Abstract
Background
The bidirectional relationship of asthma and obstructive sleep apnea (OSA) has been confirmed in recent years. However, in the clinical practice, majority of asthma patients did not pay adequate attention to their sleep apnea condition. Berlin questionnaire (BQ) and STOP-Bang questionnaire (SBQ) are two most common OSA screening questionnaires to screen high-risk patients for OSA. This study aimed at evaluating the predictive performance of BQ and SBQ for OSA in asthma patients.
Methods
Asthma outpatients of Zhongshan Hospital were enrolled into the study. All patients were asked to fill in the BQ and SBQ and clinical characteristics and asthma characteristics were recorded. Univariate and multivariate logistic regression analyses were applied to identify risk factors of OSA in asthma patients. With the gold standard of laboratory-based overnight polysomnography (PSG), the predictive performance of SBQ and BQ was evaluated and compared. The probability of OSA severity was predicted by various SBQ scores in asthma patients.
Results
A total of 123 asthma patients (average age 47.56±12.12 years; 57.72% males) were enrolled and underwent PSG diagnosis overnight at Sleep Center. Logistic regression analyses showed that rhinitis (adjusted OR =4.30; 95% CI: 1.50–12.37, P=0.007) and dyslipidemia (adjusted OR =2.75; 95% CI: 1.16–6.51, P=0.021) were associated with OSA in asthma patients after adjusting for known OSA risk factors. No asthma functional characteristic differences were found to be associated with OSA severity in the study. The prevalence of moderate-to-severe OSA (AHI ≥15) in the asthmatic population sample was 36.59% (45/123). Questionnaires predictive results showed that compared with BQ, SBQ has higher diagnostic sensitivity (84.4% vs. 60%), lower specificity (79.5% vs. 91%) lower positive predictive value (PPV): (70.4% vs. 79.4%) and higher negative predictive value (NPV) (90% vs. 80%) to detect moderate-to-severe OSA at the cut-off as AHI of 15/h. OSA probability results showed that with the increasing of the questionnaire scores, the moderate and severe OSA probability of SBQ rose significantly.
Conclusions
SBQ is a preferable sleep questionnaire better than BQ for detecting moderate and severe OSA in asthma patients which should be validated in larger population sample.
Keywords: Asthma, OSAHS, Berlin questionnaire (BQ), STOP-Bang questionnaire (SBQ)
Introduction
Asthma is a chronic respiratory disorder associated with reversible air flow obstruction and bronchial hyper-responsiveness affecting all age groups. The typical symptoms of asthma include wheezing, breathlessness, chest tightness and coughing that vary over time and intensity. 1% to 18% of the general population suffered from asthma among different regions of the world (1). Over the last quarter century, a dramatic increase in the morbidity and economic burden of asthma has become a public concern (2).
Increasing evidence indicate that the presence of comorbidities can influence asthma control and asthma outcomes (3). Obstructive sleep apnea (OSA), one of the most common comorbidities, is associated with the occurrence of asthma, poor asthma control, frequent exacerbations and asthma-related quality of life (4-6). It is a common disorder of repetitive pharyngeal collapse during sleep. Pharyngeal collapse could be complete (causing apnoea) or partial (causing hypopnoea). Epidemiologic data showed that the prevalence of OSA among asthmatic populations ranges from 38% up to as high as 70% (7). There is growing evidence that continuous positive airway pressure (CPAP) in adults and adenotonsillectomy in children which are recommended as first line treatment of OSA can improve their asthma symptoms (8-10). Mild OSA in severe asthma patients is associated with increased proportion of neutrophils in sputum and changes in airway remodeling (11,12). OSA and asthma co-morbidities are independent predictors of new heart failure and atrial fibrillation in patients with diabetes mellitus and preserved ejection fraction (13). And OSA was associated with systemic hypertension in a young asthma population (14). Recently a follow-up research for more than 5 years has suggested that asthmatic patients with OSA had greater declines in FEV1 than those without OSA and CPAP treatment alleviated the decline of FEV1 in asthma patients with severe OSA (15). Periodic OSA evaluation in patients with asthma is recommended and special consideration is needed for the care and treatment of asthmatic patients with OSA (16).
Due to miscellaneous nocturnal asthmatic symptoms, majority of asthma patients did not pay enough attention to their sleep apnea condition. Moreover, the gold standard for OSA diagnosis is attended overnight polysomnography (PSG), which is rather expensive and time-consuming. Utilizing validated screening instruments to affirm high risk of OSA in asthmatic patients will allow clinicians to cost-effectively test and treat appropriate patients, potentially improving asthma outcomes. The Berlin questionnaire (BQ), a reliable screening tool, first generated at the Conference on Sleep in Primary Care in Berlin, Germany in April 1996, has been widely used to determine high-risk patients for OSA (17). The STOP-Bang questionnaire (SBQ) was first developed in 2008 (18). Due to its ease using and high sensitivity, SBQ has been widely used in preoperative clinics, sleep clinics, the general population and other special populations to detect patients at high-risk of OSA (19). However, their screening performance in asthma patients has not yet been assessed so far. In this study, the predictive performance of BQ and SBQ were validated in asthmatic population.
Method
Participants
The study was approved by the ethics committees of Fudan University Zhongshan Hospital (B2012-073R), Shanghai, China. From January 2015 to January 2016, altogether 229 consecutive asthma patients collected from the general respiration clinic of Zhongshan Hospital were invited to participate in this study. According to the inclusion criteria, 123 patients were enrolled into the study (Figure 1). Characteristics of patients who were enrolled or removed in the study were showed in the supplementary information (Table S1).
Figure 1.
Flow chart of asthma patients screening in the study.
Table S1. Characteristics of patients who were enrolled or removed in the study.
| Variables | All patients (n=229) | Enrolled patients (n=123) | Removed patients (n=106) | P value |
|---|---|---|---|---|
| Men/Total | 140/229 | 71/123 | 69/106 | 0.254 |
| Age (years) | 47.21±15.84 | 47.56±12.12 | 46.79±19.25 | 0.724 |
| BMI (kg/m2) | 26.30±3.08 | 26.42±2.99 | 26.15±3.19 | 0.507 |
| Neck circumference (cm) | 36.38±3.50 | 36.27±2.97 | 36.51±4.02 | 0.612 |
| Asthma onset age | 15.82±8.58 | 16.43±7.80 | 15.10±9.35 | 0.244 |
| Regular medical control of asthma | 163 (71.18%) | 81 (66.1%) | 82 (77.36%) | 0.055 |
| Using ICS | 123 (53.71%) | 62 (50%) | 61 (57.55%) | 0.280 |
| Inhaled glucocorticoids, equivalent doses of budesonide (mcg/day) | 362.67±109.24 | 361.29±122.69 | 361.97±108.02 | 0.196 |
| Asthma grade | 0.053* | |||
| Mild | 98 (42.79%) | 61 (49.59%) | 37 (34.91%) | |
| Moderate | 110 (48.03%) | 54 (43.9%) | 56 (52.83%) | |
| Severe | 21 (9.17%) | 8 (6.5%) | 13 (12.26%) | |
| In asthma exacerbation within past 6 months | 27/229 | 0/123 | 27/106 | 0.000* |
Data represent as mean ± SD or number (%). *, P<0.05 for comparison with “Removed patients” (independent sample t-test or Mann-Whitney rank sum test and Chi-square test or Fisher’s exact tests for two samples testing). BMI, body mass index, BMI = Weight (Kg) ÷ Height2 (m2); ICS, inhaled corticosteroids.
The inclusion criteria were as follow: (I) asthma medical history was diagnosed definitely previously and not in asthma exacerbation within the past 6 months; (II) did not be diagnosed OSA previously or accepted OSA treatment; (III) did not combine with other chronic respiratory diseases (such as COPD, bronchiectasia, interstitial lung diseases, pulmonary fibrosis and lung neoplasms etc.) and cooperated related examinations smoothly by themselves; (IV) ranging from 18 to 80 years old; (V) complete asthma related tests and PSG tests fully; (VI) understood this study with written informed consents.
The exclusion criteria were as follow: (I) in asthma exacerbation within the past 6 months; (II) be diagnosed OSA previously or accepted OSA treatment; (III) combine with other chronic respiratory diseases (such as COPD, bronchiectasia, interstitial lung diseases, pulmonary fibrosis and lung neoplasms etc.) and cooperated related examinations smoothly by themselves; (IV) not ranging from 18 to 80 years old; (V) not complete asthma related tests and PSG tests fully; (VI) reject to participate this study.
Data collection
Collecting patients’ data and completing related examinations:
Clinical characteristics: the demographic data and clinical characteristics were collected: sex (women/men), age (age ≥50), BMI (BMI ≥35 or ≥30), neck circumference (neck circumference ≥40), Smoking status (current, Ex and never), alcohol status (current, Ex and never), high blood pressure (HBP), coronary heart disease (CHD), arrhythmia, gastroesophageal reflux disease (GERD), diabetes, dyslipidemia and rhinitis. Neck circumference, height and weight were measured by the technicians in the Sleep Center. Other concomitant disorder records were enquired by patients personally and based on patients’ medical records.
Asthma functional characteristics: all the patients were asked for the following information: Asthma Control Test (ACT), FeNO value, lung function parameters (FEV1/predicted %, FVC/predicted %, FEV1/FVC % and PEF/predicted %), asthma onset age, regular medical control of asthma, using inhaled corticosteroids (ICS), inhaled corticosteroids (equivalent doses of budesonide), asthma grade evaluation (mild, moderate and severe). FeNO and lung function tests were completed in the Zhongshan Hospital. ACT was assessed by the same sleep medicine specialist before PSG test in the Sleep Center. The total ACT score ranges from 5 to 25 points with higher score indicating good asthma control. Dose conversion: beclomethasone dipropionate 1,000 µg = budesonide 800 µg = fluticasone propionate 500 µg. The classification of Asthma Grade was according to Global Initiative for Asthma (GINA) guidelines [2015].
BQ and SBQ: before the PSG test, patients were asked to fill in the BQ and SBQ by themselves. The screening questionnaires were applied by one independent trained observer unaware of any patient’s clinical data. The BQ is a 10-item validated tool used for the screening of OSAHS risk. It includes five questions on snoring (category 1), four questions on daytime somnolence (category 2) and one question on the history of hypertension or BMI ≥30 kg/m2 (category 3). Subjects with positive two or more categories are accepted as high-risk subjects for OSA. The SBQ includes the four questions used in the STOP questionnaire plus four additional demographic queries, for a total of eight dichotomous (yes/no) questions related to the clinical features of sleep apnea [snoring (S), tiredness (T), observed apneas (O), high blood pressure (H), body mass index (BMI) over 35 kg/m2 (B), age over 50 years (A), neck circumference over 40 cm (N), and gender male (G)]. For each question, answering “yes” scores 1, a “no” response scores 0, and the total score ranges from 0 to 8. Patients can be classified for OSA risk based on their respective scores. In our study, the item of “BMI more than 35 kg/m2” was replaced with “BMI more than 30 kg/m2” to be comparable with BQ. There is no performance difference between Using BMI cutoff of 30 and cutoff of 35 for Asians (20). According to the previous literatures, subjects with 3 or more scores are accepted as high-risk subjects for OSA.
PSG test: PSG test was undertook in Sleep Center of Zhongshan Hospital. Sleep Time was 5 h at least monitored by Alice-4 18-channel PSG (Alice-4 Respironics, Pittsburgh, Pennsylvania, USA) in a same light- and temperature-controlled examination rooms. The reports were analyzed by another sleep medicine specialist. PSG tests were not taken in the acute exacerbation stage of asthma. PSG test items include the electroencephalogram, electrooculogram, submental electromyogram, electrocardiogram, oronasal airflow by a nasal pressure transducer, arterial oxygen saturation by transcutaneous pulse oximetry, thoracic and abdominal bands and a body position electrode. Sleep stages are divided into non-rapid-eye-movement (NREM) sleep and rapid-eye-movement (REM) sleep. The sleep monitor report parameters include four parts: sleep architecture, respiratory condition, SpO2 condition and position situation. AHI refers to the number of apneas and hypopneas per hour during sleep time: apnoea was defined as the absence of oronasal airflow for ≥10 seconds associated with continued or increased inspiratory effort and hypopnoea was defined as a ≥50% reduction in oronasal airflow accompanied by oxygen desaturation more than 4%. ODI refers to the total number of episodes of oxyhemoglobin desaturation ≥4% from the immediate baseline per hour during sleep time. REM (%TST) refers to the proportion of REM stage sleep time to total sleep time. Scoring of respiratory events was done according to 2007 American Academy of Sleep Medicine (AASM) criteria (21). AHI is the accepted international criteria to grade the severity of OSA according to the Chinese Respiratory Society (22) and 2007 AASM guidelines: 5≤ AHI <15, the grade is mild; 15≤ AHI <30, the grade is moderate; 30≤ AHI, the grade is severe.
Statistical analysis
Data were presented as means ± standard deviations and number (proportions %) unless otherwise stated. Two sample means comparison was assessed by independent sample t-test or Mann-Whitney rank sum test (for continuous variables) and Chi-square test or Fisher’s exact tests (for categorical variables). Multiple sets of samples comparison were assessed by one-way analysis of variance (ANOVA) or Mann-Whitney rank sum test (for continuous variables) and Chi-square test or Fisher’s exact tests (for categorical variables). The post hoc of ANOVA referred to the least-significant difference (LSD) test. Before the independent sample t-test or ANOVA for continuous variables, Kolmogorov-Smirnov test was tested for the data normality: if data does not conform to the normal distribution, rank sum test will be applied. Stepwise method will be chosen to screen variables. Univariate and multivariate logistic regression models were applied to identify the risk factors associated with OSA by odds ratio (OR) and a 95% confidence interval (CI). Covariates were entered into multivariate models if they were significant in univariate models. Enter method was used in the multivariate logistic regression model. Spearman correlation analyses were used to determine relationships between AHI with BQ and SBQ scores. Sensitivities, specificities, positive predictive value (PPV), and negative predictive value (NPV), positive and negative likelihood ratios (PLR and NLR), and area under the receiver operating characteristic (ROC) curve (AUC) were calculated including respective confidence intervals. Kappa test was used for testing the consistency of screening questionnaires and PSG results. Ordinal logistic regression analysis was applied to predict the OSA probability of various BQ and SBQ scores. All the results and figures were analyzed and established by SPSS 19.0 software package (SPSS, Inc., Chicago, IL, USA).
Results
Baseline demographic and medical characteristics
From January 2015 to January 2016, 123 out of 229 asthma patients in the Respiratory Clinic of Zhongshan Hospital were enrolled into the study. Eighty-four patients were excluded at the Respiratory Clinic and 22 patients’ data were deleted at the Sleep Center. Table 1 showed the baseline demographic and history characteristics of the enrolled population. The enrolled population consisted of 71 men (57.72%) and 52 women (42.28%). The PSG test diagnosed 20 (16.26%) subjects as severe OSA with AHI ≥30, 25 (20.33%) as moderate OSA with 15≤ AHI <30 and 33 (26.83%) as mild OSA with 5≤ AHI <15. There was a significant difference of Age, BMI, neck circumference, smoker, drinker, HBP between the OSA and non-OSA groups. All these clinical characteristics have been proved previously to be closely related with the occurrence and development of OSA by considerable epidemiology surveys. AHI and ODI are two frequently-used index to quantify the severity of OSA. The values of REM (%TST) of OSA and Non-OSA groups had no difference. Increasing OSA severity was associated with a higher BQ and SBQ scores and a higher prevalence of men, neck circumference ≥40, BMI ≥30, current and ex-drinker, hypertension, rhinitis and dyslipidemia. 49.59% asthma patients were mild grade, 43.9% were moderate and only 6.5% were severe. In the OSA group, inhaled glucocorticoids dose were slightly higher (387.37±132.21 vs. 320±96.48, P=0.044) and the proportion of using ICS was higher (54.55% vs. 41.46%) but without statistical significance (P=0.315). FEV1 and FEV1/FVC were slightly poorer in the OSA group. No other asthma functional characteristics differences were found to be different between OSA and non-OSA groups.
Table 1. Baseline demographic and medical history characteristics of 123 asthma patients.
| Variables | Total (n=123) | Non-OSA (n=45) | OSA (n=78) |
|---|---|---|---|
| Clinical characteristics | |||
| Men/Total | 71 (57.72) | 14 (31.11) | 57 (73.08) |
| Age (years) | 47.56±12.12 | 40.93±10.03 | 51.38±11.62 |
| Age ≥50 | 54 (43.9) | 8 (17.78) | 46 (58.97) |
| BMI (kg/m2) | 26.42±2.99 | 24.6±2.06 | 27.47±2.94 |
| BMI ≥30 | 15 (12.20) | 0 (0) | 15 (19.23) |
| BMI ≥35 | 2 (1.63) | 0 (0) | 2 (2.56) |
| Neck circumference (cm) | 36.27±2.97 | 33.82±2.22 | 37.67±2.38 |
| Neck circumference ≥40 | 19 (15.45) | 0 (0) | 19 (24.36) |
| Current and Ex-smoker | 37 (30.08) | 8 (13.33) | 29 (37.18) |
| Current and Ex-drinker | 73 (59.32) | 21 (46.34) | 52 (66.23) |
| HBP | 39 (31.36) | 6 (12.20) | 33 (41.56) |
| CHD | 26 (22.03) | 5 (12.20) | 21 (27.27) |
| Arrhythmia | 14 (11.02) | 4 (7.32) | 10 (12.99) |
| GERD | 28 (23.73) | 13 (31.71) | 15 (19.48) |
| Rhinitis | 80 (65.04) | 12 (26.67) | 68 (87.18) |
| Diabetes | 12 (10.17) | 2 (4.88) | 10 (12.99) |
| Dyslipidemia | 48 (40.68) | 8 (19.51) | 40 (51.95) |
| Asthma functional characteristics | |||
| Asthma onset age | 16.43±7.80 | 15.84±8.55 | 16.71±7.41 |
| FeNO (ppb) | 25.54±22.44 | 23.53±22.47 | 26.87±22.59 |
| Lung function data | |||
| FEV1/predicted% | 89.45±12.43 | 92.58±9.75 | 87.63±13.47 |
| FVC/predicted% | 90.51±11.72 | 92.51±9.24 | 89.36±12.85 |
| FEV1/FVC% | 77.23±9.42 | 79.91±9.26 | 75.68±9.22 |
| PEF/predicted% | 86.51±13.09 | 89.30±10.62 | 84.90±14.13 |
| Positive bronchial dilation test | 85 (69.11) | 30 (66.67) | 55 (71.51) |
| Regular medical control of asthma | 81 (66.1) | 31 (68.29) | 50 (64.94) |
| Using ICS | 62 (50.0) | 20 (41.46) | 42 (54.55) |
| Inhaled glucocorticoids, equivalent doses of budesonide (mcg/day) | 361.29±122.69 | 320.00±96.48 | 387.37±132.21 |
| Asthma grade | |||
| Mild | 61 (49.59) | 26 (57.78) | 35 (44.87) |
| Moderate | 54 (43.9) | 17 (37.78) | 37 (47.44) |
| Severe | 8 (6.5) | 2 (4.44) | 6 (7.69) |
| Questionnaire scales | |||
| Asthma control test | 20.71±1.74 | 20.87±1.97 | 20.6±1.58 |
| Berlin questionnaire | 1.16±0.94 | 0.53±0.59 | 1.52±0.91 |
| STOP-Bang questionnaire | 2.80±1.93 | 1.22±1.18 | 3.65±1.73 |
| Polysomnography data | |||
| AHI (/h) | 15.07±12.87 | 2.88±1.50 | 22.28±11.11 |
| ODI (/h) | 15.74±13.24 | 3.54±1.76 | 22.98±11.73 |
| REM (%TST) | 13.00±5.90 | 12.73±6.11 | 13.12±5.83 |
| Average SaO2 | 92.85±3.94 | 95.82±1.71 | 91.13±3.84 |
| Min SaO2 | 82.16±9.45 | 90.44±3.86 | 77.38±8.36 |
Data represent as mean ± SD or number (%). BMI, body mass index, BMI = Weight (Kg) ÷ Height2 (m2); FEV1, forced expiratory volume in one second; FVC, forced vital capacity; PEF, peak expiratory flow; HBP, high blood pressure; CHD, coronary heart disease; GERD, gastroesophageal reflux disease; FeNO, exhaled nitric oxide fraction; ICS, inhaled corticosteroids; AHI, apnea-hypopnea index; ODI, oxygen desaturation index; REM, rapid eye movement sleep; TST, total sleep time; SaO2, arterial oxygen saturation.
Univariate and multivariate logistic regression analyses were applied to identify risk factors of OSA in asthma patients (Table 2). Combined with the above results, rhinitis (OR =1.94; 95% CI: 1.23–3.04, P=0.004; adjusted OR =4.30; 95% CI: 1.50–12.37, P=0.007) and dyslipidemia (OR =1.70; 95% CI: 1.14–2.53, P=0.009; adjusted OR =2.75; 95% CI: 1.16–6.51, P=0.021) were associated with OSA in asthma patients after adjusting for sex, age, BMI, neck circumference, smoke, alcohol and HBP which are the definite risk factors of OSA. As a concomitant disease of asthma, the morbidity of rhinitis in asthma patients was quite high (65.04%), especially in OSA group, up to 87.18%. No asthma functional characteristics differences were found to be associated with OSA severity in the study.
Table 2. Logistic regression analysis between OSA and clinical, asthma functional characteristics.
| Variables | OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Clinical characteristics | ||
| Rhinitis | 1.94 (1.23–3.04)* | 4.30 (1.50–12.37)* |
| Dyslipidemia | 1.70 (1.14–2.53)* | 2.75 (1.16–6.51)* |
| CHD | 2.95 (1.03–8.47)* | 0.44 (0.058–3.93) |
| Arrhythmia | 1.51 (0.44–5.12) | 0.33 (0.04–2.99) |
| GERD | 0.59 (0.25–1.38) | 1.69 (0.40–7.13) |
| Diabetes | 3.16 (0.66–15.13) | 1.37 (0.13–14.31) |
| Asthma functional characteristics | ||
| Asthma onset age | 1.02 (0.98–1.07) | 1.97 (0.35–11.01) |
| FeNO (ppb) | 1.01 (0.99–1.02) | 1.02 (0.98–1.05) |
| FEV1/predicted% | 0.97 (0.94–1.00)* | 1.01 (0.93–1.09) |
| FVC/predicted% | 0.98 (0.95–1.01) | 1.02 (0.95–1.11) |
| FEV1/FVC% | 0.95 (0.91–0.99)* | 0.99 (0.91–1.07) |
| PEF/predicted% | 0.97 (0.95-1.00) | 1.00 (0.93–1.07) |
| Positive bronchial dilation test | 0.53 (0.25–1.13) | 0.27 (0.07–1.10) |
| Regular medical control of asthma | 0.85 (0.39-1.87) | 0.51 (0.10–2.72) |
| Using ICS | 1.05 (0.50–2.18) | 0.36 (0.08–1.63) |
Model was adjusted for sex, age, BMI, neck circumference, smoke, alcohol and HBP. *, P<0.05. OSA, obstructive sleep apnea; OR, odds ratio; CI, confidence intervals; CHD, coronary heart disease; GERD, gastroesophageal reflux disease; FeNO, exhaled nitric oxide fraction; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; PEF, peak expiratory flow; ICS, inhaled corticosteroids; BMI, body mass index; HBP, high blood pressure.
Predictive performance of BQ and SBQ to detect OSA in asthma patients
Before the PSG test, 123 patients filled in BQ and SBQ. The questionnaire characteristics of the study subjects are shown in Table 3. The mean score of the BQ was 1.02±0.88, and 34 subjects (27.64%) were classified as being at high risk for OSA with a score of ≥2. The mean score of the SBQ was 2.55±1.97, and 54 (43.9%) subjects were classified as being at high risk for OSA with a score of ≥3. AHI value was significantly correlated with SBQ items and BQ categories but the item of Do you often feel tired, fatigued, or sleepy during daytime (P=0.55) in SBQ and category 2 in BQ (P=0.51). The sensitivity, specificity, PPV, NPV, positive likelihood ratio (PLR), negative likelihood ratio (NLR), accuracy, Kappa and AUC of the two to predict OSA (cut-off points set as AHI of 5/h, 15/h, and 30/h) were shown in Table 4. As the main purpose of questionnaire screening is to identify patients at risk of moderate or severe OSA, where CPAP treatment is recommended, the predictive performance of BQ and SBQ at the cut-off as AHI of 15/h was particularly focused on.
Table 3. Subject responses and relationship between AHI with questionnaire items and scores.
| Questionnaire items | Subjects with positive response | Correlation coefficient | P |
|---|---|---|---|
| Subjects with SBQ score ≥3 | 54 (43.9) | ||
| SBQ score | 2.55±1.97 | 0.77* | <0.001 |
| Do you snore loudly (louder than talking or loud enough to be heard through closed doors)? | 51 (41.46) | 0.57 | <0.001 |
| Do you often feel tired, fatigued, or sleepy during daytime? | 30 (24.39) | −0.05 | 0.55 |
| Has anyone observed you stop breathing during your sleep? | 39 (31.71) | 0.72 | <0.001 |
| Do you have or are you being treated for high blood pressure? | 37 (30.08) | 0.34 | <0.001 |
| BMI more than 30 kg/m2? | 15 (12.20) | 0.49 | <0.001 |
| Age over 50 years old? | 54 (43.90) | 0.30 | 0.001 |
| Neck circumference greater than 40 cm? | 16 (13.01) | 0.50 | <0.001 |
| Gender male? | 71 (57.72) | 0.49 | <0.001 |
| Subjects with BQ score ≥2 | 34 (27.64) | ||
| BQ score | 1.02±0.88 | 0.62 | <0.001 |
| BQ category 1 | 51 (41.46) | 0.57 | <0.001 |
| BQ category 2 | 29 (23.58) | 0.06 | 0.51 |
| BQ category 3 | 45 (36.59) | 0.46 | <0.001 |
Data are presented as mean ± SD or as number (%). *, Spearman correlation analysis was used to determine the relationship. In the study, the item of “BMI more than 35 kg/m2” was replaced with “BMI more than 30 kg/m2” to be comparable with BQ. BQ, Berlin questionnaire; SBQ, STOP-Bang questionnaire; BMI, body mass index.
Table 4. Predictive indicators of BQ and SBQ to detect OSA.
| Predictive parameters | AHI ≥5 | AHI ≥15 | AHI ≥30 | |||||
|---|---|---|---|---|---|---|---|---|
| BQ | SBQ ≥3 | BQ | SBQ ≥3 | BQ | SBQ ≥3 | |||
| Prevalence of OSA, % | 63.41 | 36.59 | 16.26 | |||||
| Sensitivity, % | 42.3 (32.8–52.3) | 62.8 (52.9–72) | 60 (46.7–72.3) | 84.4 (72.8–92.5) | 80 (59.9–92.9) | 100 (86.1–) | ||
| Specificity, % | 97.8 (89.9–99.9) | 88.9 (78–95.5) | 91.0 (83.8–95.7) | 79.5 (70.5–86.7) | 82.5 (75.2–88.4) | 67.0 (58.6–74.6) | ||
| PPV, % | 97.1 (86.8–99.8) | 90.7 (81.5–96.3) | 79.4 (64.8–89.9) | 70.4 (58.5–80.4) | 47.1 (32.2–62.3) | 37.0 (26.1–49.1) | ||
| NPV, % | 49.4 (40.3–58.6) | 58.0 (47.4–68.1) | 80.0 (71.5–86.5) | 90.0 (81.8–95.1) | 95.5 (90–98.5) | 100 (95.8–) | ||
| PLR, % | 0.7 | 1.7 | 1.5 | 5.4 | 4 | |||
| NLR, % | 44 | 8 | 10.1 | 3.9 | 5.7 | 2.0 | ||
| Accuracy, % | 62.6 (54.8–69.9) | 72.4 (65–78.9) | 79.7 (72.8–85.5) | 81.3 (74.6–86.9) | 82.1 (75.5–87.6) | 72.4 (65–78.9) | ||
| AUC | 0.74 (0.65–0.82) | 0.83 (0.76–0.90) | 0.82 (0.75–0.90) | 0.91 (0.86–0.96) | 0.86 (0.78–0.94) | 0.92 (0.86–0.97) | ||
| Kappa | 0.332 | 0.465 | 0.538 | 0.613 | 0.488 | 0.398 | ||
Data are presented as average (95% confidence interval). BQ, Berlin questionnaire; SBQ, STOP-Bang questionnaire; OSA, obstructive sleep apnea; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio; AUC, area under the curve.
At the cut-off as AHI of 5/h, compared with BQ, SBQ had higher diagnostic sensitivity (62.82% vs. 42.31%), higher NPV (57.97% vs. 49.44%), higher PLR (1.69 vs. 0.73), higher accuracy (72.36% vs. 62.6%) and larger AUC (0.83 vs. 0.74). The kappa of SBQ was greater than BQ (0.465 vs. 0.332) indicating the consistency tendency of SBQ was better. Although SBQ had a better predictive performance than BQ to detect any OSA in asthma patients, the sensitivity and NPV were relatively low at the cut-off as AHI of 5/h.
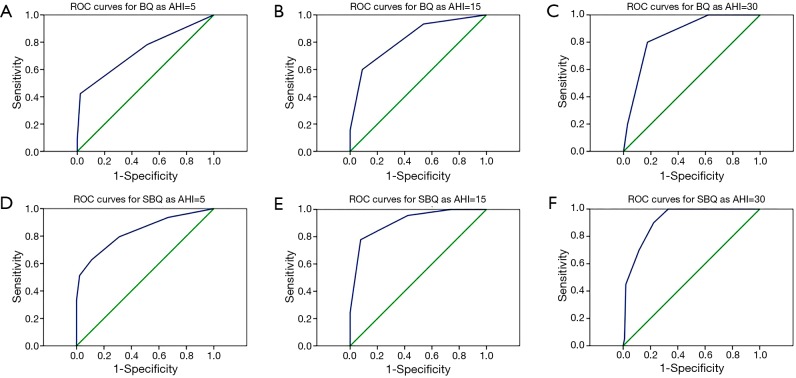
At the cut-off as AHI of 15/h, compared with BQ, SBQ had higher diagnostic sensitivity (84.44% vs. 60%), higher NPV (89.86% vs. 79.78%), higher PLR (5.43 vs. 1.5), higher accuracy (81.3% vs. 79.67%) and larger AUC (0.91 vs. 0.82). Compared with the cut-off as AHI of 5/h, the sensitivity, NPV, PLR, accuracy, Kappa and AUC of BQ and SBQ increased at the cut-off as AHI of 15/h respectively. The kappa of SBQ was greater than BQ (0.613 vs. 0.538) indicating the consistency tendency of SBQ was better. Both BQ and SBQ had an acceptable predictive performance to detect moderate and severe OSA in asthma patients at cut-off as AHI of 15/h and SBQ was better evidently. At the cut-off as AHI of 30/h, the sensitivity, NPV and AUC of BQ and SBQ increased significantly, meanwhile the Kappas of SBQ decreased to 0.398. ROC curves for BQ and SBQ as AHI =5, 15 and 30 are provided in the supplementary information (Figure S1).
Figure S1.
ROC curves for BQ and SBQ as AHI =5, 15 and 30. (A) ROC curves for BQ as AHI =5 (AUC =0.74); (B) ROC curves for BQ as AHI =15 (AUC =0.83); (C) ROC curves for BQ as AHI =30 (AUC =0.82); (D) ROC curves for SBQ as AHI =5 (AUC =0.91); (E) ROC curves for SBQ as AHI =15 (AUC =0.86); (F) ROC curves for SBQ as AHI =30 (AUC =0.92).
In conclusion, SBQ was a valid sleep questionnaire better than BQ to distinguish moderate and severe OSA (AHI =15).
Relationship between various SBQ scores and OSA probability in asthma patients
The probability of moderate and severe OSA was predicted by various SBQ scores in asthma patients (Figure 2). When the score was 3, the moderate and severe OSA probability of SBQ was 34.95%, the severe OSA probability was 7.08%; When the score was 7, the moderate and severe OSA probability of SBQ increased to 97.85%, the severe OSA probability increased to 86.58%. With the increasing of the questionnaire scores, the moderate and severe OSA probability of SBQ rose significantly. It indicated that the patients who have a higher score in the SBQ would have a greater probability of moderate and severe OSA.
Figure 2.
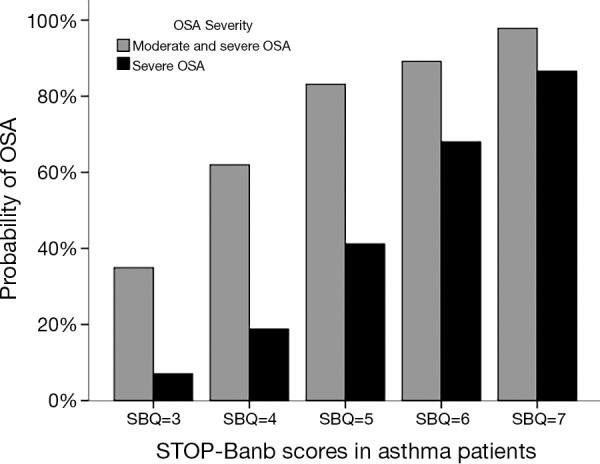
The relationship between the various STOP-Bang scores and OSA probability. OSA, obstructive sleep apnea.
Discussion
In the study, 123 asthma patients in the Respiratory Clinic were enrolled. Baseline demographic and medical characteristics were compared between the OSA and Non-OSA groups. The predictive performance of BQ and SBQ was evaluated and compared at cut-off points set as AHI of 5/h, 15/h, and 30/h before PSG test. Results showed that SBQ had a good predictive performance to detect moderate and severe OSA at the cut-off as AHI of 15/h. To the best of our knowledge, this is the first study to validate the predictive performance of the two most common OSA screening questionnaires—BQ and SBQ to detect OSA syndrome in asthmatic population.
The motivation of screening OSA in asthma patients is the high prevalence of OSA in asthma patients and the good effect of available treatment within asthma and OSA patients. As the “Overlap Syndrome” referred to OSA and COPD coexisting, the term of “Alternative Overlap Syndrome” is granted for the coexisting of Asthma and OSA (23). Their coexistence has synergistic effects on patient symptoms, response to therapy, and general outcomes. Asthma has been confirmed to be a risk factor of OSA occurrence and development in large sample clinical studies and OSA might aggravate asthma reciprocally. In clinic-based epidemiologic studies, OSA symptoms, such as sleepiness, snoring and apnea, were more frequently reported by patients with asthma than by the general population and high OSA prevalences were found in patients with difficult-to-control asthma. The prevalence of moderate-to-severe OSA (36.59%) in our asthmatic population sample is considerably higher than what has been reported in the general population (28.1%) (19). A study conducted on 1,941 subjects: 740 with asthma alone and 1,201 with asthma and allergic rhinitis. SBQ revealed that 52.6% of the subjects were at increased risk of OSA syndrome: 47.3% of subjects with asthma alone and 55.9% of patients with asthma and allergic rhinitis (24). In our study, SBQ (≥3) revealed that 43.9% of the subjects were at high risk of OSA.
As a significant comorbidity of asthma, OSA is associated with poor asthma control, frequent exacerbations, increased health resource utilization and poorer inpatient outcomes (5,25). Asthma patients with OSA are more likely to experience severe exacerbations than those without OSA. The AHI is significantly associated with the occurrence of severe asthma exacerbations (4). Researchers have found that long-term treatment of CPAP in patients with both asthma and obstructive sleep apnoea can decrease the asthma symptoms and improve asthma control and quality of life (9,10). Since evaluating OSA in patients with asthma has been included in the Guidelines for the Diagnosis and Management of Asthma (21), there is a need for clinical investigations of early and periodic detection of OSA among asthma populations. However patients with OSA have different patterns of clinical presentation, disturbed sleep, excessive daytime sleepiness and minimally symptomatic (26), which brings diagnosis confusion for clinical primary care. Asthma and OSA co-existing could make the presentation confused further. Considering the importance of OSA diagnosis and treatment among asthmatics, reliable, concise, and easy-to-use OSA screening tools can provide good choice instead of PSG test.
Although Berlin questionnaire has been a familiar sleep screening questionnaire all around the world, the results are conflicting in different study populations with sensitivity and specificity ranging from 0.43 to 0.89 and 0.33 to 0.79, respectively (27). In a sleep clinic population (28), BQ predicted an AHI ≥5 with a sensitivity of 0.73 and a specificity of 0.44 and sensitivity of BQ (0.80) was highest when the cut-off value of AHI was 30. In a large cohort of resistant hypertensive patients (29), the Berlin questionnaire had a low accuracy with the specificity, sensitivity, positive and NPV in detecting moderate-to-severe OSA was 40%, 69%, 58% and 50%, respectively and the PLR and NLR were 1.15 and 0.78, with a very low agreement (kappa =0.081). In our study, BQ showed relatively preferable predictive performances at the cut-off value of AHI as 15 with a relatively low sensitivity of 0.60 and a specificity of 0.91 and kappa =0.538. These various results can be explained by heterogeneous design of different studies as the type of PSG and its blind execution, different definitions of scoring criteria, the use of modified questionnaires, and populations at different baseline risks of having OSA (sleep clinic population, general population and special populations, such as hypertension and obstructive lung diseases). In summary, the Berlin questionnaire can improves the identification of patients with OSA, however, its performance seems to be variable and uncertain in studied populations.
The sensitivity of different questionnaires in predicting moderate-to-severe OSA varied from 54% to 93%, and SBQ showed the highest sensitivity (27). Another systematic review and meta-analysis showed that in the sleep clinic population, the sensitivity was 90%, 94% and 96% to detect any OSA (AHI ≥5), moderate-to-severe OSA (AHI ≥15), and severe OSA (AHI ≥30), respectively. With a stepwise increase of the STOP-Bang score to 4, 5, 6 and 7/8, the probability of severe OSA rose proportionally to 35%, 45%, 55% and 75%, respectively (19). In our study, when the score increased from 3 to 7, the moderate and severe OSA probability of SBQ increased from 34.95% to 97.85%, the severe OSA probability increased from 7.08% to 86.58%. The available data in the literature support that there is a correlation existing between a higher STOP-Bang score and the severity of OSA. Experts argue that the SBQ should be considered the optimal screening tool at the present time and that the score can be used for making more reasoned clinical decisions (30).
Compared with previous researches, in our study, a relatively low sensitivity and relatively high specificity of the SBQ were found in the asthmatics: At the cut-off as AHI of 15/h, the sensitivity and specificity were 84.4% and 79.5%, respectively. The clinical significance for sensitivity is to detect patients among actual patients and for specificity is to detect healthy subjects among actual non-patients. Compared with the sleep clinic patients and preoperative patients in the relevant studies, lower sensitivity and higher specificity were found in our study OSA subjects among asthmatics might be less likely to report that they feel sleepy during daytime meanwhile non-OSA subjects are more apt to complain their symptoms of sleepiness due to miscellaneous nocturnal asthmatic symptoms. These situations will decrease the sensitivity. Furthermore, in our study, only 12.20% have BMI ≥30 kg/m2 and the average BMI was 26.42±2.99 kg/m2. This was a generally non-obese population. Compared with obese population, non-obese population would have less OSA patients and screening questionnaires would screen less patients with OSA risk which could decrease sensitivity and increase specificity to varying degrees. Meanwhile, the subjects with AHI <15/h in the study include more young and slim female patients (45/78, 57.69%) will complaint less sleep symptoms and comorbidities which could increase the specificity. Silva et al. found that a reduced sensitivity and increased specificity of the questionnaire when used in the community setting as compared to the preoperative population (31). In a population-based cohort in Singapore also showed that the sensitivity of a STOP-Bang score of ≥3 was 66.2% to detect AHI ≥15 and 69.2% to detect AHI ≥30. The relatively high specificities were 74.7% and 67.1%, respectively (20). Compared with the general population in the relevant studies, higher sensitivity was found in our study. One of the reasons may be that asthmatics have a higher OSA morbidity which has an association with the sensitivity. Subjects in the sleep clinic usually have more sleep symptoms, such as feel tired, fatigued, or sleepy during daytime and preoperative patients would be older and be heavier, have more comorbidities, such as HBP. Both of these situations will increase the sensitivity. A recent population-based study found that the majority of subjects with moderate-to-severe OSA in the general population or community setting were mostly asymptomatic and not sleepy (26). In our study, AHI was not correlated with the SBQ item of Do you often feel tired, fatigued, or sleepy during daytime (P=0.55) and BQ category 2 on daytime somnolence (P=0.51). Recently the performance of SBQ was found to be different in men vs. women with type 2 diabetes mellitus (32): the sensitivity to identify men with AHI ≥15 was 74% with a specificity of 56%, while for women, the sensitivity reached only 29% with a specificity of 82%. The risk of OSA brought by male sex (or protective effect of female sex) is not proportionally reflected in the SBQ. Another important predictive in director is NPV which can be used to rule out patients with very little chance of moderate-to-severe OSA and to prioritize patients for further testing. In the study, at the cut-off as AHI of 15/h, the NPV of SBQ was 0.9: That means if subject’s SBQ score <3, the probability of AHI <15 is 0.9; at the cut-off as AHI of 30/h, the NPV of SBQ was 1.0: that means if your SBQ score <3, the probability of AHI <30 is 1.0.
Another two important sleep related questionnaires are Pittsburgh Sleep Quality Index and Epworth Sleep Scale questionnaires. In Simone Scarlata’ study, PSQI was not helpful in the pre-polysomnographic assessment of people with suspected OSAHS. ESS was significantly, but weakly, correlated with the AHI (AHI vs. ESS: R=0.308; P<0.001), whereas PSQI was not (R=0.037; P=0.581). Both PSQI and ESS performed unsatisfactorily: sensitivity 37.8% and 69.7%; Specificity 76.1% and 31.0% as AHI ≥5 (33). And the PSQI and ESS should no longer be used as a screening or diagnostic instrument for the four PSG-defined sleep disorders, especially in a low-risk population (34). Although in our study, patients were not evaluated with PSQI and ESS, hypersomnolence was considered as a variable or confounding factor which is known to influence the accuracy of screening questionnaires in a relevant manner. There are too many facets influencing the somnolence that might be an important reason for uncertainty about the accuracy or clinical utility of all potential screening tools (35).
In our study, 2007 AASM criteria were used for OSA scoring criteria. Apneas were defined as a 90% reduction in airflow for at least 10 s, and hypopneas were defined as a 30% reduction in airflow for at least 10 s with an oxyhemoglobin desaturation of either 3% [2012 AASM criteria (36)] or 4% [2007 AASM criteria (21)]. 2007 AASM criteria are known to be the most restrictive and least inclusive of the various published criteria. That is to say, non-OSA group could include OSA patients, asthmatic population could have a higher prevalence of OSA. In term of predictive performance of screening questionnaires, restrictive OSA scoring criteria might increase the sensitivity and decrease the specificity.
An important risk factor of OSA in asthma patients is rhinitis which acts as a bidirectional catalysator within the interaction of OSA and asthma. Nasal obstruction could be considered as one of most contributing factors. That is one of the reasons why asthma population has a higher OSA mortality. Recent a meta-analysis showed that patients who received intranasal corticosteroid therapy had a significant improvement in OSA although bias and heterogeneity of the selected RCTs existed (37). Both immunoglobulin E-mediated and irritant-induced inflammation in either airway location play a significant role in the OSA, rhinitis and asthma (38). Recent a population-based study has indicated that a history of allergic rhinitis is associated with increased risk of SDB in the elderly (39). In the study, rhinitis was associated with the increasing AHI and was an independent risk factor of OSA in asthma population (OR =1.665, 95% CI: 1.038–2.671, P=0.035; adjusted OR =3.080, 95% CI: 1.066–8.901, P=0.038). Further rhinitis treatment could be recommended to these asthma, OSA and rhinitis comorbidities patients.
Recent studies have suggested that the systemic and inhaled corticosteroid therapy on the pharyngeal airway could have a long-term impact on OSA risk and other UAW functions. A population-based cohort study in Taiwan showed that the risk of OSA is proportional to asthma control and patients with inhaled steroid treatment have a higher risk for OSA than those without steroid treatment (40). 16-week high dose FP treatment elicited Pcrit changes in healthy subjects with well-controlled asthma and stiff upper airways (41). In our study, inhaled glucocorticoids dose were slightly higher (=0.044) in the OSA group and the proportion of using ICS was higher but without statistical significance (P=0.315). The glucocorticoids effect on the pharyngeal airway includes two facets: dose and duration. However, in the clinical practice, the actual count of used medication is only followed in medical records and clinical studies. It was extremely difficult to quantify the glucocorticoids effect as a whole.
Asthma can induce OSA occurrence and development directly or indirectly and OSA could aggravate asthma reciprocally. Repeated episodes of upper airway obstruction during sleep lead to intermittent hypoxia and reoxygenation which could lead to local and systemic inflammation. Obviously, high levels of systemic inflammatory markers, such as TNF-α, C-reactive protein (CRP), and interleukin-6 (IL-6), could predispose to asthma and increased the risk of acute or sudden-onset fatal asthma exacerbations (42). The association among OSA/obesity, GERD, underlying asthma and a complex interplay of transcription factors, such as T cells, macrophages, neutrophils, and endothelial cells determine the degree of airway inflammation and the severity of airway and vascular inflammatory processes (43). Gastroesophageal reflux disease (GERD) is another indispensable link between OSA and asthma. Upper airway obstruction can predispose to retrograde movement of gastric contents and induce acid reflux which plays a role in triggering asthma symptoms. OSA was associated with an increased risk of Barrett’s Esophagus, potentially through BMI and GERD independent mechanisms (44). The potential mechanisms involving how GERD trigger asthma are micro-aspiration, acid stimulation of the oesophagus and vagal nerve stimulation. However, the association among these three conditions remains complex.
The limitation of present study is the asthma subjects were enrolled from respiratory clinic not from population-based community and majority of them were mild and moderate grades, and non-obese population, our conclusion could be extended to these asthmatic populations only. And during the whole study, clinical characteristics and asthma functional characteristics were based on patients’ subjective reports and medical record. There were a small number of subjects enrolled in the study.
Conclusions
In the study, we studied the predictive performance of BQ and SBQ in asthmatic population. Although there were many limitations in our research, SBQ could be used as a screening tool for detecting moderate and severe OSA in asthma patients which should be validated in larger population sample. There is an urgent need for clinical investigations of early and periodic detection of OSA among asthma populations.
Acknowledgements
Funding: This work is supported by a research grant from Shanghai Committee of Science and Technology (No. 13430720500), Shanghai Leading Academic Discipline Project (No. B115) and National Natural Science Foundation of China (No. 81570081).
Ethical Statement: The study was approved by the ethics committees of Fudan University Zhongshan Hospital (B2012-073R), Shanghai, China.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Loftus PA, Wise SK. Epidemiology of asthma. Curr Opin Otolaryngol Head Neck Surg 2016;24:245-49. 10.1097/MOO.0000000000000262 [DOI] [PubMed] [Google Scholar]
- 2.Becker AB. Challenges to treatment goals and outcomes in pediatric asthma. J Allergy Clin Immunol 2002;109:S533-8. 10.1067/mai.2002.124567 [DOI] [PubMed] [Google Scholar]
- 3.Tay TR, Radhakrishna N, Hore-Lacy F, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology 2016;21:1384-90. 10.1111/resp.12838 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Liu K, Hu K, et al. Impact of obstructive sleep apnea on severe asthma exacerbations. Sleep Med 2016;26:1-5. 10.1016/j.sleep.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 5.Becerra MB, Becerra BJ, Teodorescu M. Healthcare burden of obstructive sleep apnea and obesity among asthma hospitalizations: Results from the US-based Nationwide Inpatient Sample. Respir Med 2016;117:230-36. 10.1016/j.rmed.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 6.Kim MY, Jo EJ, Kang SY, et al. Obstructive sleep apnea is associated with reduced quality of life in adult patients with asthma. Ann Allergy Asthma Immunol 2013;110:253-7, 257.e1. [DOI] [PubMed]
- 7.Abdul Razak MR, Chirakalwasan N. Obstructive sleep apnea and asthma. Asian Pac J Allergy Immunol 2016;34:265-71. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharjee R, Choi BH, Gozal D, et al. Association of adenotonsillectomy with asthma outcomes in children: a longitudinal database analysis. Plos Med 2014;11:e1001753. 10.1371/journal.pmed.1001753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kauppi P, Bachour P, Maasilta P, et al. Long-term CPAP treatment improves asthma control in patients with asthma and obstructive sleep apnoea. Sleep and Breathing 2016;20:1217-24. 10.1007/s11325-016-1340-1 [DOI] [PubMed] [Google Scholar]
- 10.Serrano-Pariente J, Plaza V, Soriano JB, et al. Asthma outcomes improve with continuous positive airway pressure for obstructive sleep apnea. Allergy 2017;72:802-12. 10.1111/all.13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taillé C, Rouvel-Tallec A, Stoica M, et al. Obstructive Sleep Apnoea Modulates Airway Inflammation and Remodelling in Severe Asthma. PLoS One 2016;11:e0150042. 10.1371/journal.pone.0150042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teodorescu M, Broytman O, Curran-Everett D, et al. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. J Allergy Clin Immunol Pract 2015;3:566-75.e1. 10.1016/j.jaip.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seicean S, Negishi K, Negishi T, et al. Obstructive sleep apnea and asthma co-morbidities are independent predictors of new heart failure and atrial fibrillation in patients with diabetes mellitus and preserved ejection fraction. J Am Coll Cardiol 2012;59:E1048 10.1016/S0735-1097(12)61049-3 [DOI] [Google Scholar]
- 14.Ferguson S, Teodorescu MC, Gangnon RE, et al. Factors associated with systemic hypertension in asthma. Lung 2014;192:675-83. 10.1007/s00408-014-9600-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TY, Lo YL, Lin SM, et al. Obstructive sleep apnoea accelerates FEV1 decline in asthmatic patients. BMC Pulm Med 2017;17(55). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teodorescu M, Barnet JH, Hagen EW, et al. Association between asthma and risk of developing obstructive sleep apnea. JAMA 2015;313:156-64. 10.1001/jama.2014.17822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485-91. 10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- 18.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire - A tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812-21. 10.1097/ALN.0b013e31816d83e4 [DOI] [PubMed] [Google Scholar]
- 19.Nagappa M, Liao P, Wong J, et al. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0143697. 10.1371/journal.pone.0143697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan A, Yin JD, Tan LW, et al. Predicting obstructive sleep apnea using the STOP-Bang questionnaire in the general population. Sleep Med 2016;27-28:66-71. 10.1016/j.sleep.2016.06.034 [DOI] [PubMed] [Google Scholar]
- 21.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma–Summary Report 2007. J Allergy Clin Immunol 2007;120:S94-138. 10.1016/j.jaci.2007.09.029 [DOI] [PubMed] [Google Scholar]
- 22.He Q, Chen B. The interpretation of the guideline of obstructive sleep apnea hypopnea syndrome. Chinese Journal of tuberculosis and Respiratory Diseases 2012;35:7-8. [PubMed] [Google Scholar]
- 23.Ioachimescu OC, Teodorescu M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology 2013;18:421-31. 10.1111/resp.12062 [DOI] [PubMed] [Google Scholar]
- 24.Braido F, Baiardini I, Lacedonia D, et al. Sleep Apnea Risk in Subjects With Asthma With or Without Comorbid Rhinitis. Respir Care 2014;59:1851-56. 10.4187/respcare.03084 [DOI] [PubMed] [Google Scholar]
- 25.T ten Brinke A, Sterk PJ, Masclee AA, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J 2005;26:812-8. [DOI] [PubMed]
- 26.Arnardottir ES, Bjornsdottir E, Olafsdottir K A, et al. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J 2016;47:194-202. 10.1183/13993003.01148-2015 [DOI] [PubMed] [Google Scholar]
- 27.Jonas DE, Amick HR, Feltner C, et al. Screening for Obstructive Sleep Apnea in Adults: An Evidence Review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US), 2017; Report No.: 14-05216-EF-1. [Google Scholar]
- 28.Ulasli SS, Gunay E, Koyuncu T, et al. Predictive value of Berlin Questionnaire and Epworth Sleepiness Scale for obstructive sleep apnea in a sleep clinic population. Clin Respir J 2014;8:292-96. 10.1111/crj.12070 [DOI] [PubMed] [Google Scholar]
- 29.Margallo VS, Muxfeldt ES, Guimarães GM, et al. Diagnostic accuracy of the Berlin questionnaire in detecting obstructive sleep apnea in patients with resistant hypertension. J Hypertens 2014;32:2030-6; discussion 2037. 10.1097/HJH.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 30.Chung F, Liao P, Farney R. Correlation between the STOP-Bang Score and the Severity of Obstructive Sleep Apnea. Anesthesiology 2015;122:1436-37. 10.1097/ALN.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 31.Silva GE, Vana KD, Goodwin JL, et al. Identification of patients with sleep disordered breathing: comparing the four-variable screening tool, STOP, STOP-Bang, and Epworth Sleepiness Scales. J Clin Sleep Med 2011;7:467-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westlake K, Plihalova A, Pretl M, et al. Screening for obstructive sleep apnea syndrome in patients with type 2 diabetes mellitus: a prospective study on sensitivity of Berlin and STOP-Bang questionnaires. Sleep Med 2016;26:71-6. 10.1016/j.sleep.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 33.Scarlata S, Pedone C, Curcio G, et al. Pre-polysomnographic assessment using the Pittsburgh Sleep Quality Index questionnaire is not useful in identifying people at higher risk for obstructive sleep apnea. J Med Screen 2013;20:220-26. 10.1177/0969141313511591 [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama T, Mizuno T, Kojima M, et al. Criterion validity of the Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale for the diagnosis of sleep disorders. Sleep Med 2014;15:422-29. 10.1016/j.sleep.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 35.Jonas DE, Amick HR, Feltner C, et al. Screening for Obstructive Sleep Apnea in Adults Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017;317:415-33. 10.1001/jama.2016.19635 [DOI] [PubMed] [Google Scholar]
- 36.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med 2012;8:597-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu HT, Lin YC, Kuan YC, et al. Intranasal corticosteroid therapy in the treatment of obstructive sleep apnea: A meta-analysis of randomized controlled trials. Am J Rhinol Allergy 2016;30:215-21. 10.2500/ajra.2016.30.4305 [DOI] [PubMed] [Google Scholar]
- 38.Calais CJ, Robertson BD, Beakes DE. Association of allergy/immunology and obstructive sleep apnea. Allergy Asthma Proc 2016;37:443-9. 10.2500/aap.2016.37.4001 [DOI] [PubMed] [Google Scholar]
- 39.Kim SH, Won HK, Moon SD, et al. Impact of self-reported symptoms of allergic rhinitis and asthma on sleep disordered breathing and sleep disturbances in the elderly with polysomnography study. PLoS One 2017;12:e0173075. 10.1371/journal.pone.0173075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen TC, Lin CL, Wei CC, et al. Risk of Obstructive Sleep Apnea in Adult Patients with Asthma: A Population-Based Cohort Study in Taiwan. PLoS One 2015;10:e0128461. 10.1371/journal.pone.0128461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teodorescu M, Xie A, Sorkness CA, et al. Effects of Inhaled Fluticasone on Upper Airway during Sleep and Wakefulness in Asthma: A Pilot Study. J Clin Sleep Med 2014;10:183-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadeem R, Molnar J, Madbouly EM, et al. Serum Inflammatory Markers in Obstructive Sleep Apnea: A Meta-Analysis. J Clin Sleep Med 2013;9:1003-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasha S, Kumar S, Chatterjee AB, et al. An obstructive sleep apnea primer What the practicing allergist needs to know. Ann Allergy Asthma Immunol 2017;118:259-68. 10.1016/j.anai.2016.07.033 [DOI] [PubMed] [Google Scholar]
- 44.Leggett CL, Gorospe EC, Calvin AD, et al. Obstructive Sleep Apnea Is a Risk Factor for Barrett's Esophagus. Clin Gastroenterol Hepatol 2014;12:583-88. 10.1016/j.cgh.2013.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]