 10.1111/j.1432-1033.1980.tb04862.x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Sur l'existence chez Escherichia coli K 12 d'une régulation commune à la biosynthèse des récepteurs du bactériophage et au métabolisme du maltose. Ann Inst Pasteur (Paris) 1967 Nov;113(5):685–704. [PubMed] [Google Scholar]
- Shultz J., Silhavy T. J., Berman M. L., Fiil N., Emr S. D. A previously unidentified gene in the spc operon of Escherichia coli K12 specifies a component of the protein export machinery. Cell. 1982 Nov;31(1):227–235. doi: 10.1016/0092-8674(82)90422-6. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Shuman H. A., Beckwith J., Schwartz M. Use of gene fusions to study outer membrane protein localization in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5411–5415. doi: 10.1073/pnas.74.12.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. A study of positive staining of ultrathin frozen sections. J Ultrastruct Res. 1978 Jun;63(3):287–307. doi: 10.1016/s0022-5320(78)80053-7. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980 Jul;143(1):151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Overduin P., Lugtenberg B., Bergmans H. Cloning of phoE, the structural gene for the Escherichia coli phosphate limitation-inducible outer membrane pore protein. J Bacteriol. 1982 Feb;149(2):668–672. doi: 10.1128/jb.149.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., van Tol H., Lugtenberg B. The ultimate localization of an outer membrane protein of Escherichia coli K-12 is not determined by the signal sequence. EMBO J. 1983;2(8):1275–1279. doi: 10.1002/j.1460-2075.1983.tb01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarejo M., Davis J. L., Granett S. Osmoregulation of alkaline phosphatase synthesis in Escherichia coli K-12. J Bacteriol. 1983 Nov;156(2):975–978. doi: 10.1128/jb.156.2.975-978.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]
- van den Hondel C. A., Keegstra W., Borrias W. E., van Arkel G. A. Homology of plasmids in strains of unicellular Cyanobacteria. Plasmid. 1979 Jul;2(3):323–333. doi: 10.1016/0147-619x(79)90016-7. [DOI] [PubMed] [Google Scholar]
- van der Ley P., Amesz H., Tommassen J., Lugtenberg B. Monoclonal antibodies directed against the cell-surface-exposed part of PhoE pore protein of the Escherichia coli K-12 outer membrane. Eur J Biochem. 1985 Mar 1;147(2):401–407. doi: 10.1111/j.1432-1033.1985.tb08764.x. [DOI] [PubMed] [Google Scholar]