Abstract
The new drugs delamanid and bedaquiline are increasingly used to treat multidrug-resistant (MDR-) and extensively drug-resistant tuberculosis (XDR-TB). As evidence is lacking, the World Health Organization recommends their use under specific conditions in adults, delamanid only being recommended in children ≥6 years of age. No systematic review has yet evaluated the efficacy, safety and tolerability of the new drugs in children. A search of peer-reviewed, scientific evidence was performed, to evaluate the efficacy/effectiveness, safety, and tolerability of delamanid or bedaquiline-containing regimens in children with confirmed M/XDR-TB. We used PubMed and Embase to identify any relevant manuscripts in English until 31 December 2016, excluding editorials and reviews. Three out of 96 manuscripts retrieved satisfied the inclusion criteria, while 93 were excluded because dealing exclusively with adults (12: 4 on delamanid and 8 on bedaquiline), being recommendations or guidelines (8 manuscripts), reviews (17 papers) or other studies (56 papers). One of the studies retrieved reported evidence on 19 M/XDR-TB children, 16 of them treated under compassionate use with delamanid (13 achieving consistent bacteriological conversion) and 3 candidates for the drug. Two studies reported details on the first paediatric case treated (and cured) with a delamanid-containing regimen. Eight trials including children were also retrieved (clinicaltrials.gov). Although the methodology used in the study was rigorous, the results are limited by the paucity of the studies available in the literature on the use of new anti-TB drugs in children. In conclusion, more evidence is needed on the use of delamanid and bedaquiline in paediatric patients.
Keywords: Multidrug-resistant tuberculosis (MDR-TB), extensively drug-resistant tuberculosis (XDR-TB), delamanid, bedaquiline, children
Introduction
With 10.4 million cases of tuberculosis (TB) estimated by the World Health Organization (WHO) to have occurred in 2015 [480,000 of whom affected by multidrug-resistant tuberculosis (MDR-TB)—10% meeting the criteria for extensively drug-resistant tuberculosis (XDR-TB) and 100,000 by rifampicin-resistant TB] and 190,000 deaths, the disease is a public health priority (1-4).
Treating MDR-/XDR- is extremely difficult, as the treatment is long and expensive, and outcomes remain sub-optimal, because of frequently observed adverse events, and high rates of treatment failure (3-10).
The WHO has recently issued new recommendations on how to design MDR-TB regimens, moving from the previous stepwise approach based on five groups of drugs in priority order (9,11) to a new approach and a new drugs classification (7,12,13).
The new drugs delamanid and bedaquiline are presently classified in group D2 (7), and their use is recommended in adults for a maximum of six months, at the recommended doses, added to an OBR (optimized background regimen), under an adequate clinical monitoring (particularly for the QT interval, e.g., measure of the time between the start of the Q wave and the end of the T wave in the heart’s electrical cycle), and in the presence of pharmacovigilance and informed consent (14-16). Recently, the WHO has approved the use of delamanid for children above 6 years of age, given PK (pharmacokinetic) data having been made available (16,17).
Evidence in adults shows that delamanid and bedaquiline increase sputum smear and culture conversions, as well as success rates at the end of treatment, although much needs to be known on the potential drug-drug interactions-related cardio-toxicity. In fact, both delamanid and bedaquiline, as well as other core second-line drugs such as fluoroquinolones and clofazimine may increase the QT interval (18-26).
In adults with M/XDR-TB, as well as in children, it is often difficult to identify at least four active drugs which are necessary to design an effective regimen (2,7,10,13,27). While today some repurposed drugs are useful in designing these regimens [e.g., linezolid (28-34) and carbapenems (35-40)], there is urgent need to know how better to use the new drugs delamanid and bedaquiline (41-43). In children the problem is even more pressing, as less information is available and recruitment of paediatric cases in clinical trials is usually more challenging (17).
As of today, the information available on the use of new drugs in children is scanty.
Aim of the present manuscript is to perform a systematic review on the use of delamanid and bedaquiline in children.
Methods
A search of peer-reviewed, scientific evidence was performed, to evaluate the efficacy/effectiveness, safety, and tolerability of delamanid or bedaquiline-containing regimens in children with M/XDR-TB.
MDR-TB was defined as resistance to at least isoniazid and rifampicin, and XDR-TB as MDR with additional resistance to a fluoroquinolone plus at least one of the three second-line injectable drugs (amikacin, capreomycin and kanamycin).
We used PubMed and Embase to identify any relevant manuscripts published until December 31st, 2016, excluding editorials and reviews.
We decided to exclude conferences’ abstracts because, on the basis of the word count, the information provided is too limited when assessing the above described objectives, whilst including letters or case-series/report in case detailed clinical information. Only papers written in English were considered.
The key-words “tuberculosis and (delamanid or bedaquiline) and (child or adolescent or young)’’ were used.
The search exclusion criteria were the following: (I) experimental studies on animals with TB; (II) reviews and editorials on delamanid and bedaquiline; (III) M/XDR-TB cases not bacteriologically confirmed.
Two authors independently performed the search and evaluated the titles and abstracts of the records according to the selection criteria. Potentially interesting articles were downloaded and critically assessed; when they fulfilled the enrolment criteria, the needed information (e.g., sputum smear and culture conversion, treatment outcomes, adverse events and their grading, demographics, epidemiological data, drug resistance patterns of the collected Mycobacterium tuberculosis isolates, drug regimen prescribed and its duration) was retrieved and collected using a pre-designed electronic template. The entire process was carried out following the guidelines of the 2009 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement (44).
In addition, clinical trials involving children were looked for in the clinicaltrials.gov website using the following key words: ‘TB and (bedaquiline or delamanid) and child’ as of January1st, 2017 (45).
Results
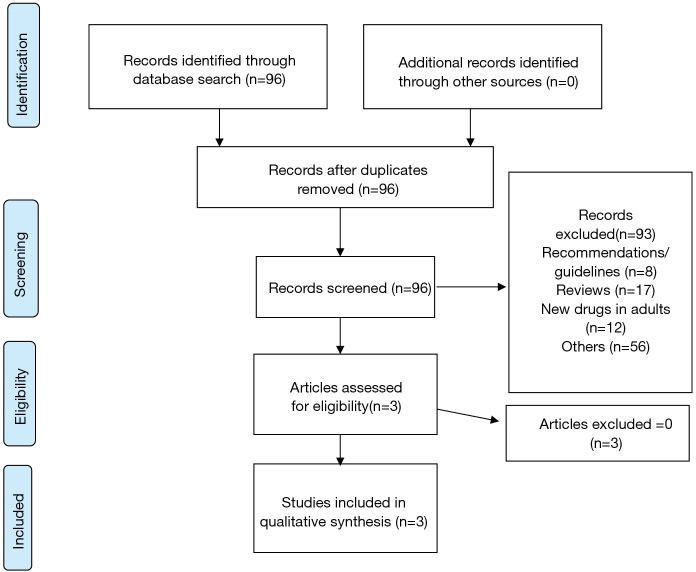
A total of 96 records were obtained from the search (PRISMA flowchart, Figure 1); 93 were excluded because dealing with the new drugs in adults (12: 4 on delamanid and 8 on bedaquiline), being recommendations or guidelines (8 manuscripts), reviews (17 papers) or other studies (56 papers).
Figure 1.
Selection process: PRISMA 2009 flow diagram.
Three studies only (20,21,46) satisfied the criteria for further analysis: all of them were published between 2014 and 2016, 2 being research letters, and reporting on a total of 19 cases. Two studies (21,46) (both from the same Authors) provided additional details on the first child treated with delamanid and being cured.
All the 19 cases described were part of the Compassionate Use Programme of the delamanid’s manufacturer; 16 of them received delamanid with a mean delay of 73.5 days (range, 14–153 days), while 3 were still waiting for the drug at the moment the article was accepted (20).
They were recruited in eight different countries in Africa, Asia and Europe. Based on limited treatment options, extensive resistance profile (from 4 to 15 drugs) or adverse events to core second line anti-TB drugs, an independent Consilium-like body supported the rationale use of delamanid, and the manufacturer provided it under compassionate use.
All 19 children had pulmonary TB, 2 additionally having extrapulmonary localizations. Their mean age was 14.4 years, ranging from 8 to 17 years. Three out of 19 were HIV co-infected. Out of 19 children, 14 met the XDR-TB defining criteria, 4 were pre-XDR (e.g., their disease was due to MDR-TB strains with additional resistance to one fluoroquinolone (3 cases) or one injectable second-line anti-TB drug (one case)) and one had MDR-TB. All cases were bacteriologically confirmed, 14 undergoing Xpert (13 being rifampicin-resistant and one indeterminate).
Delamanid was prescribed at the dose of 100 mg twice a day, with one single exception where the dose was reduced to 50 mg (as the body weight was 22 kg) (20).
Out of the 16 children treated with delamanid, 6 completed 24 weeks of delamanid treatment while 10 were still completing it at the moment the paper was accepted. Thirteen out of 16 children achieved a consistent bacteriological conversion (1 being cured), the remaining 3 having just started their delamanid treatment. No adverse event was reported in fifteen children. A single child, treated with a combination of delamanid, capreomycin, ethionamide, cycloserine, clofazimine, imipenem, amoxicillin/clavulanate and pyrazinamide experienced vomiting, renal impairment, electrolyte disturbances and QTcF (QT interval in the electrocardiogram corrected according to Fredericia formula) ≥500 ms requiring temporary interruption of delamanid. After managing vomiting and the electrolyte imbalance, the child was able to complete delamanid treatment without further QtcF prolongation (20).
Details on the studies are reported in Tables 1-3.
Table 1. Epidemiological characteristics of the selected studies.
| First author | Publication year | Country | Study design | Clinical setting | Study duration |
|---|---|---|---|---|---|
| Tadolini (20) | 2016 | Italy, South Africa, Georgia, India, Namibia, Swaziland, Russia and Armenia | Retrospective case reports | Reference hospitals in the different countries | February 2014–March 2016 |
| Esposito (21) | 2014 | Italy | Case 1 of 19, detailed report | Reference Hospital, Milano, Italy | October 2013–March 2014 |
| Esposito (46) | 2016 | As above | As above | As above | October 2013–March 2016 |
Table 2. Clinical features of the selected studies.
| First author | Number of individuals exposed to delamanid/HIV status | Age (years)/sex | XDR-TB/previous anti-TB treatment | Clinical form | Drug resistance profile | Regimen administered/delamanid dose |
|---|---|---|---|---|---|---|
| Tadolini (20) | 16 out of 19, 3 HIV + | Mean: 14.4 11 males; 8 females |
15 XDR/yes | 19 Pulmonary TB (2 also extra-pulmonary TB) | Resistant to 4–15 drugs | Individualised regimens available in the Table of the original article/Dlm: 100 mg twice a day (1 case: half dose because of low body weight) |
| Esposito (21) | 1, HIV negative | 12/male | XDR/ yes | Pulmonary and laryngeal TB | Resistant to 8 drugs | Delamanid, ethionamide, clofazimine, amikacin, meropenem, para-aminosalicylic acid, linezolid, clarithromycin, terizidone, amoxi/clavulanate, moxifloxacin/Dlm: 100 mg twice a day |
| Esposito (46) | Same as above | Same as above | Same as above | Same as above | Same as above | Same as above |
XDR-TB, extensively drug-resistant tuberculosis; Dlm, delamanid.
Table 3. Effectiveness, safety and tolerability profiles of delamanid in the selected studies.
| First author | Sputum smear conversion | Sputum culture conversion | Treatment outcome | QT interval prolongation (>500 msec) | Interruption of delamanid due to adverse events |
|---|---|---|---|---|---|
| Tadolini (20) | Yes (13/16) | Yes (13/16) | Favourable clinical, microbiological and radiological responses in all; 6 completed 24 weeks of delamanid, 1 cured | Yes (1 case) | Yes (1 cases, temporary discontinuation) |
| Esposito (21) | Yes | Yes | Favourable clinical, microbiological and radiological response | No | No |
| Esposito (46) | Same as above | Same as above | Cured | No | No |
QT interval: measure of the time between the start of the Q wave and the end of the T wave in the heart’s electrical cycle.
A summary of the ongoing clinical trials on new drugs in children is available in Table 4.
Table 4. Clinical trials on new drugs in children [modified from (45)].
| Title | Code/status | Phase | Design | Children (years of age) | Outcome measures | Planned/Estimated number for enrolment | Start/completion dates |
|---|---|---|---|---|---|---|---|
| A 6-month safety, efficacy, and PK trial of delamanid in paediatric patients with multidrug resistant tuberculosis | NCT01859923 (Recruiting) | 2 | Non-randomized; safety study; parallel assignment; open label | Yes (≤17) | Safety and tolerability: pharmacokinetics (PK); pharmacokinetics/pharmacodynamics (PK/PD) relationship analysis of delamanid and DM-6705 and change in corrected QT interval; efficacy of delamanid; palatability of the paediatric formulation (Groups 3/ 4 only) | 36 | August 2013–November 2019 |
| Pharmacokinetic study to evaluate anti-mycobacterial activity of TMC207 in combination with background regimen (BR) of multidrug resistant tuberculosis (MDR-TB) medications for treatment of children/adolescents pulmonary MDR-TB | NCT02354014 (Recruiting) | 2 | Safety study; single group assignment; open label | Yes (≤18) | Number of participants with adverse events (AEs) or serious AE (SAEs); maximum plasma concentration (Cmax); time to reach maximum plasma concentration (Tmax); minimum plasma concentration (Cmin); Area Under the plasma concentration-time Curve from the time of dose administration up to X hours (AUCtime-h); elimination half-life (t1/2); Area Under the plasma concentration-time curve from the time of dose administration up to 168 hours [AUC168h]; volume of distribution (Vd); apparent clearance (CL); percentage of participants with favourable treatment outcome (sustained positive clinical cure); time to first confirmed sputum culture conversion or other microbiology specimen sample | 40 | May 2016–December 2022 |
| Evaluating the pharmacokinetics, safety, and tolerability of bedaquiline in HIV-infected and HIV-uninfected infants, children, and adolescents with multidrug-resistant tuberculosis | NCT02906007 (Not yet recruiting) | 1/2 | Safety study; single group assignment; open label | Yes (≤18) | Frequency of participant termination from treatment due to a drug-related AE; frequency of Grade 3/4 AEs severity; absolute QTc interval ≥500 msec; frequency of unstable dysrhythmias requiring hospitalization/treatment; incidence of death; frequency of AEs ≥ Grade 3/4 severity; frequency of AEs ≥ Grade 3/4 severity | 72 estimated enrollment | December 2016–June 2020 |
| Pharmacokinetic and safety trial to determine the appropriate dose (of delamanid) for paediatric patients with multidrug resistant tuberculosis | NCT01856634 (Recruiting) | 1 | Non-randomized; pharmacokinetics study; parallel assignment; open label | Yes (≤17) | Plasma concentrations; AEs; safety palatability of the paediatric formulation | 36 | July 2013–November 2017 |
| A phase 3 study assessing the safety and efficacy of bedaquiline plus PA-824 plus linezolid in subjects with drug resistant pulmonary tuberculosis | NCT02333799 (Recruiting) | 3 | Safety/efficacy study; single group assignment; open label | Yes (≥14) | Incidence of bacteriologic/clinical failure or relapse through follow-up until 24 months after the end of treatment.; time to sputum culture conversion; proportion of subjects with sputum culture conversion at 4, 6, 8, 12, 16 and 26 or 39 weeks.; incidence of treatment emergent adverse events (TEAEs) by incidence, and seriousness, leading to TB-related or non-TB related death.; all subjects- pre-dose sampling at weeks 2, 8 and 16 to measure Ctrough levels of bedaquiline, bedaquiline metabolite M2, linezolid and PA-824.; time to sputum culture conversion | 200 | March 2015–October 2021 |
| A prospective patient registry of patients exposed to bedaquiline | NCT02274389 (Recruiting) | 4 | Observational perspective cohort study | Yes | Percentage of participants with medical indication for bedaquiline (BDQ) treatment and frequency of use of expert medical consultation with BDQ; number of participants with BDQ susceptibility based on minimum inhibitory concentration (MIC); maximum dose of BDQ; total duration of BDQ treatment; percentage of participants with different drug distribution mechanisms utilized for drug administration; number of participants with clinical and microbiologic reported outcomes; number of participants with AEs/SAEs; comparison of rate of observed outcomes with nationally available reported rates | 300 | December 2013–December 2018 |
| Evaluating newly approved drugs for multidrug-resistant TB | NCT02754765 (Not yet recruiting) | 3 | Randomized; efficacy study; parallel assignment; open label | Yes (≥15) | Week 73 efficacy; week 104 efficacy; early treatment response (culture conversion); week 39 efficacy; week 73 survival; week 104 survival; week 73 safety; week 104 safety; week 73 QTc interval prolongation | 750 estimated enrollment | July 2016–April 2021 |
| Pharmacokinetic study of antiretroviral drugs and related drugs during and after pregnancy | NCT00042289 (Recruiting) | 4 | Non-randomized; pharmacokinetics study; parallel open label | Yes | AUC from 0 to 12 hours (AUC 0-12); AUC from 0 to 24 hours (AUC 0-24); Cmax; pre-dose concentration (Cdose); Cmin; Tmax; clearance over systemic availability (Cl/F); volume of distribution over systemic availability (V/F); t1/2); ARV concentrations in vaginal secretions and plasma; for contraceptives: plasma concentration; ratio of cord blood concentration to maternal blood concentration; ratio of unbound/total drug concentrations; rate of detection of study drugs in vaginal secretions; ratio of vaginal drug concentrations to simultaneous blood concentrations; rate of detection of HIV RNA/DNA in vaginal secretions and comparison to level in blood; ARV exposure (as measured by AUC or other PK parameters) during pregnancy and postpartum according to genotype; AEs ≥ Grade 3; infant neurological events ≥ Grade; adverse pregnancy outcome: preterm birth; adverse pregnancy outcome: low birth weight; adverse pregnancy outcome: fetal demise; adverse pregnancy outcome: congenital anomalies; infant HIV infection status | 1,786 | June 2018- completion date not available |
PK, pharmacokinetics; AEs, adverse events; AUC, area under the curve; Cmax, maximum concentration; Cmin, minimum concentration; Tmax, time after administration of drug when maximum plasma concentration is reached; ARV, antiretroviral
Out of 8 trials, 2 are evaluating delamanid, 4 bedaquiline and 2 both of them.
The outcome measures are safety and tolerability in two trials, PK parameters in two trials, safety/tolerability/efficacy in three trials and drug-drug interaction between both delamanid/bedaquiline and antiretrovirals in one trial. The different trials (in all phases from 1 to 5) have a sample size ranging from 36 to 1,786 cases. Out of 8 trials, 6 are presently recruiting. No information on ‘ad-interim’ analyses is available as of today.
Discussion
Aim of the present study is to systematically review the available scientific evidence on the use of delamanid or bedaquiline in M/XDR-TB children.
Only three papers met the inclusion criteria (20,21,46), all focused on delamanid, while no study is unfortunately available on bedaquiline in children.
Although several reviews discussing the new anti-TB drugs were checked, none of them was systematic providing a methodology justifying the choice of the articles cited (thus preventing the reader from understanding if other articles were eventually available in the literature).
The core study (20) included information on 19 children treated with delamanid under compassionate use, while the two ancillary studies (21,46) provide details on the first case of the series. This case is rather emblematic, being the first child who accessed delamanid under compassionate use, the first who underwent delamanid for more than 6 months and, more importantly, the first child who was cured with the new drug (20,21,46).
The information identified is really scarce, due to the lack of studies on new drugs in children. In spite of the format used (Letter), the core details of the individual patients have been provided. Unfortunately, only a few cases (6) completed the 24 weeks of delamanid treatment, and a single case the whole treatment regimen (therefore having the treatment outcome available). In spite of its poverty, this piece of evidence is one of the few allowing WHO to revise its recommendations for the use of delamanid in children and adolescents (16).
The main conclusions which can be drawn from this systematic review are the following: (I) information on delamanid in children is increasingly available, while no published data on bedaquiline can be found yet. We need to wait for the results of the ongoing trials (45), although out of the 4 trials on bedaquiline and the 2 on both the new drugs, 2 have not yet started to recruit patients; (II) delamanid in children seems to be promising, as it was well tolerated (a single, reversible QT prolongation above 500 msec was described, which has been adequately managed) in the 16 children treated (20) and apparently effective, as all the 13 children undergoing treatment for more than a few days achieved smear and culture conversion. However, more information is necessary to draw consistent conclusions, and the results of the ongoing trials will be most welcome; (III) electrolytes (potassium and magnesium) as well as albumin should be monitored, as electrolytes disturbance and/or hypoalbuminemia and any problem need to be adequately managed to prevent QTc prolongation or other problems; (IV) no published information is presently available on the combined use of delamanid and bedaquiline in children.
Unfortunately, even the recent large study describing safety and effectiveness of bedaquiline used programmatically was performed on adults only (47).
Although the methodology used in the study was rigorous, the results are limited by the paucity of the studies available in the literature on the use of new anti-TB drugs in children.
In conclusion, more evidence is needed on the use of delamanid and bedaquiline in paediatric patients.
Acknowledgements
This article has been developed within the ERS/ALAT SinTB project and ERS/SBPT project.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.World Health Organization. Global tuberculosis report 2016. WHO/HTM/TB/2016.13. Geneva: World Health Organization, 2016. [Google Scholar]
- 2.Tiberi S, D'Ambrosio L, De Lorenzo S, et al. Tuberculosis elimination, patients' lives and rational use of new drugs: revisited. Eur Respir J 2016;47:664-7. 10.1183/13993003.01297-2015 [DOI] [PubMed] [Google Scholar]
- 3.Falzon D, Gandhi N, Migliori GB, et al. Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 2013;42:156-68. 10.1183/09031936.00134712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migliori GB, Sotgiu G, Gandhi NR, et al. Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. Drug resistance beyond extensively drug-resistant tuberculosis: individual patient data meta-analysis. Eur Respir J 2013;42:169-79. 10.1183/09031936.00136312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diel R, Rutz S, Castell S, et al. Tuberculosis: cost of illness in Germany. Eur Respir J 2012;40:143-51. 10.1183/09031936.00204611 [DOI] [PubMed] [Google Scholar]
- 6.Diel R, Vandeputte J, de Vries G, et al. Costs of tuberculosis disease in the European Union: a systematic analysis and cost calculation. Eur Respir J 2014;43:554-65. 10.1183/09031936.00079413 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis 2016 update. WHO/HTM/TB 2016.04 Geneva: World Health Organization, 2016. [Google Scholar]
- 8.Migliori GB, De Iaco G, Besozzi G, et al. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveill 2007;12:E070517.1. [DOI] [PubMed] [Google Scholar]
- 9.Caminero JA, Scardigli A. Classification of antituberculosis drugs: a new proposal based on the most recent evidence. Eur Respir J 2015;46:887-93. 10.1183/13993003.00432-2015 [DOI] [PubMed] [Google Scholar]
- 10.Sotgiu G, Tiberi S, D’Ambrosio L, et al. International Carbapenem Study Group Faster for less, the new ‘Shorter’ regimen for multidrug-resistant tuberculosis. Eur Respir J 2016;48:1503-7. 10.1183/13993003.01249-2016 [DOI] [PubMed] [Google Scholar]
- 11.Falzon D, Jaramillo E, Schünemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011;38:516-28. 10.1183/09031936.00073611 [DOI] [PubMed] [Google Scholar]
- 12.Tiberi S, Scardigli A, Centis R, et al. Classifying new anti-TB drugs: rationale and future perspectives. Int J Infect Dis 2017;56:181-4. 10.1016/j.ijid.2016.10.026 [DOI] [PubMed] [Google Scholar]
- 13.Sotgiu G, Pontali E, Centis R, et al. New anti-tuberculosis drugs for special populations: a difficult-to-address issue. Eur Respir J 2016;48:957-8. 10.1183/13993003.01289-2016 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. The use of delamanid in the treatment of multidrug-resistant tuberculosis. Interim policy guidance. WHO/HTM/TB2014.23. Geneva: World Health Organization, 2014. [PubMed] [Google Scholar]
- 15.World Health Organization. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis. Interim policy guidance. WHO/HTM/TB/2013.6. Geneva: World Health Organization, 2013. [PubMed] [Google Scholar]
- 16.World Health Organization. The use of delamanid in the treatment of multidrug-resistant tuberculosis in children and adolescents: interim policy guidance. WHO/HTM/TB/2016.14. Geneva, World Health Organization, 2016. [PubMed] [Google Scholar]
- 17.Harausz EP, Garcia-Prats A, Seddon JA, et al. Sentinel Project on Pediatric Drug-Resistant Tuberculosis New Drugs, Repurposed Drugs, and Novel Regimens for Children with Multidrug-Resistant Tuberculosis: Practice-Based Recommendations. Am J Respir Crit Care Med 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Pontali E, Sotgiu G, D’Ambrosio L, et al. Bedaquiline and MDR-TB: a systematic and critical analysis of the evidence. Eur Respir J 2016;47:394-402. 10.1183/13993003.01891-2015 [DOI] [PubMed] [Google Scholar]
- 19.Sotgiu G, Pontali E, Centis R, et al. . Delamanid (OPC-67683) for treatment of multi-drug-resistant tuberculosis. Expert Rev Anti Infect Ther 2015;13:305-15. 10.1586/14787210.2015.1011127 [DOI] [PubMed] [Google Scholar]
- 20.Tadolini M, Garcia-Prats AJ, D’Ambrosio L, et al. Compassionate use of new drugs in children and adolescents with multidrug-resistant and extensively-drug resistant tuberculosis: early experiences and challenges. Eur Respir J 2016;48:938-43. 10.1183/13993003.00705-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito S, D'Ambrosio L, Tadolini M, et al. ERS/WHO Tuberculosis Consilium assistance with extensively drug-resistant tuberculosis management in a child: case study of compassionate delamanid use. Eur Respir J 2014;44:811-5. 10.1183/09031936.00060414 [DOI] [PubMed] [Google Scholar]
- 22.Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J 2013;41:1393-400. 10.1183/09031936.00125812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 2012;366:2151-60. 10.1056/NEJMoa1112433 [DOI] [PubMed] [Google Scholar]
- 24.Pym AS, Diacon AH, Tang SJ, et al. TMC207-C209 Study Group Bedaquiline in the treatment of multi- and extensively drug-resistant tuberculosis. Eur Respir J 2016;47:564-74. 10.1183/13993003.00724-2015 [DOI] [PubMed] [Google Scholar]
- 25.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009;360:2397-405. 10.1056/NEJMoa0808427 [DOI] [PubMed] [Google Scholar]
- 26.Diacon AH, Pym A, Grobusch MP, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 2014;371:723-32. 10.1056/NEJMoa1313865 [DOI] [PubMed] [Google Scholar]
- 27.Dalcolmo M, Gayoso R, Sotgiu G, et al. Resistance profile of drugs composing the "shorter" regimen for multidrug-resistant tuberculosis in Brazil, 2000-2015. Eur Respir J 2017;49(4). 10.1183/13993003.02309-2016 [DOI] [PubMed] [Google Scholar]
- 28.Sotgiu G, Pontali E, Migliori GB. Linezolid to treat MDR-/XDR-Tuberculosis: available evidence and future scenarios. Eur Respir J 2015;45:25-9. 10.1183/09031936.00145014 [DOI] [PubMed] [Google Scholar]
- 29.Villar M, Sotgiu G, D'Ambrosio L, et al. Linezolid safety, tolerability and efficacy to treat multidrug- and extensively drug-resistant tuberculosis. Eur Respir J 2011;38:730-3. 10.1183/09031936.00195210 [DOI] [PubMed] [Google Scholar]
- 30.De Lorenzo S, Centis R, D'Ambrosio L, et al. On linezolid efficacy and tolerability. Eur Respir J 2012;39:770-2. 10.1183/09031936.00116011 [DOI] [PubMed] [Google Scholar]
- 31.Sotgiu G, Centis R, D'Ambrosio L, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J 2012;40:1430-42. 10.1183/09031936.00022912 [DOI] [PubMed] [Google Scholar]
- 32.Sotgiu G, Centis R, D'Ambrosio L, et al. ; International Group for the study of Linezolid. Linezolid to treat extensively drug-resistant TB: retrospective data are confirmed by experimental evidence. Eur Respir J 2013;42:288-90. 10.1183/09031936.00191712 [DOI] [PubMed] [Google Scholar]
- 33.Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012;367:1508-18. 10.1056/NEJMoa1201964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sotgiu G, Centis R, D'Ambrosio L, et al. Low minimal inhibitory concentrations of linezolid against multidrug-resistant tuberculosis strains. Eur Respir J 2015;45:287-9. 10.1183/09031936.00135014 [DOI] [PubMed] [Google Scholar]
- 35.De Lorenzo S, Alffenaar JW, Sotgiu G, et al. Efficacy and safety of meropenem-clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J 2013;41:1386-92. 10.1183/09031936.00124312 [DOI] [PubMed] [Google Scholar]
- 36.Tiberi S, Payen MC, Sotgiu G, et al. Effectiveness and safety of meropenem/clavulanate-containing regimens in the treatment of multidrug and extensively drug-resistant tuberculosis. Eur Respir J 2016;47:1235-43. 10.1183/13993003.02146-2015 [DOI] [PubMed] [Google Scholar]
- 37.Tiberi S, Sotgiu G, D'Ambrosio L, et al. Effectiveness and Safety of Imipenem-Clavulanate Added to an Optimized Background Regimen (OBR) Versus OBR Control Regimens in the Treatment of Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis. Clin Infect Dis 2016;62:1188-90. 10.1093/cid/ciw088 [DOI] [PubMed] [Google Scholar]
- 38.Tiberi S, Sotgiu G, D’Ambrosio L, et al. Comparison of effectiveness and safety of imipenem/clavulanate- versus meropenem/clavulanate-containing regimens in the treatment of multidrug and extensively drug-resistant tuberculosis. Eur Respir J 2016;47:1758-66. 10.1183/13993003.00214-2016 [DOI] [PubMed] [Google Scholar]
- 39.Tiberi S, D'Ambrosio L, De Lorenzo S, et al. Ertapenem in the treatment of multidrug-resistant tuberculosis: first clinical experience. Eur Respir J 2016;47:333-6. 10.1183/13993003.01278-2015 [DOI] [PubMed] [Google Scholar]
- 40.Matteelli A, D'Ambrosio L, Centis R, et al. Compassionate and optimum use of new tuberculosis drugs. Lancet Infect Dis 2015;15:1131-2. 10.1016/S1473-3099(15)00296-0 [DOI] [PubMed] [Google Scholar]
- 41.Tadolini M, Lingtsang RD, Tiberi S, et al. First case of extensively drug-resistant tuberculosis treated with both delamanid and bedaquiline. Eur Respir J 2016;48:935-8. 10.1183/13993003.00637-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tadolini M, Lingtsang RD, Tiberi S, et al. Cardiac safety of extensively drug-resistant tuberculosis regimens including bedaquiline, delamanid, and clofazimine. Eur Respir J 2016;48:1527-9. 10.1183/13993003.01552-2016 [DOI] [PubMed] [Google Scholar]
- 43.Wallis RS. Cardiac safety of extensively drug-resistant tuberculosis regimens including bedaquiline, delamanid and clofazimine. Eur Respir J 2016;48:1526-7. 10.1183/13993003.01207-2016 [DOI] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;21;6:e1000097. [DOI] [PMC free article] [PubMed]
- 45.ClinicalTrials.gov. A service of the U.S. National Institutes of Health. Available online: www.clinicaltrials.gov (last access: January 2nd, 2017).
- 46.Esposito S, Bosis S, Tadolini M, et al. Efficacy, safety, and tolerability of a 24-month treatment regimen including delamanid in a child with extensively drug-resistant tuberculosis: A case report and review of the literature. Medicine (Baltimore) 2016;95:e5347. 10.1097/MD.0000000000005347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borisov SE, Dheda K, Enwerem M, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J 2017;49(5). 10.1183/13993003.00387-2017 [DOI] [PubMed] [Google Scholar]