Abstract
Background
the right- and left-approach open esophagectomies remain the general procedures among patients with operable thoracic esophageal squamous cell carcinoma (ESCC). The choice between the two approaches for elderly patients is controversial.
Methods
we performed a 1:1 propensity score matching (PSM) analysis to compare the impact of right- and left-approach esophagectomies on survival and perioperative complications of elderly ESCC patients. Patients aged over 70 receiving esophagectomy to treat the thoracic ESCC were retrospectively retrieved.
Results
a total of 276 patients were included in the study. Among them, 75 (27.2%) patients received right-approach esophagectomy. After match, 114 patients (57 pairs) undertook right or left-approach esophagectomy displayed no difference among clinicopathological characteristics. Both the overall survival (54.6% vs. 32.6%, P=0.036) and disease-free survival (52.7% vs. 20.2%, P=0.021) were significant better in right-approach group, along with better lymph node resection, and lower incidence of recurrence. However, increased incidences of postoperative pneumonia (P=0.040), respiratory failure (P=0.028), and sub-clinical anastomotic leak (P=0.032) were found in right-approach group as well, although the perioperative mortality was similar between groups.
Conclusions
Right-approach esophagectomy should be accepted as a preferential surgical approach for elderly patients with ESCC.
Keywords: Surgical approaches, elderly patient, esophageal squamous cell carcinoma
Introduction
Aging and longer life expectancy leads to an increased number of patients with esophageal squamous cell carcinoma (ESCC) at elderly ages. Surgical resection remains a pivotal curative treatment for ESCC patients so far (1-4). Elderly patients have worse physical status and more attendant comorbidities than young patients, which usually requires surgeons to choose a less aggressive surgical procedure. Comparing to open esophagectomy, the minimal invasive esophagectomy, with lower incidence of morbidity and survival benefits, might be a preferential technique for elderly ESCC patients (5). Yet, no randomized trials have assessed whether minimally invasive esophagectomy improves outcome among elderly patients when compared with open surgery. Furthermore, open procedures may be preferred if the patient has previous thoracic, abdominal surgery or difficulty in lymph node dissection.
The Ivor-Lewis, Mckeown, Sweet, and left-approach dual-incisional (chest and neck) esophagectomies are the most commonly used open procedures for middle and lower thoracic ESCC so far. However, right- and left-approach procedures act like “double-blade swords”: Sweet esophagectomy has been applied to treat middle or lower thoracic ESCC, though it has the disadvantage on lymphadenectomy of the upper mediastinum; on the other hand, it is difficult to dissect the right mediastinal lymph node through the left approach. Inversely, Mckeown and Ivor-Lewis esophagectomy can provide extended lymph node dissection, however they are applied less often among elderly patients, for potential higher risks of perioperative complications. There have been no standard criteria or guideline for choosing the favorable approach for elderly patients in clinical practice. Therefore, we perform the propensity matched study to compare right- or left-approach esophagectomy for elderly patients with middle or lower thoracic ESCC, based on evaluations on the risk of postoperative complications and prognostic impacts.
Methods
Patient selection
Between June 1990 and June 2010, a total of 304 ESCC patients aged over 70 undertook esophagectomy in thoracic surgery department of Sun Yat-sen University Cancer Center. Patients with less than 7 dissected lymph nodes were excluded. This study was approved by the ethics committee of Sun Yat-sen University Cancer Center (No. B2015-048-01).
Preoperative workup
Preoperative evaluations include patient history taking, physical examination, and detailed risk assessments based on history of chronic pulmonary, cardiovascular, hepatic and renal disease. All patients had routine biochemical profile, electrocardiography, chest X-ray, pulmonary function tests with spirometry, and arterial blood gas analysis. Staging techniques included neck, chest and abdominal CT, abdominal ultrasonography, barium esophagography, endoscopy of the entire upper gastrointestinal tract with biopsy. Endoscopic ultrasonography was routinely performed after 2005 and positron emission tomography was applied when patients have signs or symptoms of distant metastasis.
Surgical procedure and postoperative care
Briefly, the Sweet procedure is performed through a single left-sided thoracic incision at the sixth or seventh intercostal space, while left-approach dual-incisional procedure has an extra neck incision. And then the tumor is resected together with at least 5cm of proximal esophagus. The stomach is mobilized and perigastric lymph nodes are dissected through trans-diaphragm incision. The alimentary tract is reconstructed by stomach pull-up technique, and esophagogastric anastomosis is fashioned at either sub- or supra-aortic space. The distal extreme of the feeding tube is placed in the jejunum, along with a nasogastric tube.
Mckeown or Ivor-Lewis procedure is right-approach tri-incisional or dual-incisional esophagectomy. Firstly, the patient is positioned in the left lateral decubitus. The esophagus is mobilized through the incision in the fourth or fifth intercostal space and tumor is then resected. The preventive thoracic duct ligation will be conducted if thoracic duct injury is suspected. And then, the patient is placed in the supine position. The abdominal cavity is accessed through an upper midline abdominal incision. The stomach is mobilized after ligating left gastric artery. Then the stomach is reconstructed to form a “gastric tube”, which will be pull-up to fashion the intrathoracic anastomosis. In McKeown esophagectomy, the cervical incision is made along the anterior border of the sternocleidomastoid muscle, in order to build the cervical anastomosis through the retrosternal pathway.
Extensive lymph node dissection in the posterior mediastinum and abdomen is systematically performed, therefore, except for those with very early stage of esophageal cancer, usually more than 7 lymph nodes were taken during the operation. Cervical LN sampling is routinely performed during the McKeown and left-approach dual-incisional surgery. Patients were taken care of by a medical team consisting of thoracic surgeons, specialized nurses and nutritionists.
After patients recovered from anesthesia, they were immediately admitted to the intensive care unit. Postoperative pulmonary complications were defined as pneumonia, respiratory failure, or acute respiratory distress syndrome (ARDS). Cardiovascular complications such as arrhythmia and heart failure were recorded. Clinical anastomotic leakage was diagnosed with apparent clinical features, confirmed by esophagography, endoscopy or methylene blue test; while sub-clinical anastomotic leakage was identified as a restricted leakage around the anastomosis, with no septic complications and heals with prolongation of enteral feeding up to 20 days after surgery. Other complications such as delayed gastric emptying, wound infection, hoarseness, chylothorax and pleural effusion and hemorrhage were recorded. Postoperative death was defined as death within 90 days of surgery (6).
Follow-up
A follow-up examination is generally carried out every 3 months for the first year, every 6 months for the next two years and once a year thereafter. Telephone follow-up is conducted with those who do not come to the outpatient clinic regularly. The overall survival time is measured in months from the date of surgery to the date of death or last follow-up. Yet, the disease-free-survival time is calculated as the duration between the date of surgery and the date patient developed tumor recurrence. Patients lost to follow-up or survived at the time of last contact were considered to be censored. Routine follow-up included physical examination, blood chemistry analysis, blood tumor markers, esophagography, and CT; bone scan or cranial MRI scan will be applied when necessary to detect recurrence and/or metastasis.
Data collection
Demographics and clinicopathologic characteristics including age, gender, smoking and alcohol abusing history, preoperative or postoperative complications, surgical approaches, tumor locations, tumor grades, adjuvant treatments and pathological stages were retrospectively collected. Preoperative comorbidities, such as hypertension, coronary heart disease (CHD), arrhythmia, chronic obstructive pulmonary disease (COPD), diabetes, tuberculosis, and cerebral infarction were recorded. And other comorbidities, including gastric ulcer, hepatitis, lithiasis, benign prostatic hyperplasia, and rheumatism were also reviewed. The staging classification used for this analysis were the seventh edition of the American Joint Committee on Cancer.
Propensity score matching (PSM) and statistical analysis
The 1:1 PSM was performed using the nearest neighbor matching method with STATA 12.0 software (Stata Corp., College Station, TX, USA) by characteristics including age, gender, tumor location, pathological T stage, pathological N stage, smoking, alcohol consumption, preoperative comorbidities, surgical approaches, and adjuvant therapy.
The statistical analyses were performed using the SPSS 19.0 software package (SPSS Standard version 19.0, SPSS, Chicago, IL, USA). The distribution differences of baseline characteristics were compared with Pearsonχ2 test or Wilcoxon rank sum test. The Kaplan- Meier method and log-rank test was used to plot the survival curve and calculate the survival differences between groups. P<0.05 were considered to represent a statistically significant difference.
Results
Patient characteristics
Two hundred and seventy-six out of 304 elderly patients with thoracic ESCC, underwent Mckeown, Ivor-Lewis, Sweet, and left-approach dual-incisional procedures were retrospectively included in the study. The median age was 74 (ranging from 71 to 88 years), and 203 (73.6%) patients were male gender. The left-approach procedure was performed on 201 (72.8%) patients, and the rest 75 patients received right-approach surgery. The clinicopathological characteristics of the entire cohort were summarized in Table 1. Age (P=0.006) and tumor location (P<0.001) are the two factors having distribution differences in right- and left-approach groups (Table 1). Thus, the right-approach surgery tended to be chosen for patients younger than 74-year old with upper thoracic ESCC. Regarding preoperative complications, more patients had arrhythmia (P=0.011), along with higher percentages of other cardiovascular comorbidities, COPD, and diabetes in left-approach group; no significant differences were found in patient smoking or alcohol history between the two groups (Table 2).
Table 1. The clinicopathological characteristics of patients of the entire cohort.
| Variable | Cases (n=276) | Surgical approaches (%) | P value | |
|---|---|---|---|---|
| Right (n=75) | Left (n=201) | |||
| Age | 0.006 | |||
| ≥74* | 123 | 26 (34.7) | 107 (53.3) | |
| <74 | 153 | 49 (65.3) | 94 (46.7) | |
| Gender | 0.507 | |||
| Male | 203 | 53 (70.7) | 150 (74.6) | |
| Female | 73 | 22 (29.3) | 51 (25.4) | |
| Tumor location | <0.001 | |||
| Upper | 19 | 15 (20.0) | 4 (2.0) | |
| Middle | 175 | 52 (69.3) | 123 (61.2) | |
| Lower | 82 | 8 (10.7) | 74 (36.8) | |
| Differentiation | 0.434 | |||
| Well | 62 | 18 (24.0) | 44 (21.9) | |
| Moderate | 124 | 37 (49.3) | 87 (43.3) | |
| Poor | 90 | 20 (26.7) | 70 (34.8) | |
| pT stage | 0.492 | |||
| T1 | 18 | 5 (6.7) | 13 (6.5) | |
| T2 | 70 | 22 (29.3) | 48 (23.9) | |
| T3 | 167 | 45 (60.0) | 122 (60.7) | |
| T4a | 21 | 3 (4.0) | 18 (9.0) | |
| pN stage | 0.968 | |||
| pN0 | 144 | 38 (50.7) | 106 (52.7) | |
| pN1 | 81 | 22 (29.3) | 59 (29.4) | |
| pN2 | 39 | 11 (14.7) | 28 (13.9) | |
| pN3 | 12 | 4 (5.3) | 8 (4.0) | |
| Pathological stage | 0.107 | |||
| pI | 28 | 3 (4.0) | 25 (12.4) | |
| pII | 129 | 39 (52.0) | 90 (44.8) | |
| pIII | 119 | 33 (44.0) | 86 (42.8) | |
*, mean age is 74.
Table 2. The preoperative characteristics of patients of the entire cohort.
| Variables | Surgical approaches (%) | P value | |
|---|---|---|---|
| Right (n=75) | Left (n=201) | ||
| Smoking | 42 (56.0) | 111 (55.2) | 0.908 |
| Alcohol | 12 (16.0) | 50 (24.9) | 0.116 |
| Cardiovascular comorbidities | |||
| Hypertension | 15 (16.0) | 48 (23.9) | 0.494 |
| CHD | 5 (6.7) | 16 (8.0) | 0.718 |
| Arrhythmia | 7 (9.3) | 46 (22.9) | 0.011 |
| Pulmonary comorbidities | |||
| COPD | 2 (2.6) | 12 (6.0) | 0.364 |
| Tuberculosis | 7 (9.3) | 15 (7.5) | 0.610 |
| Cerebral infarction | 7 (9.3) | 20 (10.0) | 0.878 |
| Diabetes | 3 (4.0) | 21 (10.4) | 0.091 |
CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease.
Patient baseline clinicopathological characteristics and preoperative comorbidities could greatly affect the incidence of postoperative complication and long-term survival, especially among elderly patients, thus we performed a 1:1 PSM analysis to counteract the variances when exploring the optimal surgical procedure for elderly ESCC patients. Features, including age, gender, tumor location, pT stage, pN stage, smoking, alcohol consumption, preoperative comorbidities, surgical approaches, and adjuvant therapy, were applied as matching parameters in PSM. After match, 114 patients (57 pairs) were eligible for analysis. No patient in the paired-cohort had diabetes, COPD, or cerebral infarction. The distribution of clinicopathological features in matched groups had no significant difference (Table 3).
Table 3. The baseline clinicopathological characteristics of the paired-cohort.
| Variables | Surgical approaches | P values | |
|---|---|---|---|
| Right (n=57) | Left (n=57) | ||
| Age* | 73.0 [71–79] | 72.4 [71–79] | 0.756 |
| Gender (M/F) | 41/16 | 40/17 | 0.836 |
| Tumor location n,(%) | 0.508 | ||
| Middle | 51 (89.5) | 53 (93.0) | |
| Lower | 6 (10.5) | 4 (7.0) | |
| pT stage, n (%) | 1.000 | ||
| T1 | 5 (8.8) | 5 (8.8) | |
| T2 | 19 (33.3) | 19 (33.3) | |
| T3 | 32 (56.1) | 31 (54.4) | |
| T4 | 1 (1.8) | 2 (3.5) | |
| pN stage, n (%) | 0.772 | ||
| N0 | 31 (54.4) | 28 (49.1) | |
| N1 | 14 (24.6) | 19 (33.3) | |
| N2 | 8 (14.0) | 7 (12.3) | |
| N3 | 4 (7.0) | 3 (5.3) | |
| Comorbidity, n (%) | |||
| Hypertention | 11 (19.3) | 13 (22.8) | 0.646 |
| CHD | 2 (3.5) | 1 (1.8) | 1.000 |
| Tuberculosis | 4 (7.0) | 5 (10.6) | 1.000 |
| Arrhythmia | 0 (0.0) | 1 (1.8) | 1.000 |
| Smoking | 33 (57.9) | 32 (56.1) | 0.850 |
| Alcohol | 12 (21.1) | 14 (24.6) | 0.655 |
| Adjuvant therapy | 0 (0.0) | 1 (1.8) | 1.000 |
*, age, mean [range]; M/F, male/female; CHD, coronary heart disease.
Prognostic impact of surgical approach on elderly ESCC patients
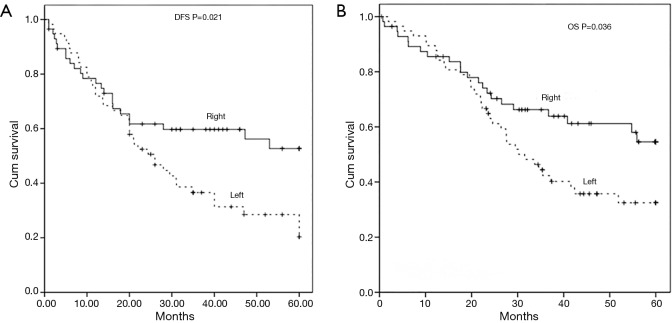
The median follow-up duration was 32.6 months (range, 1–60 months) in paired-cohort. The median disease-free survival in left-approach group was 26±4.1 months (95% CI, 18.0–34.0 months). Fewer than half of the patients in the right-approach group had recurrence during follow-up, thus the median disease-free survival time was greater than the median follow-up time. The 5-year disease-free survival rate in right-approach group was 52.7%, which was significantly higher than the rate 20.2% in left-approach group (P=0.021, Figure 1A). Moreover, patients in right-approach group had a significantly better 5-year overall survival (OS, 54.6% vs. 32.6%, P=0.036, Figure 1B) than those in left-approach group.
Figure 1.
Associations between surgical approaches and the survival of elderly patient in paired-cohort. The 5-year disease-free survival (A) and overall survival (B) of patients in paired right- and left-approach groups.
Effects of surgical approaches on postoperative complications
After match, the right-approach esophagectomy provided better lymph node resections (23.7±13.7 vs. 14.8±9.0, P<0.001), however, the operative time and length of ICU stay were significantly longer (P=0.012, Table 4). The incidence of postoperative pneumonia (P=0.040), respiratory failure (P=0.028) and subclinical anastomotic leakage (P=0.032) were higher in right-approach group. Yet, the perioperative mortality rate had no significant difference between the two groups.
Table 4. The postoperative patient characteristics of the paired-cohort.
| Variables | Surgical approaches | P value | |
|---|---|---|---|
| Right | Left | ||
| ICU stay (days) | 3.8±2.5 | 2.4±1.8 | 0.012 |
| Operation time (hours) | 6.2±2.0 | 3.2±0.8 | <0.001 |
| Number of lymph node | 23.7±13.7 | 14.8±9.0 | <0.001 |
| Perioperative death, n (%) | 4 (7.0) | 2 (3.5) | 0.402 |
| Secondary surgery, n (%) | 1 (1.8) | 1 (1.8) | 1.000 |
| Cardiac complications, n (%) | |||
| Arrhythmia | 5 (8.8) | 5 (8.8) | 1.000 |
| Heart failure | 4 (7.0) | 3 (5.3) | 0.696 |
| Pulmonary complications, n (%) | |||
| Pneumonia | 13 (22.8) | 5 (8.8) | 0.040 |
| ARDS | 1 (1.8) | 0 (0.0) | 1.000 |
| Respiratory failure | 7 (12.3) | 1 (1.8) | 0.028 |
| Gastrointestinal complications, n (%) | |||
| Clinical anastomotic leak | 4 (7.0) | 1 (1.8) | 0.364 |
| Sub-clinical anastomotic leak | 8 (14.0) | 1 (1.8) | 0.032 |
| Delayed gastric emptying | 2 (3.5) | 1 (1.8) | 1.000 |
| Other complications, n (%) | |||
| Wound infection | 3 (5.3) | 2 (3.5) | 1.000 |
| Hoarseness | 3 (5.3) | 0 (0.0) | 0.234 |
| Chylothorax | 0 (0.0) | 0 (0.0) | 1.000 |
| Pleural effusion | 8 (14.0) | 6 (10.5) | 0.568 |
| Haemorrhage | 0 (0.0) | 1 (1.8) | 1.000 |
ICU, intensive care unit; ARDS, acute respiratory distress syndrome.
Discussions
The PSM analysis revealed that right-approach esophagectomy can be accepted as a beneficial choice for elderly patients, because it provides better lymph node dissections, and extended disease-free survival along with longer overall survival. Additionally, though right-approach procedures come with the raised incidences of pneumonia, respiratory failure and sub-clinical anastomotic leak, they do not increase the risk of severe postoperative complications, and incidence of postoperative mortality.
Open procedures have been the foundation for esophagectomy. Nevertheless, the video-assisted thoracoscopic surgeries, with shorter hospital stay, decreased morbidity, and improved postoperative recovery and overall survival (7,8), might be preferential techniques for general ESCC patients. Yet, the superiority of perioperative or long-term benefits coming with video-assisted thoracoscopic surgery over open procedures to elderly patients remains uncertain. On the other hand, open procedures may be preferred if the patient has previous thoracic, abdominal surgery or difficulty in lymph node dissection.
Although studies differ in the definition of elderly patients, increased age is an adverse factor against esophagectomy and postoperative recovery of patients with ESCC (9,10). In the current series, we defined elderly as greater than 70 years of age for that it was the criteria of “old” in a majority of published studies (9,11,12) and the study results would be comparable if the same criteria was applied. Furthermore, the mean age of patients with ESCC treated in our cancer center was 63-years old and patients aged over 70 might have increased incidence of pulmonary complications, or even higher perioperative mortality rates.
The 5-year overall survival rate was 32.9% for elderly patients with esophageal carcinoma after esophagectomy in present study, which was comparable to previous studies (4,13,14), Nevertheless, the survival rate was poorer than studies about patients of all ages or younger patients (1,3,12,15,16). The right-approach esophagectomy has a reputation of better lymph node dissection, but is also considered to be more “aggressive” regarding the incidence of postoperative complications, when compared with the left-approach surgery. However, the left-approach has limitations on dissection of upper mediastinum lymph nodes, which may increase the recurrence and reduce the length of survival. Therefore, the clinical decision making between right- or left-approach is a process of balancing safety and effectiveness based on the surgeon’s experience, yet it could be very objective and confusing.
Nonetheless, no study have reported comparisons of the left and right approaches for elderly patients with ESCC. Recently, Li et al. compared Ivor-Lewis and Sweet esophagectomy in a randomized clinical trial and the early results showed that Ivor-Lewis, with lower rates of postoperative complications and more retrieval lymph nodes, is a better choice for ESCC patients at all ages (17). Yu and colleagues (18) compared the therapeutic efficacy of left dual-incisional transthoracic esophagectomy and Ivor-Lewis esophagectomy for patients with middle thoracic ESCC, and found that there was no statistically significant difference between these two approaches in overall 5-year survival. We found that right-approach surgery was associated with a favorable survival than left-approach among elderly patients. The difference in lymph node dissection region between right- and left-approaches may explain the disparity of survival. The compromised variances of patient baseline characteristics in our 1:1 PSM study effectively balanced the confounding factors in survival comparisons.
Randomized controlled trial is regarded as the standardized approach for analysis the effects of interventions, but many difficulties come with the randomized controlled trials concerned with surgical approach. First of all, patients and surgeons would know exactly the surgical method, which made it difficult to perform a double-blind trial. Secondly, if patients already had their choice of surgical approaches, they might refuse to take the randomized assigned approach, which impaired the reliability of randomized trials. There has been no randomized controlled trial comparing the short-term or long-term outcomes of elderly patients receiving right- or left-approach esophagectomy. Recently, there has been increasing interest in use of the PSM in observational studies (19), because PSM can reduce a larger proportion of the discrepancy in baseline characteristics between two treatment groups (20,21). In the present study, we used PSM to consider all potential covariates that could affect the group allocation, in order to draw a more reasonable and reliable results.
The variables in the propensity score model were chosen base on the criteria that the variables were patient baseline characteristics before surgery, unrelated to the surgery, but associated with the outcome (22). Therefore, in the present study, we included age, gender, tumor location, pathological T stage, pathological N stage, smoking, alcohol consumption, preoperative comorbidities, surgical approaches, and adjuvant therapy to generate a propensity score model. Due to the chemotherapy or radiotherapy, a small portion of elderly patient received the therapy from 1990 to 2010. Among the 276 cases, only 6 patients received neoadjuvant therapy, therefore neoadjuvant therapy was not used as one of the matching criteria.
Due to the nature of retrospective study, our study had several limitations. Despite the use of PSM, selection bias existed. Additionally, the study was based on single institution experience. Other limitations included the lack of detailed data on pathologic characteristics, such as vascular and perineural invasion as well as lymph node micro-invasion, which might be identified as confounding factors.
In conclusion, our study provided evidence for application of right-approach esophagectomy in elderly ESCC patients, regarding its efficacy and tolerable minor postoperative complications. To the best of our knowledge, this is the first study comparing right- and left-approach esophagectomy in elderly ESCC patients. However, further large-scare randomize controlled trials is necessary to confirm the results.
Acknowledgements
None.
Ethical Statement: This study was approved by the ethics committee of Sun Yat-sen University Cancer Center (No. B2015-048-01).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Fang W, Igaki H, Tachimori Y, et al. Three-field lymph node dissection for esophageal cancer in elderly patients over 70 years of age. Ann Thorac Surg 2001;72:867-71. 10.1016/S0003-4975(01)02896-X [DOI] [PubMed] [Google Scholar]
- 2.Pultrum BB, Bosch DJ, Nijsten MW, et al. Extended esophagectomy in elderly patients with esophageal cancer: minor effect of age alone in determining the postoperative course and survival. Ann Surg Oncol 2010;17:1572-80. 10.1245/s10434-010-0966-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruol A, Portale G, Zaninotto G, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg 2007;133:1186-92. 10.1016/j.jtcvs.2006.12.040 [DOI] [PubMed] [Google Scholar]
- 4.Yang HX, Ling L, Zhang X, et al. Outcome of elderly patients with oesophageal squamous cell carcinoma after surgery. Br J Surg 2010;97:862-7. 10.1002/bjs.7005 [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Yang H, Wen J, et al. NHE9 induces chemoradiotherapy resistance in esophageal squamous cell carcinoma by upregulating the Src/Akt/beta-catenin pathway and Bcl-2 expression. Oncotarget 2015;6:12405-20. 10.18632/oncotarget.3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMillan RR, Berger A, Sima CS, et al. Thirty-day mortality underestimates the risk of early death after major resections for thoracic malignancies. Ann Thorac Surg 2014;98:1769-74; discussion 1774-5. [DOI] [PMC free article] [PubMed]
- 7.Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. 10.1097/SLA.0b013e3182590603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 9.Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1-8. 10.1111/j.1442-2050.2008.00901.x [DOI] [PubMed] [Google Scholar]
- 10.Chang AC, Lee JS. Resection for esophageal cancer in the elderly. Thorac Surg Clin 2009;19:333-43. 10.1016/j.thorsurg.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson A. Oesophageal resection in the elderly. Ann R Coll Surg Engl 1989;71:73. [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Zhu J, Pan J, et al. Postoperative radiotherapy improved survival of poor prognostic squamous cell carcinoma esophagus. Ann Thorac Surg 2010;90:435-42. 10.1016/j.athoracsur.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 13.Kinugasa S, Tachibana M, Yoshimura H, et al. Esophageal resection in elderly esophageal carcinoma patients: improvement in postoperative complications. Ann Thorac Surg 2001;71:414-8. 10.1016/S0003-4975(00)02333-X [DOI] [PubMed] [Google Scholar]
- 14.Paulson EC, Ra J, Armstrong K, et al. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg 2008;143:1198-203; discussion 1203. 10.1001/archsurg.143.12.1198 [DOI] [PubMed] [Google Scholar]
- 15.Li H, Zhang Q, Xu L, et al. Factors predictive of prognosis after esophagectomy for squamous cell cancer. J Thorac Cardiovasc Surg 2009;137:55-9. 10.1016/j.jtcvs.2008.05.024 [DOI] [PubMed] [Google Scholar]
- 16.Vallböhmer D, Holscher AH, Brabender J, et al. Clinicopathologic and prognostic factors of young and elderly patients with esophageal adenocarcinoma: is there really a difference? Dis Esophagus 2008;21:596-600. 10.1111/j.1442-2050.2008.00817.x [DOI] [PubMed] [Google Scholar]
- 17.Li B, Xiang J, Zhang Y, et al. Comparison of Ivor-Lewis vs Sweet esophagectomy for esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg 2015;150:292-8. 10.1001/jamasurg.2014.2877 [DOI] [PubMed] [Google Scholar]
- 18.Yu Y, Wang Z, Liu XY, et al. Therapeutic efficacy comparison of two surgical procedures to treat middle thoracic esophageal carcinoma. World J Surg 2010;34:272-6. 10.1007/s00268-009-0341-7 [DOI] [PubMed] [Google Scholar]
- 19.Cheng YJ, Wang MC. Estimating propensity scores and causal survival functions using prevalent survival data. Biometrics 2012;68:707-16. 10.1111/j.1541-0420.2012.01754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37-48. 10.1097/00001648-199901000-00008 [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-55. 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 22.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149-56. 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]