Abstract
The Mediterranean Sea is an invasion hotspot, with non-indigenous species suspected to be a major driver behind community changes. We used size spectra, a reliable index of food web structure, to examine how the influx of Red Sea fishes into the Mediterranean Sea has impacted the indigenous species community. This is the first attempt to use changes in the size spectra to reveal the effect of biological invasions. We used data from trawl catches along Israel's shoreline spanning 20 years to estimate changes in the community size spectra of both indigenous and non-indigenous species. We found that the relative biomass of non-indigenous species increased over the 20 years, especially for small and large species, leading to a convergence with the indigenous species size spectra. Hence, the biomass of indigenous and non-indigenous species has become identical for all size classes, suggesting similar energetic constraints and sensitivities to fishing. However, over this time period the size spectrum of indigenous species has remained remarkably constant. This suggests that the wide-scale invasion of non-indigenous species into the Mediterranean may have had little impact on the community structure of indigenous species.
Keywords: size spectra, biological invasions, Mediterranean Sea, Lessepsian migration
1. Introduction
Biological invasions are increasing globally and generating much debate regarding their effect on indigenous biodiversity [1,2]. The Mediterranean Sea is subject to an ongoing invasion of Red Sea species through the Suez Canal in one of modern history's most important biogeographic transitions [3]. Among these invasives are over 100 fish species, more than documented for any other marine ecosystem in the world [4]. These invasive species now constitute more than 50% of the fish biomass in some Eastern Mediterranean habitats [5]. While invasives are suspected to be a major driver behind ichtyofaunal community change [5], direct evidence for their effect on indigenous community structure is generally lacking. The unparalleled magnitude of invasion in the Eastern Mediterranean provides a unique opportunity to understand how massive establishment of new species impacts existing communities.
The community size spectrum describes the relationship between the summed biomass of all species within a biomass class and the body size class [6]. Given the size-based trophic structuring of aquatic ecosystems, size spectra serve as a reliable index of food web structure [6,7]. Size spectra are ubiquitously negative and mostly log-linear, with the intercept determined by the community's productivity and the slope by the efficiency of biomass transfer across trophic levels [8]. In addition, steepening of the size-spectra slope may serve as an indicator of increased fishing for large-sized fish [9,10]. The effects of biological invasions on the community size spectrum, however, have not been studied.
Here, we used data on trawl catches along Israel's shoreline from two time periods spanning 20 years to examine how the influx of Red Sea species into the Mediterranean Sea has impacted the local community's size structure. As marine systems are predicted to be increasingly exposed to invasion, our results have implications for understanding the future of other, currently less impacted, regions.
2. Methods
We used data from trawl surveys conducted, using identical methods, in the years 1990–1994 and 2008–2012 along the Israeli continental shelf, between latitudes 31°20′ N and 33°05′ S, with a depth range of 15–300 m [5,11]. Data from 1990–1994 consisted of 241 hauls, and from 2008–2012 of 253 hauls. For each haul, a random sample of one box representative of the total catch (totalling approximately 5 kg and 260 ± 10 (s.e.) individuals) was identified to the species level, counted and measured for total length (TL) to the nearest 0.5 cm. Since the sample's proportion of the total catch biomass is estimated but not measured directly, we treat total catch biomass for this study as unknown and thus have data only on the relative abundances. Altogether, the dataset contains 127 805 individuals of 168 species of fish, along with the total length for each. We used species-specific a and b coefficients acquired from FishBase [12] to transform TL into biomass using the length–weight relationship W = aLb.
For each group of indigenous or non-indigenous species within each time period, we assigned fish to log2 weight classes and summed the cumulative biomass within each weight class. Fishes of less than 8 g were excluded from the analysis as they are underrepresented due to trawl size selectivity, and fish of more than 8192 g were excluded because surveyors during the second sampling period may have overrepresented large individuals (D. Edelist 2017, personal communication; electronic supplementary material, figure S1). We examined trends in each group and weight class's proportional biomass, calculated by dividing cumulative biomass of each weight class by the total biomass sampled within each period. While this division does not influence the size-spectrum slopes, it is necessary for the comparison of size-spectrum intercepts across time. Lastly, to transition from measures of proportion to ecologically relevant measures of biomass, we multiplied the calculated proportions by the mean sampled total biomass of the two time periods (i.e. half the sum of total biomass sampled in both time periods). This step does not affect further analyses. Cumulative biomass was normalized by dividing biomass within each size class by the size class width [13], log10-transformed and plotted against the log10-transformed mid-point of each size class in order to produce a size spectrum. This was done for each origin group (indigenous versus non-indigenous) and sampling period (1990–1994 versus 2008–2012).
To examine the effect of origin and period on size-spectra parameters, we used linear models with the normalized cumulative biomass as the response variable, the mid-point of each size class as a covariate, and period and origin as categorical predictors. When needed, a second-order polynomial term was added to size class. To determine differences in size-spectra slopes, models were tested with and without interaction terms (size class × period or size class × origin). Model comparisons were based on AICc values.
Since indigenous and non-indigenous differ in species richness, a null model was used to test whether the differences in size spectra across groups may be an artefact of dividing a community into two groups of unequal size. The null model was based on 9999 random splits of the community into two groups, maintaining the observed non-indigenous and indigenous species richness. Each randomized community was subjected to a model similar to the original analyses that resulted in a distribution of regression coefficients that we then compared to the observed coefficients.
3. Results
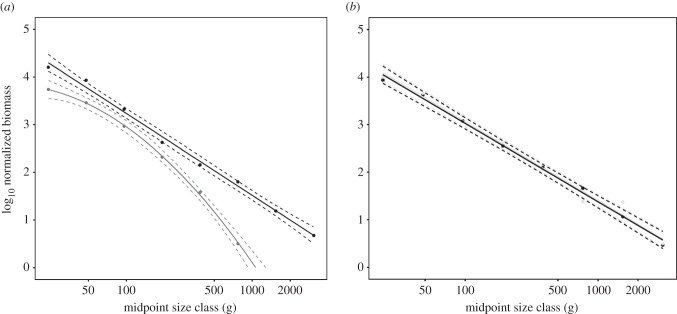
Eastern Mediterranean fishes display overall negative size spectra, with indigenous and non-indigenous communities showing different temporal trends (figure 1). In 1990–1994 (figure 1a), indigenous species exhibited a linear size spectrum, while the non-indigenous species exhibited a nonlinear one (ΔAICc = 8.35 in favour of the model with a second-order interaction; electronic supplementary material, table S1), with underrepresentation of smaller and larger size classes. Relative biomass of non-indigenous species, across all size classes, was lower than that of the indigenous species (p < 0.01; figure 1a).
Figure 1.
Size spectra of Mediterranean fishes. (a) Indigenous (black) and non-indigenous (grey) species in 1990–1994. (b) Indigenous and non-indigenous species in 2008–2012. Dashed lines represent 95% CI.
In 2008–2012, both indigenous and non-indigenous species showed linear size spectra (figure 1b). Importantly, neither the size-spectra intercept nor the slope differs between indigenous and non-indigenous species, producing nearly perfectly overlapping curves (ΔAICc = 3.26 in favour of model without origin; electronic supplementary material, table S1). The resemblance of the two groups' size spectra translates into a one-to-one ratio of indigenous to non-indigenous biomass across all examined size classes.
Across time periods, the size-spectra slopes for indigenous species were similar (ΔAICc = 3.02 in favour of the model without a period × size class interaction term; electronic supplementary material, table S1). The intercept, however, decreased significantly over time (p < 0.01). However, since we used relative biomass as opposed to total biomass, a decrease in the indigenous size-spectrum intercept is statistically inevitable given increasing proportion of non-indigenous biomass. The size spectrum for non-indigenous species, however, varied between time periods (ΔAICc = 5.64 in favour of the model with period × size class2 interaction term; electronic supplementary material, table S1) and with a higher intercept (ΔAICc > 3 for models with period; electronic supplementary material, table S1).
To test for the robustness of these results to the unequal species richness across origin groups, we applied a null. In 2008–2012, we found that for non-indigenous species the null models predicted similar size-spectrum intercepts (electronic supplementary material, figure 2Sa) and slopes (electronic supplementary material, figure S2b) to those observed. This suggests that the size spectrum does not differ from that expected for a community of indigenous species of similar richness. However, in 1990–1994 the null model for non-indigenous species predicted higher size-spectrum intercepts (electronic supplementary material, figure S2c), lower slopes (electronic supplementary material, figure S2d) and smaller curvatures (electronic supplementary material, figure S2e) than observed.
4. Discussion
We used the community size spectra, a well-established indicator of aquatic community structure [10], to assess the effect of the continuous influx of non-indigenous fish species on the Mediterranean indigenous fish community. This is the first attempt to use size spectra to address the impact of biological invasions on community structure. We found that the size spectrum of indigenous species maintained its slope in the face of a large and size-specific increase in non-indigenous fish biomass. This suggests that the massive invasion has a relatively minor effect on the structure of the indigenous community.
It has been proposed that indigenous Mediterranean fish species may be more sensitive to size-selective fishing than non-indigenous species [14]. A difference in sensitivity to fishing is expected to result in divergent size-spectra slopes between indigenous and non-indigenous species. However, our results indicate that indigenous and non-indigenous size spectra converge over time, while the slope for indigenous species did not change. This suggests that fishing may have a similar effect on indigenous and non-indigenous fishes.
Increased competition for local resources has been hypothesized as a major impact of biological invasions on indigenous species. If invasives increase unevenly across size groups we may expect a reduction in indigenous species biomass at the size groups impacted, altering the shape of the size spectrum. We found that the size-spectrum slope of indigenous species has remained constant, even though the relative biomass of non-indigenous species increased considerably for small and large size classes (figure 1). This pattern suggests either weak or size-independent competition between indigenous and non-indigenous species and a remarkably robust size spectrum for indigenous species. One possible reason for the apparently weak competition between indigenous and non-indigenous species is that non-indigenous species tend to occupy ecological niches that are underutilized by indigenous species [15]. Given the long history of exploitation in the Mediterranean Sea, underutilized niches may be more common than in less impacted environments, which might experience stronger competition between indigenous and non-indigenous species.
The increase in biomass of non-indigenous species over the time period examined could result from two non-mutually exclusive processes: the continuous introduction of new species, or increases in already introduced, rapidly growing populations. We found that both of these processes are taking place, with 14 new species documented in the 2008–2012 time period paralleled by a 25% increase in relative biomass for the non-indigenous species present in the first time period (electronic supplementary material, table S2). This is corroborated by the null model analyses that found that in the first time period the size spectrum was dramatically lower than null expectations. The difference from null was most noticeable in smaller and larger species, as shown by the greater than expected non-linearity of the size spectrum (electronic supplementary material, figure S2). However, by the later period, the non-indigenous species size spectrum did not differ from that of the null expectations, which suggests a convergent community structure of indigenous and non-indigenous species, in terms of relative biomass distribution across species.
The converged size-spectrum slope of −2.65 is lower than the widespread value of −2 hypothesized by Sheldon [6,13]. The steeper slope could result from both a long history of human exploitation and temperature-induced reduction in body sizes given the warm nature of the eastern Mediterranean Sea [16]. This convergence of size spectra suggests that indigenous and non-indigenous species are subjected to similar energetic constraints and similar fishing impacts, although they may not strongly compete for resources. While similarity of slopes could stem from similar constraints, we have no a priori reason to expect the identical biomass of indigenous and non-indigenous species across size classes implied by the similar size-spectrum intercepts. Hence, it is hard to predict future patterns associated with increasing numbers of non-indigenous species. One possibility is that non-indigenous biomass will continue to increase across size groups with no apparent effect on the indigenous species' size spectrum. However, it is also possible that non-indigenous species have managed to fill all trophic positions in the community. In this case, subsequent growth of non-indigenous biomass may exert stronger competition on indigenous species and cause a future shift in indigenous species community structure.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data accessibility
The data used in the study were archived in BioTIME: a database of biodiversity time series for the Anthropocene [17]. The supplementary material available online contains the model selection table output, the table describing species-specific changes in biomass of non-indigenous species, as well as the entire catch size spectra and the null model output figures.
Authors' contributions
Y.B. carried out the data analysis and drafted the manuscript; J.B. and J.P.D. conceived and designed the study; I.v.R. and S.A.B. participated in the data analysis; D.E. and O.S. managed the data collection; J.P.D. revised the draft for important intellectual content. All authors contributed to the writing, gave final publication approval, and agree to be held accountable for the content therein.
Competing interests
We have no competing interests.
Funding
This study was funded by the Bi-National Science Foundation (BSF, grant no. 2014295), Marie Curie Integration grant no. 614352 and the Israel Science Foundation (grant no. 1356/15) to J.B.
References
- 1.Simberloff D. et al 2013. Impacts of biological invasions: what's what and the way forward. Trends Ecol. Evol. 28, 58–66. ( 10.1016/j.tree.2012.07.013) [DOI] [PubMed] [Google Scholar]
- 2.Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR. 2016. Invasive predators and global biodiversity loss. Proc. Natl Acad. Sci. USA 113, 11 261–11 265. ( 10.1073/pnas.1602480113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azzurro E, Maynou F, Belmaker J, Golani D, Crooks JA. 2016. Lag times in Lessepsian fish invasion. Biol. Invasions 18, 2761–2772. ( 10.1007/s10530-016-1184-4) [DOI] [Google Scholar]
- 4.Golani D, Orsi-Relini L, Massutí E, Quignard J-P. 2002. CIESM Atlas of exotic species in the Mediterranean: Vol 1. Fishes. CIESM; See http://www.ciesm.org/online/atlas/intro.htm [Google Scholar]
- 5.Edelist D, Rilov G, Golani D, Carlton JT, Spanier E. 2013. Restructuring the Sea: profound shifts in the world's most invaded marine ecosystem. Divers. Distrib. 19, 69–77. ( 10.1111/ddi.12002) [DOI] [Google Scholar]
- 6.Sheldon RW, Prakash A, Sutcliffe WH. 1972. The size distribution of particles in the ocean. Limnol. Oceanogr. 17, 327–340. ( 10.4319/lo.1972.17.3.0327) [DOI] [Google Scholar]
- 7.Petchey OL, Belgrano A. 2010. Body-size distributions and size-spectra: universal indicators of ecological status? Biol. Lett. 6, 434–437. ( 10.1098/rsbl.2010.0240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennings S, Blanchard JL. 2004. Fish abundance with no fishing: predictions based on macroecological theory. J. Anim. Ecol. 73, 632–642. ( 10.1111/j.0021-8790.2004.00839.x) [DOI] [Google Scholar]
- 9.Jennings S, Greenstreet SPR, Hill L, Piet GJ, Pinnegar JK, Warr KJ. 2002. Long-term trends in the trophic structure of the north sea fish community: evidence from stable-isotope analysis, size-spectra and community metrics. Mar. Biol. 141, 1085–1097. ( 10.1007/s00227-002-0905-7) [DOI] [Google Scholar]
- 10.Shin Y-J, Rochet M-J, Jennings S, Field JG, Gilasone H. 2005. Using size-based indicators to evaluate the ecosystem effects of fishing. ICES J. Mar. Sci. 62, 384–396. ( 10.1016/j.icesjms.2005.01.004) [DOI] [Google Scholar]
- 11.Edelist D, Sonin O, Golani D, Rilov G, Spanier E. 2011. Spatiotemporal patterns of catch and discards of the Israeli Mediterranean trawl fishery in the early 1990s: ecological and conservation perspectives. Sci. Mar. 75, 641–652. ( 10.3989/scimar.2011.75n4641) [DOI] [Google Scholar]
- 12.Froese R, Pauly D.2015. FishBase [Internet]. World Wide Web electronic publication. See www.fishbase.org .
- 13.Edwards AM, Robinson JPW, Plank MJ, Baum JK, Blanchard JL. 2016. Testing and recommending methods for fitting size spectra to data. Methods Ecol. Evol. 8, 57–67. ( 10.1111/2041-210X.12641) [DOI] [Google Scholar]
- 14.Goren M, Galil BS, Diamant A, Stern N, Levitt-Barmats Y. 2016. Invading up the food web? Invasive fish in the southeastern Mediterranean Sea. Mar. Biol. 163, 91 ( 10.1007/s00227-016-2950-7) [DOI] [Google Scholar]
- 15.Givan O, Parravicini V, Kulbicki M, Belmaker J. 2016. Trait structure reveals the processes underlying fish establishment in the Mediterranean. Glob. Ecol. Biogeogr. 26, 142–153. ( 10.1111/geb.12523) [DOI] [Google Scholar]
- 16.van Rijn I, Buba Y, DeLong J, Kiflawi M, Belmaker J. 2017. Large but uneven reduction in fish size across species in relation to changing sea temperatures. Glob. Chang. Biol., 1–8.. ( 10.1111/gcb.13688) [DOI] [PubMed] [Google Scholar]
- 17.Dornelas M, et al. In review. BioTIME: a database of biodiversity time series for the Anthropocene. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Dornelas M, et al. In review. BioTIME: a database of biodiversity time series for the Anthropocene. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data used in the study were archived in BioTIME: a database of biodiversity time series for the Anthropocene [17]. The supplementary material available online contains the model selection table output, the table describing species-specific changes in biomass of non-indigenous species, as well as the entire catch size spectra and the null model output figures.