Abstract
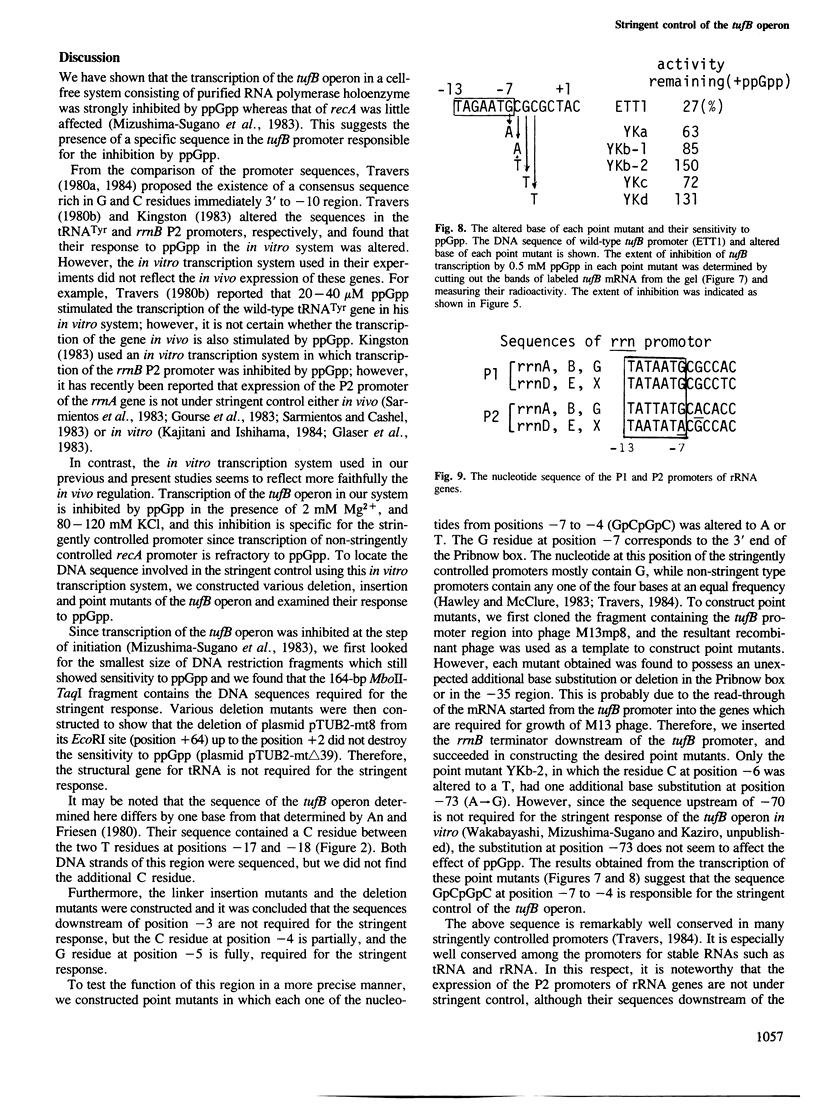
We have located the DNA sequence involved in the stringent control of the Escherichia coli tufB operon. Various deletion and insertion mutants of the promoter locus were constructed by in vitro mutagenesis, and their response to guanosine-5'-diphosphate-3'-diphosphate (ppGpp) was examined in a cell-free transcription system consisting of purified RNA polymerase holoenzyme. The nucleotide sequence (GpCpGpC) from positions -7 to -4 (designating the initiation site of mRNA as position +1) is responsible for the selective inhibition by ppGpp of tufB transcription. Point mutations were then constructed in which each one of the above four nucleotides was replaced by an A or T residue and tested for their response to ppGpp in the in vitro transcription system. The results indicated that the alteration of any nucleotide in the GpCpGpC sequence leads to the loss of the stringent response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Glaser G., Sarmientos P., Cashel M. Functional interrelationship between two tandem E. coli ribosomal RNA promoters. Nature. 1983 Mar 3;302(5903):74–76. doi: 10.1038/302074a0. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., Stark M. J., Dahlberg A. E. Regions of DNA involved in the stringent control of plasmid-encoded rRNA in vivo. Cell. 1983 Apr;32(4):1347–1354. doi: 10.1016/0092-8674(83)90315-x. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase. Differential stringent control of the multiple promoters from ribosomal RNA and protein operons. J Biol Chem. 1984 Feb 10;259(3):1951–1957. [PubMed] [Google Scholar]
- Lee J. S., An G., Friesen J. D., Fill N. P. Location of the tufB promoter of E. coli: cotranscription of tufB with four transfer RNA genes. Cell. 1981 Jul;25(1):251–258. doi: 10.1016/0092-8674(81)90250-6. [DOI] [PubMed] [Google Scholar]
- Lee J. S., An G., Friesen J. D., Fill N. P. Location of the tufB promoter of E. coli: cotranscription of tufB with four transfer RNA genes. Cell. 1981 Jul;25(1):251–258. doi: 10.1016/0092-8674(81)90250-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Kaziro Y. Coordination of levels of elongation factors Tu, Ts, and G, and ribosomal protein SI in Escherichia coli. J Biochem. 1978 Feb;83(2):453–462. doi: 10.1093/oxfordjournals.jbchem.a131932. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Shibuya M., Kaziro Y. Construction and characterization of the two hybrid Co1E1 plasmids carrying Escherichia coli tufB gene. FEBS Lett. 1979 Jun 15;102(2):207–210. doi: 10.1016/0014-5793(79)80001-0. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Shibuya M., Kuchino Y., Kaziro Y. Transcription of the E. coli tufB gene: cotranscription with four tRNA genes and inhibition by guanosine-5'-diphosphate-3'-diphosphate. Mol Gen Genet. 1981;183(1):13–19. doi: 10.1007/BF00270131. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Yokota T., Takebe Y., Nakamura M., Kaziro Y. A deletion mutant lacking three out of four transfer RNA genes upstream of the coding region of tufB. J Biochem. 1983 Apr;93(4):1101–1108. doi: 10.1093/oxfordjournals.jbchem.a134235. [DOI] [PubMed] [Google Scholar]
- Mizushima-Sugano J., Miyajima A., Kaziro Y. Selective inhibition of transcription of the E. coli tufB operon by guanosine-5'-diphosphate-3'-diphosphate. Mol Gen Genet. 1983;189(2):185–192. doi: 10.1007/BF00337802. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmientos P., Cashel M. Carbon starvation and growth rate-dependent regulation of the Escherichia coli ribosomal RNA promoters: differential control of dual promoters. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7010–7013. doi: 10.1073/pnas.80.22.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmientos P., Sylvester J. E., Contente S., Cashel M. Differential stringent control of the tandem E. coli ribosomal RNA promoters from the rrnA operon expressed in vivo in multicopy plasmids. Cell. 1983 Apr;32(4):1337–1346. doi: 10.1016/0092-8674(83)90314-8. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Kaziro Y. Studies on stringent control in a cell-free system. Regulation by guanosine-5'-diphosphate-3'-diphosphate of the synthesis of elongation factor Tu. J Biochem. 1979 Aug;86(2):403–411. doi: 10.1093/oxfordjournals.jbchem.a132539. [DOI] [PubMed] [Google Scholar]
- Temple G. F., Dozy A. M., Roy K. L., Kan Y. W. Construction of a functional human suppressor tRNA gene: an approach to gene therapy for beta-thalassaemia. Nature. 1982 Apr 8;296(5857):537–540. doi: 10.1038/296537a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A. A tRNATyr promoter with an altered in vitro response to ppgpp. J Mol Biol. 1980 Jul 25;141(1):91–97. doi: 10.1016/s0022-2836(80)80030-1. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Conserved features of coordinately regulated E. coli promoters. Nucleic Acids Res. 1984 Mar 26;12(6):2605–2618. doi: 10.1093/nar/12.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Promoter sequence for stringent control of bacterial ribonucleic acid synthesis. J Bacteriol. 1980 Feb;141(2):973–976. doi: 10.1128/jb.141.2.973-976.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Derbyshire R., Guy A., Molko D., Roget A., Téoule R., Chambon P. Specific in vitro transcription of conalbumin gene is drastically decreased by single-point mutation in T-A-T-A box homology sequence. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7024–7028. doi: 10.1073/pnas.77.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]