Abstract
Rising ocean temperatures are predicted to cause a poleward shift in the distribution of marine fishes occupying the extent of latitudes tolerable within their thermal range boundaries. A prevailing theory suggests that the upper thermal limits of fishes are constrained by hypoxia and ocean acidification. However, some eurythermal fish species do not conform to this theory, and maintain their upper thermal limits in hypoxia. Here we determine if the same is true for stenothermal species. In three coral reef fish species we tested the effect of hypoxia on upper thermal limits, measured as critical thermal maximum (CTmax). In one of these species we also quantified the effect of hypoxia on oxygen supply capacity, measured as aerobic scope (AS). In this species we also tested the effect of elevated CO2 (simulated ocean acidification) on the hypoxia sensitivity of CTmax. We found that CTmax was unaffected by progressive hypoxia down to approximately 35 mmHg, despite a substantial hypoxia-induced reduction in AS. Below approximately 35 mmHg, CTmax declined sharply with water oxygen tension (PwO2). Furthermore, the hypoxia sensitivity of CTmax was unaffected by elevated CO2. Our findings show that moderate hypoxia and ocean acidification do not constrain the upper thermal limits of these tropical, stenothermal fishes.
Keywords: critical oxygen tension (Pcrit), critical thermal maximum (CTmax), oxygen- and capacity-limited thermal tolerance (OCLTT), oxygen limit for thermal tolerance (PCTmax), oxygen uptake ( ṀO2), hypercapnia (water CO2)
1. Introduction
Climate warming is predicted to impact the abundance and distribution of aquatic species via increases in the frequency and severity of heat waves where temperatures exceed species' upper thermal limits, and via poleward shifts in latitudinal thermal range boundaries because of rising ocean temperatures [1]. Coral reef fishes have evolved in a stable thermal environment and are already shifting their distribution ranges poleward as a result of climate warming [2]. Furthermore, coral reef fishes live close to their upper thermal limits and in 2016, the Great Barrier Reef experienced the highest transient sea surface temperatures ever recorded [3]. Climate change involves not only rising temperatures, but also declining pH (ocean acidification) due to elevated CO2 and increases in the frequency and severity of environmental hypoxia [4]. Understanding the physiological responses of fishes to the synergistic effects of multiple environmental stressors (e.g. heat waves, ocean acidification and hypoxia) is essential if we are to make accurate predictions on the impact of climate change on species and whole ecosystems [4].
The critical thermal maximum (CTmax) is the temperature at which the fishes exhibits loss of equilibrium (LOE) due to the temperature-induced collapse of vital physiological functions [5]. The CTmax defines the upper boundary of a species' fundamental thermal niche and is a commonly used metric in studies investigating the thermal tolerance limits in marine fishes and the impacts of climate warming on their distribution [1]. Oxygen supply capacity is the ability of the cardiorespiratory system to maximize the delivery of oxygen from the environment to the tissues, by increasing gill performance, heart performance and blood-oxygen carrying capacity. Oxygen supply capacity can be quantified via aerobic scope (AS), which is the difference between the maximum rate of oxygen consumption (maximum metabolic rate, MMR) and the rate of oxygen consumption required to sustain basal metabolism (standard metabolic rate, SMR). In fishes, exposure to progressive hypoxia causes a gradual decline in AS from 100% in normoxia to 0% at the animal's critical oxygen tension (Pcrit). Building on this principle, we demonstrated that the upper thermal limits of fishes, estimated via CTmax, can be classified as either oxygen-dependent or oxygen-independent [6]. In species with oxygen-dependent upper thermal limits, SMR surpasses MMR at the critical temperature (Tcrit) due to a temperature-induced collapse of cardiorespiratory performance [7]. Above Tcrit, survival becomes reliant on unsustainable anaerobic metabolism, and LOE occurs at the temperature where ATP deficiency becomes critical (i.e. CTmax). Consequently, the CTmax of these species and their resilience to transient heat waves should decline in habitats where water oxygen tensions (PwO2) are low. In species with oxygen-independent upper thermal limits, CTmax is determined by a temperature-induced collapse of vital physiological functions not directly related to tissue oxygen supply [6,8]. As a result, these species maintain AS at their upper thermal limits and CTmax is relatively insensitive to environmental hypoxia. The oxygen limit for thermal tolerance (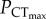 ) is the PwO2 where an organism's CTmax starts to decline and can be used to determine the oxygen-dependence of CTmax in fishes and other water-breathing ectotherms [6]. Ocean acidification may constrain the AS of fishes by increasing the energetic cost of acid–base regulation, which could increase SMR, and reduce the oxygen binding affinity of haemoglobin, which could decrease MMR [9]. In a number of coral reef fishes, exposure to elevated CO2 reduces MMR and AS [10], indicating that ocean acidification may exacerbate the effects of environmental hypoxia on their upper thermal limits [6]. However, other species maintain MMR and AS in elevated CO2 [10], indicating that the impact of ocean acidification on upper thermal limits may be species-specific.
) is the PwO2 where an organism's CTmax starts to decline and can be used to determine the oxygen-dependence of CTmax in fishes and other water-breathing ectotherms [6]. Ocean acidification may constrain the AS of fishes by increasing the energetic cost of acid–base regulation, which could increase SMR, and reduce the oxygen binding affinity of haemoglobin, which could decrease MMR [9]. In a number of coral reef fishes, exposure to elevated CO2 reduces MMR and AS [10], indicating that ocean acidification may exacerbate the effects of environmental hypoxia on their upper thermal limits [6]. However, other species maintain MMR and AS in elevated CO2 [10], indicating that the impact of ocean acidification on upper thermal limits may be species-specific.
To assess the effects of hypoxia and ocean acidification on the upper thermal nice boundaries of stenothermal fishes, we examined the oxygen-dependence of CTmax in black-axil chromis (Chromis atripectoralis), five-lined cardinalfish (Cheilodipterus quinquelineatus), and spiny chromis damselfish (Acanthochromis polyacanthus), and the synergistic effects of elevated CO2 (simulating ocean acidification) on the hypoxia sensitivity of CTmax in C. atripectoralis. As proof of concept we also determined AS and Pcrit across a range of water temperatures and oxygen tensions and assessed the effects of temperature and hypoxia on oxygen supply capacity.
2. Material and methods
Fish were collected in the waters around Lizard Island, Australia (Department of Primary Fisheries permit #170251 and Great Barrier Reef Marine Park Authority collection permit G13/35909.1). Prior to experimentation, animals were maintained in aerated, normocapnic water at 30°C; the average summer temperature for this location [3]. The CTmax was measured at normoxia and at multiple levels of hypoxia, using a warming rate of 2°C h−1 [6]. This protocol was repeated on C. atripectoralis acclimated for two weeks to 1000 µatm hypercapnia (electronic supplementary material, table S2).
Oxygen consumption rates (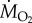 ) were determined using fibre-optic oxygen sensors, meters and software (Pyro Science GmbH, Aachen, Germany or Loligo Systems, Tjele, Denmark). MMR, SMR and Pcrit were estimated in C. atripectoralis at 29, 31, 33 and 35°C, respectively. For each group, the temperature was increased or decreased from the 30°C acclimation temperature (2°C h−1). Measurements were initiated once the temperature reached the target temperature. MMR at normoxia and at a PwO2 of 75 mmHg was estimated using a chase protocol [11]. SMR was estimated using the mean of the lowest 10% of
) were determined using fibre-optic oxygen sensors, meters and software (Pyro Science GmbH, Aachen, Germany or Loligo Systems, Tjele, Denmark). MMR, SMR and Pcrit were estimated in C. atripectoralis at 29, 31, 33 and 35°C, respectively. For each group, the temperature was increased or decreased from the 30°C acclimation temperature (2°C h−1). Measurements were initiated once the temperature reached the target temperature. MMR at normoxia and at a PwO2 of 75 mmHg was estimated using a chase protocol [11]. SMR was estimated using the mean of the lowest 10% of 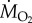 measurements performed over a 12 h period using intermittent-flow respirometry [11]. At the end of this period,
measurements performed over a 12 h period using intermittent-flow respirometry [11]. At the end of this period, 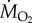 measurements were performed on fish gradually exposed to increasing hypoxia, and Pcrit was determined as the PwO2 where
measurements were performed on fish gradually exposed to increasing hypoxia, and Pcrit was determined as the PwO2 where 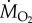 decreased below SMR. See electronic supplementary material for full experimental protocol and statistical analysis.
decreased below SMR. See electronic supplementary material for full experimental protocol and statistical analysis.
3. Results
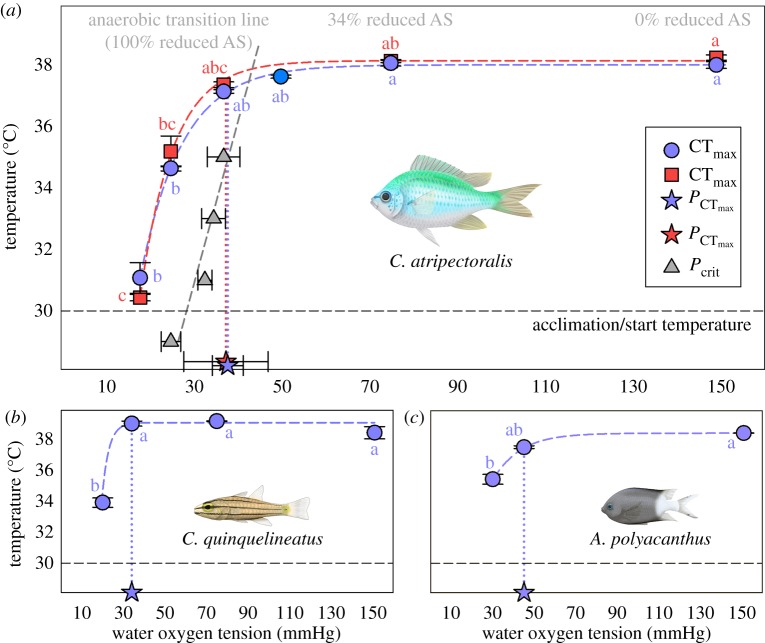
In C. atripectoralis, the 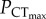 was estimated using a piecewise, two-segmented linear regression [6]. The
was estimated using a piecewise, two-segmented linear regression [6]. The 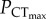 was 37.9 ± 3.5 and 37.5 ± 9.6 mmHg in normocapnia and elevated CO2, respectively, with no significant difference between the two groups (figure 1a; electronic supplementary material, table S1). These values should be taken with some caution, however, owing to the low number of points on either side of the intercept. As such,
was 37.9 ± 3.5 and 37.5 ± 9.6 mmHg in normocapnia and elevated CO2, respectively, with no significant difference between the two groups (figure 1a; electronic supplementary material, table S1). These values should be taken with some caution, however, owing to the low number of points on either side of the intercept. As such, 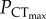 in C. quinquelineatus and A. polyacanthus was defined as the lowest oxygen tension that did not cause a significant decrease in CTmax, when compared to the species' CTmax under normoxic conditions (figure 1b,c). In C. atripectoralis, AS in normoxia was maintained between 29 and 35°C (electronic supplementary material, table S1). Exposure to a PwO2 of 75 mmHg reduced AS by approximately 34%, which was independent of water temperatures between 29 and 35°C (figure 1; electronic supplementary material, table S1). The
in C. quinquelineatus and A. polyacanthus was defined as the lowest oxygen tension that did not cause a significant decrease in CTmax, when compared to the species' CTmax under normoxic conditions (figure 1b,c). In C. atripectoralis, AS in normoxia was maintained between 29 and 35°C (electronic supplementary material, table S1). Exposure to a PwO2 of 75 mmHg reduced AS by approximately 34%, which was independent of water temperatures between 29 and 35°C (figure 1; electronic supplementary material, table S1). The 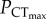 of C. atripectoralis was 37 mmHg, which is far below the oxygen tension sufficient to reduce AS by 34% (75 mmHg, figure 1a; electronic supplementary material, table S1). Consequently, when C. atripectoralis reach their CTmax under normoxic conditions, they still retain approximately 66% of their AS; the CTmax of C. atripectoralis can therefore be classified as oxygen-independent [6]. In C. atripectoralis, CTmax measured at corresponding PwO2 levels were not significantly different in animals acclimated and measured at 1000 µatm CO2 when compared to animals at normocapnia. In C. quinquelineatus and A. polyacanthus, CTmax values were maintained at oxygen tensions of 33.8 and 45.7 mmHg, respectively (figure 1b,c). These PwO2 values are far below oxygen tensions shown to reduce the AS in C. atripectoralis and other fish species [6,12]. This suggests that the CTmax for C. quinquelineatus and A. polyacanthus may also be classified oxygen-independent.
of C. atripectoralis was 37 mmHg, which is far below the oxygen tension sufficient to reduce AS by 34% (75 mmHg, figure 1a; electronic supplementary material, table S1). Consequently, when C. atripectoralis reach their CTmax under normoxic conditions, they still retain approximately 66% of their AS; the CTmax of C. atripectoralis can therefore be classified as oxygen-independent [6]. In C. atripectoralis, CTmax measured at corresponding PwO2 levels were not significantly different in animals acclimated and measured at 1000 µatm CO2 when compared to animals at normocapnia. In C. quinquelineatus and A. polyacanthus, CTmax values were maintained at oxygen tensions of 33.8 and 45.7 mmHg, respectively (figure 1b,c). These PwO2 values are far below oxygen tensions shown to reduce the AS in C. atripectoralis and other fish species [6,12]. This suggests that the CTmax for C. quinquelineatus and A. polyacanthus may also be classified oxygen-independent.
Figure 1.
Change in the critical thermal maximum (CTmax) (blue circles) with declining water oxygen tension (PwO2) (one-way ANOVA, p < 0.05), and the oxygen limit for thermal tolerance (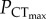 ) (stars) in (a) C. atripectoralis (N = 5), (b) C. quinquelineatus (N = 5) and (c) A. polyacanthus (N = 5). For (a) C. atripectoralis (N = 8), the critical oxygen tension (Pcrit) (grey triangles) increased with temperature, and the aerobic scope (AS) was maintained at normoxia (i.e. 0% reduction), reduced by 34% at 75 mmHg, and by 100% at the anaerobic transition line (grey dashed line) (see test and electronic supplementary material, table S1 for details). In (a) C. atripectoralis, the CTmax that was measured above the anaerobic transition line was not significantly different from the CTmax measured at normoxia, but decreased significantly with PwO2 when measured below the anaerobic transition line (one-way ANOVA, p < 0.05). In (a) C. atripectoralis (N = 5), the hypoxia sensitivity of CTmax was not affected by 14 days acclimation to elevated CO2 (red squares) (two-way ANOVA, p < 0.05). Values are means ± 1 s.e.m. Illustrations by Erin Walsh.
) (stars) in (a) C. atripectoralis (N = 5), (b) C. quinquelineatus (N = 5) and (c) A. polyacanthus (N = 5). For (a) C. atripectoralis (N = 8), the critical oxygen tension (Pcrit) (grey triangles) increased with temperature, and the aerobic scope (AS) was maintained at normoxia (i.e. 0% reduction), reduced by 34% at 75 mmHg, and by 100% at the anaerobic transition line (grey dashed line) (see test and electronic supplementary material, table S1 for details). In (a) C. atripectoralis, the CTmax that was measured above the anaerobic transition line was not significantly different from the CTmax measured at normoxia, but decreased significantly with PwO2 when measured below the anaerobic transition line (one-way ANOVA, p < 0.05). In (a) C. atripectoralis (N = 5), the hypoxia sensitivity of CTmax was not affected by 14 days acclimation to elevated CO2 (red squares) (two-way ANOVA, p < 0.05). Values are means ± 1 s.e.m. Illustrations by Erin Walsh.
4. Discussion
The upper thermal limits of C. atripectoralis, C. quinquelineatus and A. polyacanthus were classified as oxygen-independent. A temperature-induced collapse of vital physiological functions not directly related to cardiorespiratory oxygen supply must therefore be the responsible for the CTmax of these tropical, stenothermal species. Rising water temperatures and decreasing PwO2 constrain AS by increasing SMR and decreasing MMR, respectively. The ‘anaerobic transition line’ defines the PwO2 where AS is zero (Pcrit) at corresponding temperatures. In C. atripectoralis, when CTmax was measured beyond the anaerobic transition, it continued to decrease with PwO2 (figure 1a), suggesting that survival beyond the anaerobic transition line would be time-limited because animals are no longer able to sustain their baseline aerobic metabolism. The 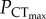 is determined by the oxygen supply capacity and a CO2-induced reduction in oxygen supply capacity should therefore increase
is determined by the oxygen supply capacity and a CO2-induced reduction in oxygen supply capacity should therefore increase 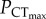 . The lack of change in
. The lack of change in 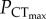 indicates that the effect of elevated CO2 on oxygen supply capacity is either absent, or insufficient to cause a significant change in
indicates that the effect of elevated CO2 on oxygen supply capacity is either absent, or insufficient to cause a significant change in 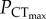 . Ocean acidification is therefore unlikely to act as a significant synergistic stressor with hypoxia on the upper thermal limits of this species. Importantly, the lack of significant change in CTmax of C. atripectoralis in normoxia suggest that the thermal tolerance of the physiological mechanisms responsible for setting CTmax is also not affected by elevated CO2.
. Ocean acidification is therefore unlikely to act as a significant synergistic stressor with hypoxia on the upper thermal limits of this species. Importantly, the lack of significant change in CTmax of C. atripectoralis in normoxia suggest that the thermal tolerance of the physiological mechanisms responsible for setting CTmax is also not affected by elevated CO2.
In conclusion, moderate environmental hypoxia, above approximately 35 mmHg, and future ocean acidification conditions should have little impact on the upper thermal limits of these species and their resilience to transient heat waves. By contrast, severe hypoxia, below approximately 35 mmHg, would be expected to constrain their upper thermal limits. To the degree these tropical, stenothermal fish species occupy the extent of latitudes tolerable within their thermal range boundaries [1], our findings suggest that moderate hypoxia and ocean acidification are unlikely to impact their latitudinal distribution ranges via direct limitations on their upper thermal limits.
Supplementary Material
Acknowledgements
We thank Tiffany Nay and Blake Spady for technical assistance.
Ethics
Fish were maintained under James Cook University Animal Ethics Committee regulations (permit: #A2089).
Data accessibility
Experimental protocols, statistical analyses and results are available in the electronic supplementary material.
Authors' contributions
R.E. conceived and designed the study, carried out the experiments and the data analyses, and drafted the manuscript; J.L.J. carried out the experiments, and revised the manuscript; J.L.R. carried out the experiments, and revised the manuscript; A.J.E. conceived and designed the study, and revised the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
R.E. acknowledges funding from the Carlsberg Foundation (CF15-0321) and the Company of Biologists (JEBTF-151205). J.L.R. acknowledges funding from the Australian Research Council (PDE150101266). A.J.E. acknowledges funding from the National Science Foundation (EF 1315290).
References
- 1.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690. ( 10.1038/nclimate1539) [DOI] [Google Scholar]
- 2.Feary DA, et al. 2013. Latitudinal shifts in coral reef fishes: why some species do and others do not shift. Fish Fish. 15, 593–615. ( 10.1111/faf.12036) [DOI] [Google Scholar]
- 3.Rummer JL, Couturier CS, Stecyk JAW, Gardiner NM, Kinch JP, Nilsson GE, Munday PL. 2014. Life on the edge: thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob. Change Biol. 20, 1055–1066. ( 10.1111/gcb.12455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IPCC, Pörtner HO, et al. 2017. Ocean systems. In Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (ed. Field CB.), pp. 411–484. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Beitinger TL, Bennett WA, McCauley RW. 2000. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fishes 58, 237–275. ( 10.1023/A:1007676325825) [DOI] [Google Scholar]
- 6.Ern R, Norin T, Gamperl AK, Esbaugh AJ. 2016. Oxygen-dependence of upper thermal limits in fishes. J. Exp. Biol. 219, 3376–3383. ( 10.1242/jeb.143495) [DOI] [PubMed] [Google Scholar]
- 7.Pörtner HO. 2010. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 213, 881–893. ( 10.1242/jeb.037523) [DOI] [PubMed] [Google Scholar]
- 8.Ern R, Huong DTT, Phuong NT, Madsen PT, Wang T, Bayley M. 2015. Some like it hot: thermal tolerance and oxygen supply capacity in two eurythermal crustaceans. Sci. Rep. 5, 10743 ( 10.1038/srep10743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heuer RM, Grosell M. 2014. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, 1061–1084. ( 10.1152/ajpregu.00064.2014) [DOI] [PubMed] [Google Scholar]
- 10.Munday PL, Crawley NE, Nilsson GE. 2009. Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar. Ecol. Prog. Ser. 388, 235–242. ( 10.3354/meps08137) [DOI] [Google Scholar]
- 11.Clark TD, Sandblom E, Jutfelt F. 2013. Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J. Exp. Biol. 216, 2771–2782. ( 10.1242/jeb.084251) [DOI] [PubMed] [Google Scholar]
- 12.Norin T, Clark TD. 2016. Measurement and relevance of maximum metabolic rate in fishes. J. Fish Biol. 88, 122–151. ( 10.1111/jfb.12796) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental protocols, statistical analyses and results are available in the electronic supplementary material.