Abstract
Background
Dengue hemorrhagic fever is the leading cause of hospitalization and death in children living in Asia and Latin America. There is an urgent need for an effective and safe dengue vaccine to reduce morbidity and mortality in this high-risk population given the lack of dengue specific treatment at present. This review aims to determine the efficacy, safety, and immunogenicity of CYD-TDV vaccine in children.
Methods
This is a systematic review including meta-analysis of randomized controlled clinical trial data from Embase, Medline, the Cochrane Library, Web of Science, and ClinicalTrials.gov. Studies that assessed CYD-TDV vaccine efficacy [(1 − RR)*100], safety (RR), and immunogenicity (weighted mean difference) in children were included in this study. Random effects model was employed to analyze patient-level data extracted from primary studies.
Results
The overall efficacy of CYD-TDV vaccine was 54% (40–64), while serotype-specific efficacy was 77% (66–85) for DENV4, 75% (65–82) for DENV3, 50% (36–61) for DENV1, and 34% (14–49) for DENV2. 15% (−174–74) vaccine efficacy was obtained for the unknown serotype. Meta-analysis of included studies with longer follow-up time (25 months) revealed that CYD-TDV vaccine significantly increased the risk of injection site reactions (RR = 1.1: 1.04–1.17; p-value = 0.001). Immunogenicity (expressed as geometric mean titers) in descending order was 439.7 (331.7–547.7), 323 (247 – 398.7), 144.1 (117.9–170.2), and 105 (88.7–122.8) for DENV3, DENV2, DENV1, and DENV4, respectively.
Conclusion
CYD-TDV vaccine is effective and immunogenic in children overall. Reduced efficacy of CYD-TDV vaccine against DENV2 notoriously known for causing severe dengue infection and dengue outbreaks cause for serious concern. Post hoc meta-analysis of long-term follow-up data (≥25 months) from children previously vaccinated with CYD-TDV vaccine is needed to make a conclusion regarding CYD-TDV vaccine safety in children. However, CYD-TDV vaccine should be considered for use in regions where DENV2 is not endemic as currently there is no specific treatment for dengue infection.
Keywords: dengue hemorrhagic fever, dengue shock syndrome, CYD-TDV, dengue virus, efficacy, safety, immunogenicity
Introduction
Continuously increasing dengue virus (DENV) related morbidity and mortality poses a serious threat to global public health and has exerted pressure on national health budgets of endemic countries. There are four types of genetically distinct dengue viruses (DENV 1–4) (1), all causing severe dengue infection (2, 3). Brady et al. estimates that four billion people are at risk of acquiring dengue infection worldwide (4) with approximately 284–528 million dengue cases being documented annually (5). Dengue hemorrhagic fever (DHF)/Dengue Shock Syndrome (DSS) comprise 500,000 to one million of these cases leading to over 20,000 fatalities mostly in children (6, 7). The goal of World Health Organization is to reduce dengue related morbidity and mortality by 2020 (8). Despite the availability of vector control programs, dengue infection has continued to rise globally (9) with significant economic burdens and might continue to do so in the future given the ongoing climate change. Introducing an efficacious and safe vaccine in endemic regions has the potential to reduce dengue related hospitalization and death in children due to severe dengue infection (DHF/DSS). The risk of developing DHF/DSS during secondary infection is increased when an individual is exposed to a dengue serotype that is different from the one previously experienced (10). This occurs due to antibody-dependent enhancement (ADE) which involves low levels of cross-reactive neutralizing antibodies produced during primary dengue infection forming complexes with target cell receptors (3, 11, 12). Consequently enhancing the number of dengue-infected cells and viremia (3, 11, 12). Therefore, a tetravalent dengue vaccine capable of eliciting a balanced immune response against all four dengue serotypes is warranted if complications due to ADE are to be averted (11, 13, 14). CYD-TDV vaccine is the most advanced live attenuated tetravalent dengue vaccine (15). However, comprehensive evidence regarding CYD-TDV vaccine efficacy, safety, and immunogenicity in children exclusively is absent.
A meta-analysis of randomized controlled trials (RCTs) by da Costa et al. has demonstrated that CYD-TDV vaccine is safe and induces a balanced immune response (16). However, safety and immunogenicity were determined for all age groups and no subgroup analysis based on the age of included participants was conducted. The fact that dengue infection is more severe in children compared to adults indicates that the two groups might respond to CYD-TDV vaccine differently with a possibility of adults confounding the true effect of the vaccine in children. The study also assessed vaccine efficacy by combining five primary studies. However, only Sabchareon et al. out of the five combined studies was designed to determine CYD-TDV vaccine efficacy in Thai children (17). The determined vaccine efficacy was 30.2% and was not statistically significant. Two large Phase III clinical studies designed to determine CYD-TDV vaccine efficacy and not included in the meta-analysis by da Costa et al. have been conducted in Asian and Latin American children showing efficacy of 56.5 and 60.8%, respectively (9, 18). However, because these studies were conducted in various age groups from different regions, the findings are not directly comparable. Therefore, to comprehensively address all the aforementioned concerns, we decided to determine the efficacy, safety, and immunogenicity of CYD-TDV vaccine in children by answering the following questions: (i) does CYD-TDV vaccine reduce the incidence of virologically confirmed dengue (VCD) cases in vaccinated compared with unvaccinated children? (ii) Does CYD-TDV vaccine increase the risk of adverse events in vaccinated as compared to the unvaccinated children? (iii) Is there a difference in geometric mean titers (GMTs) between children exposed to CYD-TDV vaccine and those unexposed?
Methods
Eligibility Criteria and Definitions
This review was conducted and reported in accordance with the Cochrane and preferred reporting items for systematic reviews and meta-analyses guidelines (19, 20). Population: children were defined as all individuals under the age of 18 years (21). Intervention: CYD-TDV vaccine manufactured by Sanofi Pasteur. Vaccine was reconstituted in 0.4% sodium chloride and 2.5% serum albumin. Comparator: standard of care, placebo, or no intervention. Outcome: the primary end assessing points were CYD-TDV vaccine efficacy in accordance with the “Guidelines for clinical trials of dengue vaccine in endemic areas” (22). Reduction in the incidence of VCD cases per protocol analysis. Safety: AEs—unfavorable medical occurrences that are not treatment related and ARs—those that might be treatment related (23). Immunogenicity: levels of dengue neutralizing antibodies expressed as GMTs and measured using plaque reduction neutralisation test with a 50% plaque reduction threshold (PRNT50). More information on the PRNT50 test can be obtained in the “guidelines for plaque reduction neutralization testing of human antibodies to dengue viruses” (24). Study design: only RCTs were included in this review. CYD-TDV vaccination interval requirement was that immunizations be conducted at months 0, 6, and 12 (three vaccine regimen). Exclusion criteria: studies which did not assess CYD-TDV vaccine efficacy, safety, or immunogenicity or did not use CYD-TDV vaccine; studies involving participants aged over 17 years; and studies that used non-RCT study designs, non-three vaccine regimen or a three vaccine regimen with a different vaccination interval.
Literature Search and Data Extraction
A comprehensive search strategy was developed in collaboration with an experienced medical librarian to identify recently published studies as presented in Appendix I (all referenced appendices are in the Supplementary Material). Embase, Medline, Web of Science, the Cochrane Library, ClinicalTrials.gov, references of included studies, and authors served as sources for published data. Gray literature was not searched because it lacks quality control. The search for published data was initiated on 01/03/2016, concluded on 11/05/2016 and pilot tested according to the method proposed by Long (25) (Appendix II, Figure 1 in Supplementary Material). This was done to ensure that the analysis includes all relevant information and fit to achieve the goal of this study. Corresponding authors of primary studies were contacted to request for numerical data or clarifications in cases where data were incomplete or graphically presented (Appendix II, Figure 2 in Supplementary Material). Information was extracted based on individual patient-level data.
Data Items and Summary Measures
Primary end points and their respective summary measures included: overall and serotype-specific CYD-TDV vaccine efficacy (per protocol analysis), CYD-TDV safety [immediate AEs, severe adverse events (SAEs), solicited ARs, solicited injection site reactions (pain, erythema, and swelling), solicited systemic reactions (fever, headache, malaise, myalgia, and asthenia) and unsolicited adverse event (UAEs)]. Relative risk (RR) was the preferred summary measure for CYD-TDV efficacy [(1 − RR)*100] (17) and safety, whereas immunogenicity (measured as GMTs) was estimated using the weighted mean difference (WMD). Relative risk was defined as the ratio of incidence VCD cases in the CYD-TDV vaccine group divided by the ratio of incidence VCD cases in the unvaccinated group (26). Mean difference was defined as an absolute difference between the GMTs in the intervention and control groups.
Risk of Bias and Statistical Analysis
The Cochrane Handbook for Systematic Reviews of Interventions tool (27) was used in this analysis to assess the quality of each of the included studies. The risk of bias was assessed both at the study and the outcome levels. Evidence tables served as the starting point for data synthesis. The tables were reviewed as well as the forest plots to determine the possibility of combining data from studies in a meta-analysis. The I2 and Q statistics were used to formally check for the presence of heterogeneity and consequently determine whether the effect sizes should be pooled. Heterogeneity was classified as low, medium and high for I2 values corresponding to 25, 50, and 75%, respectively (28). If heterogeneity was either low/medium or reduced to these levels after being resolved, the pooled effect sizes of outcomes were explored. However, if variations in the effect size between pooled studies remained high (I2 ≥ 75%) after efforts to resolve heterogeneity, meta-analysis was not conducted. The heterogeneity was investigated using meta-regression and subgroup analysis to explain its possible cause. The Cochrane Collaboration recommends that studies with divergent effect sizes from the rest should be excluded to resolve heterogeneity (27). The influence of individual studies on the overall effect size was formally investigated using meta-influence. Identified studies were removed systematically to reduce heterogeneity across the combined studies. Sensitivity analysis was performed to explore the robustness of the findings using the fixed effects model (Appendices III and IV in Supplementary Material). The random effects model was preferred because the true effect size may not be constant across all the included studies (29) given that they were conducted in different age groups, countries, regions, and ethnic groups. Extracted data were exported from Excel spreadsheet to STATA version 13.0, where all statistical analyses were conducted. Publication bias was explored by employing Harbord’s and Egger’s tests.
Results
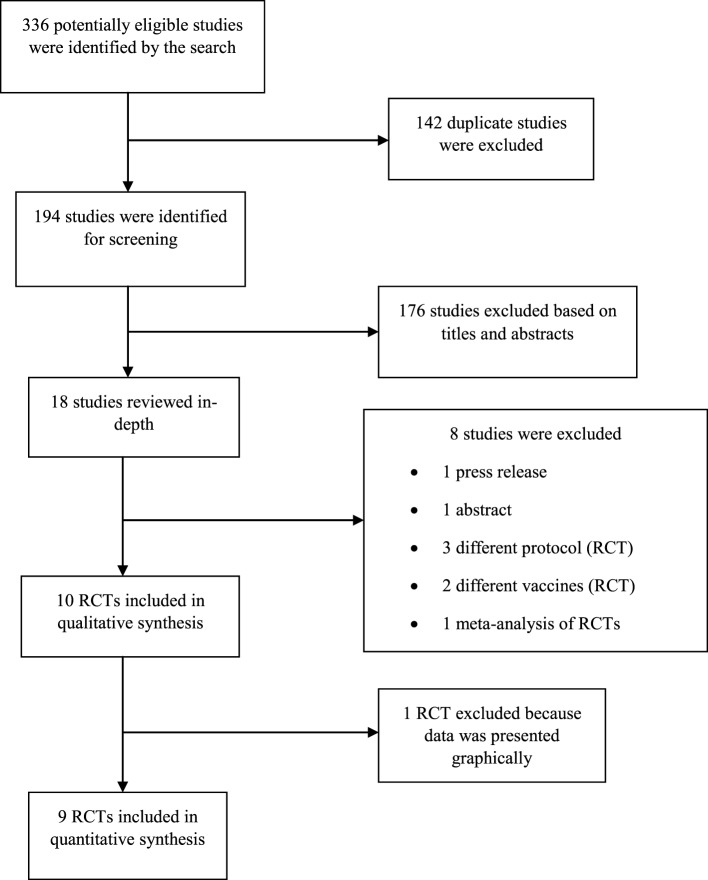
336 articles were selected for this study. Embase, Medline, Web of Science, the Cochrane Library, and ClinicalTrials.gov yielded 104, 80, 53, 46, and 53 articles, respectively (Figure 1 below). Duplicate studies were removed using Mendeley reference manager leaving 194 published articles for further analysis. Of these, 174 articles were removed based on titles and abstracts. After an in-depth review, additional eight articles were excluded, including a press release (30), an abstract (31), one paper used a two regimen vaccination protocol (32), two papers used different vaccines, Acambis (33) and PVRV (34), and one meta-analysis of RCTs previously mentioned (16). Also, two studies utilizing a different protocol of the vaccination regimen and vaccine reconstitution protocol were excluded (35, 36). At the end of the selection procedure, nine studies were found eligible for inclusion in meta-analysis (9, 17, 18, 37–42) after one study was excluded (43) because it presented all data graphically.
Figure 1.
Study selection process.
A summary of the included studies’ characteristics is presented in Table 1. Additional study activities are presented in Appendix II, Table 1 in Supplementary Material. All three studies that assessed vaccine efficacy used reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay tests to confirm dengue cases. Six studies did not specify how information regarding CYD-TDV vaccine safety was collected, while four requested parents/guardians of participants to record safety profiles. PRNT50 was utilized by all included studies to determine immunogenicity. Details regarding the aforesaid are summarized in Appendix II, Table 2 in Supplementary Material. Quality assessment of eligible studies is presented in Table 2. Regarding RR and WMD analyses the values of no difference were 1 and 0, respectively. Therefore, 95% confidence intervals (95% CI) that traversed 1 regarding the former and 0 the latter were considered statistically insignificant (alpha > 0.05). This review considered three hypotheses: CYD-TDV vaccination does not reduce the incidence of VCD cases in children; CYD-TDV vaccination increases the risk of ARs in children; and there is no difference in GMT levels between vaccinated and unvaccinated children. Variables used in meta-regression and subgroup analyses included: gender, sample size, randomization ratio, blinding method, placebo type, age group, study location, RCT phase, and flavivirus (DENV, yellow fever, and Japanese encephalitis) seroprevalence at baseline.
Table 1.
Study characteristics of the vaccine trials that meet the inclusion criteria.
| Reference | Study design (phase) | Sample size |
Age range (years) | Males |
Outcome determined | Seroprevalence at baseline |
|||
|---|---|---|---|---|---|---|---|---|---|
| CYD-TDV | Placebo | CYD-TDV (%) | Placebo (%) | CYD-TDV | Placebo | ||||
| Crevat et al. (43) | RCT (II) | 60 | 30 | 1–1.25 | 63 | 60 | Safety and immunogenicity | DENV or JE; 45% | DENV or JE; 50% |
| Villar et al. (9) | RCT (III) | 12,574 | 6,261 | 9–16 | 49.7 | 49.6 | Efficacy and safety | DENV; 80% | DENV; 77% |
| Capeding et al. (18) | RCT (III) | 6,851 | 3,424 | 2–14 | 48 | 48 | Efficacy and safety | DENV or JE; 79% | DENV or JE; 77% |
| Amar-Singh et al. (40) | RCT (III) | 199 | 51 | 2–11 | 48 | 62.7 | Safety and immunogenicity | FV-positive; 44% | |
| FV-negative; 35% | |||||||||
| Villar et al. (41) | RCT (II) | 401 | 199 | 9–16 | 49.1 | 45.7 | Safety and immunogenicity | FV; 78.8% | FV; 80.8% |
| Dayan et al. (37) | RCT (II) | 100 | 50 | 9–16 | 40 | 55 | Safety and immunogenicity | DENV or YF; 81% | DENV or YF; 84% |
| Leo et al. (38) | RCT (II) | 898 | 300 | 2–17 | 44.0 | 54.4 | Safety and immunogenicity | Not stated | Not stated |
| Tran et al. (39) | RCT (II) | 120 | 60 | 2–17 | 48 | 58 | Safety and immunogenicity | DENV or JE; 71% | DENV or JE; 78% |
| Sabchareon et al. (17) | RCT (IIb) | 2,669 | 1,333 | 4–11 | 48 | 48 | Efficacy, safety, and immunogenicity | DENV or JE; 91% | DENV or JE; 92% |
| Lanata et al. (42) | RCT (II) | 199 | 99 | 2–11 | 51 | 46 | Safety and immunogenicity | DENV; 37% | DENV; 48% |
RCT, randomized controlled trial; DENV, dengue virus; YF, yellow fever; JE, Japanese encephalitis; FV, flavivirus.
Table 2.
Risk of bias assessment.
| Reference | Selection bias |
Blinding |
Attrition | Reporting bias | Other bias | Researcher’s judgment | ||
|---|---|---|---|---|---|---|---|---|
| Randomization | Concealment | Performance | Detection | |||||
| Crevat et al. (43) | Low | Unknown | Low | Unknown | Low | Low | Unknown | Unknown |
| Villar et al. (9) | Low | Low | Low | Low | Low | Low | Unknown | Low |
| Capeding et al. (18) | Low | Low | Low | Low | Low | Low | Unknown | Low |
| Amar-Singh et al. (40) | High | Low | High | Low | Low | Low | Unknown | High |
| Villar et al. (41) | Low | Low | High | Low | Low | Low | Unknown | High |
| Dayan et al. (37) | Low | Low | High | Low | Low | Low | Unknown | High |
| Leo et al. (38) | Low | Low | High | Low | Low | Low | Unknown | High |
| Tran et al. (39) | Low | Low | High | Low | Low | Low | Unknown | High |
| Sabchareon et al. (17) | Low | Low | High | Low | Low | Low | Unknown | High |
| Lanata et al. (42) | Low | Low | Low | Low | Low | Low | Unknown | Low |
| Risk of bias | Interpretation | Within trial bias | Across trial bias | |||||
| Low | Bias, if present, is unlikely to alter the results seriously | Low risk of bias for all key domains | Most information is from trials at low risk of bias | |||||
| Unknown | A risk of bias that raises some doubt about the results | Low or unclear risk of bias for all key domains | Most information is from trials at low or unclear risk of bias | |||||
| High | Bias may alter the results seriously | High risk of bias for one or more key domains | The proportion of information from trials at high risk of bias is sufficient to affect the interpretation of results | |||||
CYD-TDV Vaccine Efficacy
The review found a statistically significant pooled overall CYD-TDV vaccine efficacy of 54% (40–64; p-value < 0.001). This result implies that the vaccine reduces the risk of acquiring dengue infection in the intervention group relative to the control group by 54%. Serotype-specific efficacy showed that CYD-TDV vaccine was more effective against DENV4 (77%: 66–85; p-value < 0.001) and DENV3 (75%: 65–82; p-value < 0.001), while it was less effective against DENV1 (50%: 36–61; p-value < 0.001), DENV2 (34%: 14–49; p-value = 0.002) and unknown DENV serotype (15%: −174–74; p-value = 0.79). There was convincing evidence to reject the pre-specified null hypothesis for all but the unknown serotype, which was not statistically significant. The main findings for CYD-TDV efficacy including evidence for the presence of heterogeneity and publication bias are summarized in Table 3.
Table 3.
Main CYD-TDV efficacy findings.
| CYD-TDV efficacy | Number of studies pooled | Intervention n/N | Control n/N | Heterogeneity (p-value) | Harbord’s test for publication bias (p-value) | RR (95% CI) | Efficacy = (RR − 1)*100 (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Overall | 3 | 338/21,736 | 386/10,832 | 61.6% (0.074) | 0.396 | 0.46 (0.36–0.60) | 54% (40–64) | <0.001 |
| DENV1 | 3 | 126/21,736 | 126/10,832 | 0.0% (0.97) | 0.482 | 0.50 (0.39–0.64) | 50% (36–61) | <0.001 |
| DENV2 | 3 | 127/21,736 | 96/10,832 | 0.0% (0.45) | 0.359 | 0.66 (0.51–0.86) | 34% (14–49) | 0.002 |
| DENV3 | 3 | 54/21,736 | 107/10,832 | 0.0% (0.91) | 0.062 | 0.25 (0.18–0.35) | 75% (65–82) | <0.001 |
| DENV4 | 3 | 35/21,736 | 78/10,832 | 0.0% (0.61) | NA | 0.23 (0.15–0.34) | 77% (66–85) | <0.001 |
| Unknown serotype | 3 | 12/21,736 | 6/10,832 | 17.3% (0.3) | NA | 0.85 (0.26–2.74) | 15% (−174–74) | 0.79 |
N, sample size; n, number of cases recorded; NA, not applicable (could not be calculated because input data contained 0 values); DENV 1–4, dengue virus serotypes.
CYD-TDV Vaccine Safety
Generally, solicited injection site reactions (any) and solicited systemic reactions (any, fever, headache, and asthenia) showed an increased but statistically insignificant risk in vaccinated children compared with unvaccinated children. Other than the aforesaid, CYD-TDV vaccine reduced the risk of ARs. However, meta-analysis of included studies with longer follow-up time (25 months) revealed that CYD-TDV vaccination increased the risk of solicited injection site reactions; RR = 1.1 (1.04–1.17; p-value = 0.001) and RR = 1.09 (0.97–1.22; p-value = 0.145) using the fixed and random effects models, respectively. However, only the fixed model showed a statistically significant risk (Appendix IV, Figure 8 in Supplementary Material). Insignificantly increased risk of solicited systemic reactions was also observed using both the random and fixed effects models (Appendix IV, Figures 10 and 11 in Supplementary Material). None of the aforementioned variables used in subgroup analyses were associated with finding of a statistically significant heterogeneity (I2 > 90%; p-value < 0.001). However, meta-regression analysis demonstrated that gender explained 95.1% (p-value = 0.049) and 96.5% (p-value = 0.002) of the variability in the studies that assessed solicited reactions and solicited injection site reactions, respectively (Appendix IV, Figures 2 and 3 in Supplementary Material). Negative gender coefficients entailed that for every unit increase in the proportion of males in the CYD-TDV group, the log RR reduced by 0.45 and 0.91 units for solicited reactions and injection site reactions, respectively. Following stratification of solicited reactions and injection site reactions by gender, heterogeneity in the subgroups of the pooled studies with more males plummeted to 0.0% (p-value < 0.41) and 61.4% (p-value = 0.035), respectively (Table 4). A study by Crevat et al. found that the most frequently reported safety profiles in the intervention group were UAEs (60–70%), solicited ARs (50–55%), solicited systemic reactions (40–50%), and solicited injection site reactions (<20%) (43). There were no immediate AEs or SAEs reported.
Table 4.
Main CYD-TDV safety findings.
| Safety profile | Number of studies combined | Intervention n/N | Control n/N | Heterogeneity (p-value) | Harbord’s test for publication bias (p-value) | RR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
| SAE | 7 | 783/24,304 | 447/12,020 | 0.0% (0.51) | 0.52 | 0.86 (0.77–0.96) | 0.009 |
| Solicited reactionsa | 2 | 133/299 | 110/149 | 0.0% (0.41) | NA | 0.64 (0.55–0.74) | <0.001 |
| UAE | 7 | 1,709/4,239 | 847/2,003 | 18.7% (0.29) | 0.02 | 0.95 (0.88–1.03) | 0.19 |
| Solicited injection site reactions occurring between day 0 and 7 post vaccination | |||||||
| Anya | 5 | 2,112/3,935 | 898/1,848 | 61.4% (0.035) | 0.64 | 1.06 (0.97–1.16) | 0.2 |
| Pain (any)a | 3 | 151/663 | 191/329 | 52.2% (0.12) | 0.48 | 0.40 (0.30–0.51) | <0.001 |
| Erythema (any) | 5 | 72/1,140 | 50/504 | 67.1% (0.016) | 0.42 | 0.55 (0.27–1.11) | 0.09 |
| Swelling (any) | 5 | 50/1,140 | 41/504 | 67.2% (0.016) | 0.37 | 0.47 (0.20–1.06) | 0.07 |
| Solicited systemic reaction occurring between day 0 and 14 post vaccination | |||||||
| Any | 7 | 2,694/4,234 | 1,266/1,998 | 33.9% (0.17) | 0.52 | 1.0 (0.95–1.06) | 0.9 |
| Fever (any) | 5 | 187/1,138 | 75/504 | 48.1% (0.1) | 0.94 | 1.08 (0.76–1.53) | 0.67 |
| Headache (any) | 5 | 401/1,140 | 173/504 | 60.8% (0.03) | 0.44 | 1.0 (0.78–1.3) | 0.97 |
| Malaise (any) | 5 | 314/1,140 | 133/504 | 55.9% (0.059) | 0.96 | 0.99 (0.75–1.32) | 0.96 |
| Myalgia (any) | 5 | 312/1,140 | 158/504 | 68.0% (0.014) | 0.788 | 0.8 (0.59–1.08) | 0.15 |
| Asthenia (any) | 5 | 177/1,140 | 69/504 | 56.8% (0.055) | 0.093 | 1.14 (0.75–1.74) | 0.55 |
N, sample size; n, number of cases recorded; NA, not applicable (could not be calculated because few studies were pooled); SAE, severe adverse events; UAE, unsolicited adverse events.
aAll parameters were determined after resolving heterogeneity by excluding divergent studies.
CYD-TDV Vaccine Induced Immunogenicity
Although it was challenging to determine the overall immunogenicity using the random effects model due to the persistence of variation in the effect sizes even after resolving serotype-specific heterogeneity, the fixed effects model generated 74.28 1/dil (69.90–78.68; p-value < 0.001). The combined serotype-specific GMT levels found after resolving heterogeneity in descending order was: DENV3 (439.7 1/dil), DENV2 (323 1/dil), DENV1 (144.1 1/dil), and DENV4 (105.71 1/dil). A different order was detected when the fixed effects model was applied; DENV3 (114.56 1/dil), DENV4 (112.34 1/dil), DENV2 (81.91 1/dil), and DENV1 (40.51 1/dil) (Appendix IV in Supplementary Material). This showed that the estimates of immunogenicity were not robust. Subgroup analysis and the systematic elimination of studies with divergent mean differences revealed that combining studies with different ages resulted in significant heterogeneity, except for DENV1. This was observed even within the same study (Appendix IV, Figures 4 and 5 in Supplementary Material). By contrast, pooling together studies with the same age resulted in low heterogeneity (I2 = 0.0%), except for DENV4 (Appendix IV, Figures 6 and 7 in Supplementary Material). The main immunogenicity findings and respective 95% CIs are summarized in Table 5. The highest neutralizing antibody titers in descending order reported by Cravat et al. were as follows: DENV3 (311–387 1/dil), DENV2 (147–213 1/dil), DENV4 (127–160 1/dil), and DENV1 (105–124 1/dil) (43). Tran et al. reported the following GMTs: DENV1—4:129, 216, 169, and 146 1/dil, respectively (39). The study further demonstrated that GMTs in children increased with increasing age: 2–5 years (64.7–143 1/dil), 6–11 years (93.9–185 1/dil) and 12–17 years (135–334 1/dil).
Table 5.
Main CYD-TDV immunogenicity findings.
| Dengue serotype | Number of studies combined | Heterogeneity (p-value) | Egger’s test for publication bias (p-value) | WMD expressed as GMTs (95% CI) | p-Value |
|---|---|---|---|---|---|
| DENV1 | 6 | 93.8% (<0.001) | 0.01 | 107.5 (70.1–144.9) | <0.001 |
| DENV1a | 5 | 11% (0.34) | 0.11 | 144.1 (117.9–170.2) | <0.001 |
| DENV2 | 6 | 95.4% (<0.001) | 0.007 | 176.9 (115.4–238.4) | <0.001 |
| DENV2a | 3 | 14.9% (0.31) | 0.53 | 323.1 (247–398.7) | <0.001 |
| DENV3 | 6 | 95.2% (<0.001) | 0.013 | 221.9 (152.6–291.2) | <0.001 |
| DENV3a | 3 | 41.6% (0.18) | 0.5 | 439.7 (331.7–547.7) | <0.001 |
| DENV4 | 6 | 94.4% (<0.001) | 0.04 | 152.9 (110.6–195.3) | <0.001 |
| DENV4a | 3 | 37.4% (0.2) | 0.65 | 105.7 (88.7–122.8) | <0.001 |
WMD, weighted mean difference expressed as GMTs; GMTs, geometric mean titers (1/dil); DENV 1–4, dengue virus serotypes.
aAll parameters were determined after resolving heterogeneity by excluding divergent studies.
Discussion
CYD-TDV Vaccine Efficacy
Although our findings show that CYD-TDV vaccine is protective against dengue infection overall, its reduced efficacy against DENV2 is extremely worrying. This is because DENV2 is known to cause severe dengue infection and is twice as likely to result in DHF/DSS compared to other serotypes (6). The Asian DENV2 has also been reported to cause outbreaks of DHF/DSS, highly pathogenic and is gradually replacing the less pathogenic Latin American variant (44). All efficacy studies conducted in Asia demonstrated reduced and statistically insignificant vaccine efficacy against DENV2 compared with the one conducted in Latin America. This might indicate that CYD-TDV vaccine induced antibodies readily neutralize the less pathogenic Latin American than the highly virulent Asian DENV2 variant. It has been reported that DENV2 neutralizing antibodies induced after primary infection with DENV1 demonstrate differential neutralizing activity against the Asian and Latin American DENV2 variants (45).
It is worth noting that the observed 32% CYD-TDV vaccine protection against DENV2 is merely the best estimate. The real vaccine efficacy in the population can be as low as 14% (Table 3). Another important point to note is that CYD-TDV vaccine induced DENV2 neutralizing antibodies had the second highest GMT levels and yet provided the least protection. The question is why? Villar et al. concluded that GMT levels elicited by CYD-TDV vaccine do not reflect serotype-specific vaccine efficacy (9). It has also been suggested that dengue antibodies either might not be the immunological correlate of protection or that each dengue serotype has its own protective titer threshold (46). Both findings correspond with our results which show that CYD-TDV vaccine protection was highest for the serotype with the lowest GMT levels and so on (Tables 3 and 5). Microevolution due to genetic recombination and natural selection occurring within individual dengue serotypes might explain lower efficacy of the CYD-TDV vaccine against DENV2 (47–49). Wahala et al. have shown that mutations occurring in the E protein (the major target for dengue neutralizing antibodies) have an effect on antibody binding and neutralizing activity (50). Therefore, reduced vaccine efficacy against DENV2 could be due to the fact that the circulating DENV2 virus acquired mutations in the E protein hence becoming antigenically and genetically different from the one included in the CYD-TDV vaccine. Reduced DENV2 vaccine efficacy might as well be as a result of dominant CYD-TDV vaccine induced antibodies that lack or have low serotype-specific neutralizing activity against the circulating DENV2 serotype. This is because high levels of serotype-specific neutralizing antibodies are known to confer protection against subsequent DENV infection (45). Evidence depicting a similar situation can be derived from the seasonal influenza vaccine which was only 23% effective in vaccinees that were infected with a subtype that was different from the one included in the vaccine (51). Although information regarding the unknown DENV serotype is limited, a study by Mustafa et al. has proposed the emergence of a DENV5 serotype (48). By contrast, Hesse argues that the aforesaid is highly unlikely because DENV mutation rate is too low to lead to the creation of a new serotype (44).
CYD-TDV Vaccine Safety
Our overall findings regarding the safety of CYD-TDV vaccine are in agreement with the findings by da Costa et al. (16) in that none of the increased AEs and ARs were statistically significant. Crevat et al. also concluded that CYD-TDV vaccine does not increase the risk of AEs and ARs in children below 2 years (43). However, this study had a small sample size (N = 90), short follow-up time (18 months), and it is not clear how allocation concealment was done. All of which might lead to the actual effect of CYD-TDV vaccine being overestimated. Contrary to the aforesaid, revelations from meta-analysis of studies with longer follow-up time indicate that CYD-TDV increases the risk of injection site reactions significantly thus making it difficult to reject the pre-specified hypothesis (Appendix IV, Figure 8 in Supplementary Material). Similarly, post hoc analysis of the data from Capeding et al. (18) has demonstrated that the risk of hospitalization and severe dengue in children aged between 2 and 5 years vaccinated with CYD-TDV was highly significant (RR = 7.45: 1.15–313.8, p-value not provided) (52). However, post hoc meta-analysis of all studies conducted to assess CYD-TDV vaccine safety in children is required before a comprehensive conclusion can be made.
CYD-TDV Vaccine Induced Immunogenicity
Our immunogenicity findings show that GMTs significantly increased in CYD-TDV vaccinated compared to unvaccinated children. Children in Latin America had higher GMT levels compared to those in Asia. Da Costa et al. (16) have reported similar results and evidence from our findings is sufficient to reject the pre-specified null hypothesis. However, there was significant variation in the effect sizes presented in the included studies. Interestingly, reduced heterogeneity was observed when studies with the same age group were combined regardless of study location except for DENV4. By contrast, combining studies with different age groups resulted in significantly increased heterogeneity regardless of study location and, surprisingly, even within the same study. Furthermore, we found that GMT effect sizes increased as the age of study participants increased, which corresponds with the findings by Tran et al. (39). Taken together, our findings clearly demonstrate that age influences CYD-TDV vaccine induced GMT levels. Included studies demonstrated that prior exposure to dengue viruses increased antibody response during subsequent infection. The observed variations in GMT levels among the included studies might be explained by variations in the burden of dengue infection across countries and between the two regions, with Asian countries experiencing a higher burden compared with Latin American countries (53). Although the PRNT is considered the gold standard, discrepancies between laboratories and regions have been reported (54, 55). Furthermore, the scientific community has different views regarding this test. Rainwater-Lovett et al. and Thomas et al. have separately reported that PRNT gives varying results based on the test conditions applied (56, 57). Endy has described as confusing the fact that PRNT does not give information on whether an individual will be protected from subsequent dengue infection using cross-reactive neutralizing antibodies (58). The former concern might be responsible for the observed variations in CYD-TDV vaccine elicited GMT levels across the included studies, while the later may be more applicable to the reduced vaccine efficacy against DENV2 as stated above. To the contrary, Timiryasova et al. have concluded that the PRNT test is fit for purpose and can “detect and measure dengue serotype-specific neutralizing antibodies in human serum samples with acceptable intra-assay and inter-assay precision, accuracy/dilutability, specificity, and with a lower limit of quantitation of 10” (59).
Strengths and Limitations
This, to the best of our knowledge, is the first systematic review to assess CYD-TDV vaccine efficacy not only in children exclusively but also using primary studies that were designed specifically to determine vaccine efficacy. Our review has demonstrated that children of varying ages respond to CYD-TDV vaccination differently and gender imbalance in the CYD-TDV group introduces heterogeneity when assessing solicited reactions and injection site reactions. Excluding graphically presented data prevented estimation bias. Since all of the included studies were conducted in children located in Asia and Latin America, the findings cannot be generalized to children of all endemic regions.
Implications for Public Health and Research
Emergence of novel and virulent dengue serotypes has been proposed. To prevent possible pandemics, there is need to strengthen vector control programs, dengue surveillance, diagnostic capabilities and management, and research through capacity building in endemic settings such as Asia, Latin America, and especially Africa, where dengue infection happens to be neglected (60, 61). DENV2 included in the CYD-TDV vaccine needs to be updated to match the currently circulating Asian and Latin American variants. In addition, understanding of dengue neutralizing antibodies by investigating whether they are correlates of protection and what titer thresholds against all four serotypes are optimal for protection is warranted. Therefore, the scientific community needs to quickly come to a consensus regarding the PRNT test. Otherwise, new serological tests capable of being standardized to enable inter laboratory comparability, reproducibility and that can accurately and specifically measure dengue correlates of protection must be developed given that this is crucial for vaccine development. The fact that dengue infection is known to cause DHF/DSS and death in children is an indication that passive immunotherapy using serotype-specific neutralizing monoclonal antibodies that target conserved regions of the E protein might be a viable alternative to vaccination. Passive immunotherapy might significantly benefit younger children who are at higher risk and yet respond poorly to vaccines immunologically. Another reason for considering passive immunotherapy is that higher titers of serotype-specific neutralizing monoclonal antibodies can be administered without enhancing ADE, which is mainly caused by low titer levels of cross-reactive antibodies as previously mentioned. Future vaccine trials should consider employing ≥25 months follow-up time, stratify and provide age specific data to facilitate comprehensive and conclusive analyses. Finally, post hoc analysis can reveal vital vaccine-related safety information missed during the duration of the clinical trial. Therefore, the aforementioned analysis should be considered and encouraged where complete clinical data of participants involved in clinical trials are available.
Conclusion
Overall, CYD-TDV vaccine is effective, but less efficacious against DENV2 in children. CYD-TDV vaccine is immunogenic in children with lower GMT levels observed in younger children compared to adolescents. Although the vaccine increased the risk of some safety parameters in vaccinated children insignificantly, meta-analysis of studies with long follow-up time revealed that CYD-TDV vaccine significantly increased the risk of solicited injection site reactions. Therefore, post hoc meta-analysis of the long term follow-up data (≥25 months) collected from the children previously vaccinated with CYD-TDV are needed before a comprehensive conclusion regarding CYD-TDV vaccine safety in children can be made. However, given the urgency for a dengue vaccine in endemic regions, CYD-TDV should be considered for use in regions where DENV2 is not endemic as currently there is no specific treatment for dengue infection.
Author Contributions
MM designed the study, performed the statistical analysis, and wrote the final report. SK, NT, and GM independently performed searches, selection, and data extraction of published articles. AR resolved disagreements from searches, selection, and data extraction. MB resolved disagreements from searches, selection, and data extraction. All authors made contributions and reviewed the final report.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Sincere gratitude goes to William Spence and Hilda Emengo from the Institute of Health and Wellbeing, University of Glasgow and Healthcare Improvement Scotland, respectively, for agreeing to supervise this work. Further gratitude goes to the Chevening Secretariat (Foreign and Commonwealth Office, United Kingdom) for its financial support that led to the realization of this work. SK and AR were supported by RSF grant 15-14-00016 and Program of Competitive Growth of Kazan Federal University. RA was also supported by state assignment 20.5175.2017/6.7 of the Ministry of Education and Science of Russian Federation.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00863/full#supplementary-material.
Abbreviations
ADE, antibody-dependent enhancement; AE, adverse events; AR, adverse reactions; CYD-TDV, live-tetravalent dengue vaccine; DENV, dengue virus; DHF, dengue hemorrhagic fever; DSS, dengue shock syndrome; ELISA, enzyme-linked immunosorbent assay; GMT, geometric mean titre; JE, Japanese encephalitis; PRISMA, preferred reporting items for systematic reviews and meta-analyses; PRNT50, plaque reduction neutralisation test with a 50% plaque reduction threshold; RCT, randomized controlled trial; RR, relative risk; RT-PCR, reverse transcription polymerase chain reaction; SAE, severe adverse events; UAE, unsolicited adverse events; UN, United Nations; VCD, virologically confirmed dengue; WHO, World Health Organization; WMD, weighted mean difference; YF, yellow fever.
References
- 1.Ramakrishnan L, Radhakrishna Pillai M, Nair RR. Dengue vaccine development: strategies and challenges. Viral Immunol (2015) 28(2):76–84. 10.1089/vim.2014.0093 [DOI] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue in the Americas and Southeast Asia: do they differ? Rev Panam Salud Publica (2006) 20(6):407–15. 10.1590/S1020-49892006001100007 [DOI] [PubMed] [Google Scholar]
- 3.Yacoub S, Mongkolsapaya J, Screaton G. The pathogenesis of dengue. Curr Opin Infect Dis (2013) 26(3):284–9. 10.1097/QCO.0b013e32835fb938 [DOI] [PubMed] [Google Scholar]
- 4.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis (2012) 6(8):e1760. 10.1371/journal.pntd.0001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature (2013) 496(7446):504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murrell S, Wu SC, Butler M. Review of dengue virus and the development of a vaccine. Biotechnol Adv (2011) 29(2):239–47. 10.1016/j.biotechadv.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 7.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine (2010) 28(3):632–49. 10.1016/j.vaccine.2009.09.098 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Global Strategy for Dengue Prevention and Control 2012–2020. (2012). Available from: http://apps.who.int/iris/bitstream/10665/75303/1/9789241504034_eng.pdf
- 9.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med (2015) 372:113–23. 10.1056/NEJMoa1411037 [DOI] [PubMed] [Google Scholar]
- 10.WHO. Dengue and Severe Dengue. (2015). Available from: http://www.who.int/mediacentre/factsheets/fs117/en/
- 11.Screaton G, Mongkolsapaya J, Yacoub S, Roberts C. New insights into the immunopathology and control of dengue virus infection. Nat Rev Immunol (2015) 15(12):745–59. 10.1038/nri3916 [DOI] [PubMed] [Google Scholar]
- 12.Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis (2007) 30(5–6):329–40. 10.1016/j.cimid.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 13.Faheem M, Raheel U, Riaz MN, Kanwal N, Javed F, Us Sahar Sadaf Zaidi N, et al. A molecular evaluation of dengue virus pathogenesis and its latest vaccine strategies. Mol Biol Rep (2011) 38(6):3731–40. 10.1007/s11033-010-0488-1 [DOI] [PubMed] [Google Scholar]
- 14.Tan GK, Alonso S. Pathogenesis and prevention of dengue virus infection: state-of-the-art. Curr Opin Infect Dis (2009) 22(3):302–8. 10.1097/QCO.0b013e328329ae32 [DOI] [PubMed] [Google Scholar]
- 15.Yauch LE, Shresta S. Dengue virus vaccine development. Adv Virus Res (2014) 88:315–72. [DOI] [PubMed] [Google Scholar]
- 16.da Costa VG, Marques-Silva AC, Floriano VG, Moreli ML. Safety, immunogenicity and efficacy of a recombinant tetravalent dengue vaccine: a meta-analysis of randomized trials. Vaccine (2014) 32(39):4885–92. 10.1016/j.vaccine.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 17.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet (2012) 380(9853):1559–67. 10.1016/S0140-6736(12)61428-7 [DOI] [PubMed] [Google Scholar]
- 18.Capeding MR, Tran NH, Hadinegoro SRS, Ismail HIHM, Chotpitayasunondh T, Chua MN, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet (2014) 384(9951):1358–65. 10.1016/S0140-6736(14)61060-6 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928–5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint – preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther (2009) 89(9):873–80. 10.1136/bmj.b2535 [DOI] [PubMed] [Google Scholar]
- 21.United Nations. Convention on the Rights of the Child. (1989). p. 1–23. Available from: http://www.hrweb.org/legal/child.html
- 22.Hombach J. Guidelines for clinical trials of dengue vaccine in endemic areas. J Clin Virol (2009) 46(Suppl 2):S7–9. 10.1016/S1386-6532(09)70287-2 [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Glossary of terms used in pharmacovigilance. WHO Program Int Drug Monit. (2013). p. 1–6. Available from: http://pvtoolkit.org/toolkit/readers/glossary_of_terms.pdf
- 24.World Health Organization. Guidelines for Plaque Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Geneva: WHO; (2007). 26 p. [DOI] [PubMed] [Google Scholar]
- 25.Long L. Routine piloting in systematic reviews – a modified approach? Syst Rev (2014) 3:77. 10.1186/2046-4053-3-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonita R, Beaglehole R, Kjellström T. Basic Epidemiology. 2nd ed Geneva: World Health Organization; (2006). Available from: http://apps.who.int/iris/bitstream/10665/43541/1/9241547073_eng.pdf [Google Scholar]
- 27.Higgins J, Green S. In: Julian PT Higgins and Sally Green , editor. Cochrane Handbook for Systematic Reviews of Interventions. West Sussex: JohnWiley & Sons Ltd; (2011). [Google Scholar]
- 28.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods (2006) 11(2):193–206. 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- 29.Borenstein M, Hedges LV, Higgins J, Rothstein HR. Fixed-effect versus random-effects models. Introduction to Meta-Analysis. West Sussex: John Wiley & Sons Ltd; (2009). p. 77–85. [Google Scholar]
- 30.Riedmann EM. Human vaccines & immunotherapeutics: news. Hum Vaccin Immunother (2013) 9(10):2034–7. 10.4161/hv.26189 [DOI] [PubMed] [Google Scholar]
- 31.Morrison D, Capeding MR, Poo JL, Forrat R, Zambrano B, Wartel-Tram A, et al. Safety and infectivity of tetravalent Chimeric live attenuated dengue vaccine in different age populations in endemic and non-endemic areas. Am J Trop Med Hyg (2007) 77(5S):99. [Google Scholar]
- 32.Crevat D, Reynolds D, Langevin E, Capeding MR. Safety and immunogenicity of a tetravalent dengue vaccine in flavivirus-naive and -immune pediatric populations with two vaccination regimens. Am J Trop Med Hyg (2009) 81(5):113. [Google Scholar]
- 33.Bouckenooghe A, Capeding MR, Morrison DN, Poo JL, Lang J, Chambonneau L, et al. Safety and immunogenicity in children and adults from endemic countries and adults from nonendemic countries of a tetravalent, live attenuated dengue vaccine. Am J Trop Med Hyg (2008) 79(6):114. [Google Scholar]
- 34.Sabchareon A, Lang J, Chanthavanich P, Yoksan S, Forrat R, Attanath P, et al. Safety and immunogenicity of a three dose regimen of two tetravalent live-attenuated dengue vaccines in five- to twelve-year-old Thai children. Pediatr Infect Dis J (2004) 23(2):99–109. 10.1097/01.inf.0000109289.55856.27 [DOI] [PubMed] [Google Scholar]
- 35.Poo J, Galan F, Forrat R, Zambrano B, Lang J, Dayan GH, et al. Live-attenuated tetravalent dengue vaccine in dengue-naive children, adolescents, and adults in Mexico City: randomized controlled phase 1 trial of safety and immunogenicity. Pediatr Infect Dis J (2011) 30(1):e9–17. 10.1097/INF.0b013e3181fe05af [DOI] [PubMed] [Google Scholar]
- 36.Capeding RZ, Luna IA, Bomasang E, Lupisan S, Lang J, Forrat R, et al. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: randomized controlled phase I trial in the Philippines. Vaccine (2011) 29(22):3863–72. 10.1016/j.vaccine.2011.03.057 [DOI] [PubMed] [Google Scholar]
- 37.Dayan GH, Garbes P, Noriega F, De Sadovsky ADI, Rodrigues PM, Giuberti C, et al. Immunogenicity and safety of a recombinant tetravalent dengue vaccine in children and adolescents ages 9-16 years in Brazil. Am J Trop Med Hyg (2013) 89(6):1058–65. 10.4269/ajtmh.13-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leo YS, Wilder-Smith A, Archuleta S, Shek LP, Chong CY, Leong HN, et al. Immunogenicity and safety of recombinant tetravalent dengue vaccine (CYD-TDV) in individuals aged 2-45: phase II randomized controlled trial in Singapore. Hum Vaccines Immunother (2012) 8(9):1259–71. 10.4161/hv.21224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran NH, Luong CQ, Vu TQH, Forrat R, Lang J, Vu QD, et al. Safety and immunogenicity of recombinant, live attenuated tetravalent dengue vaccine (CYD-TDV) in healthy Vietnamese adults and children. J Vaccines Vaccin (2012) 3(7):1–8. 10.4172/2157-7560.1000162 [DOI] [Google Scholar]
- 40.Amar-Singh HSS, Koh MT, Tan KK, Chand LG, Zhou L, Bouckenooghe A, et al. Safety and immunogenicity of a tetravalent dengue vaccine in healthy children aged 2-11 years in Malaysia: a randomized, placebo-controlled, phase III study. Vaccine (2013) 31(49):5814–21. 10.1016/j.vaccine.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 41.Villar LA, Rivera-Medina DM, Arredondo-García JL, Boaz M, Starr-Spires L, Thakur M, et al. Safety and immunogenicity of a recombinant tetravalent dengue vaccine in 9-16 year olds a randomized, controlled, phase II trial in Latin America. Pediatr Infect Dis J (2013) 32(10):1102–9. 10.1097/INF.0b013e31829b8022 [DOI] [PubMed] [Google Scholar]
- 42.Lanata CF, Andrade T, Gil AI, Terrones C, Valladolid O, Zambrano B, et al. Immunogenicity and safety of tetravalent dengue vaccine in 2-11 year-olds previously vaccinated against yellow fever: randomized, controlled, phase II study in Piura, Peru. Vaccine (2012) 30(41):5935–41. 10.1016/j.vaccine.2012.07.043 [DOI] [PubMed] [Google Scholar]
- 43.Crevat D, Brion JD, Gailhardou S, Laot TM, Capeding MR. First experience of concomitant vaccination against dengue and MMR in toddlers. Pediatr Infect Dis J (2015) 34(8):884–92. 10.1097/INF.0000000000000752 [DOI] [PubMed] [Google Scholar]
- 44.Hesse RR. Dengue virus evolution and virulence models. Clin Infect Dis (2007) 44(11):1462–6. 10.1086/517587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moi ML, Takasaki T, Kurane I. Human antibody response to dengue virus: implications for dengue vaccine design. Trop Med Health (2016) 44(1):1. 10.1186/s41182-016-0004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas SJ, Rothman AL. Trials and tribulations on the path to developing a dengue vaccine. Am J Prev Med (2015) 49(6):S334–44. 10.1016/j.amepre.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 47.Haven N. Molecular evolution of dengue type 2 virus in Thailand. Trop Med (1998) 58(1):96–101. [DOI] [PubMed] [Google Scholar]
- 48.Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of dengue virus (denv-5): a new public health dilemma in dengue control. Med J Armed Forces India (2015) 71(1):67–70. 10.1016/j.mjafi.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malavige GN, Fernando S, Fernando DJ, Seneviratne SL. Dengue viral infections. Postgrad Med J (2004) 80(948):588–601. 10.1136/pgmj.2004.019638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wahala MPB, Donaldson EF, de Alwis R, Accavitti-Loper MA, Baric RS, de Silva AM. Natural strain variation and antibody neutralization of dengue serotype 3 viruses. PLoS Pathog (2010) 6(3):e1000821. 10.1371/journal.ppat.1000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization. Questions and Answers: Vaccine Effectiveness Estimates for Seasonal Influenza Vaccines. (2015). p. 1–4. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/201502_qanda_vaccineeffectiveness.pdf
- 52.World Health Organization. Safety of CYD-TDV Dengue Vaccine: Weekly Epidemiological Record. Geneva: WHO; (2016). p. 421–8. Available from: http://www.who.int/vaccine_safety/committee/reports/wer9034.pdf [Google Scholar]
- 53.L’Azou M, Moureau A, Sarti E, Nealon J, Zambrano B, Wartel TA, et al. Symptomatic dengue in children in 10 Asian and Latin American countries. N Engl J Med (2016) 374(12):1155–66. 10.1056/NEJMoa1503877 [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. Prev Control (2009) 409(3):160. [PubMed] [Google Scholar]
- 55.Tang KF, Ooi EE. Diagnosis of dengue: an update. Expert Rev Anti Infect Ther (2012) 10(8):895–907. 10.1586/eri.12.76 [DOI] [PubMed] [Google Scholar]
- 56.Thomas SJ, Nisalak A, Anderson KB, Libraty DH, Kalayanarooj S, Vaughn DW, et al. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: how alterations in assay conditions impact performance. Am J Trop Med Hyg (2009) 81(5):825–33. 10.4269/ajtmh.2009.08-0625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rainwater-Lovett K, Rodriguez-Barraquer I, Cummings DA, Lessler J. Variation in dengue virus plaque reduction neutralization testing: systematic review and pooled analysis. BMC Infect Dis (2012) 12(1):233. 10.1186/1471-2334-12-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Endy TP. Human immune responses to dengue virus infection: lessons learned from prospective cohort studies. Front Immunol (2014) 5:183. 10.3389/fimmu.2014.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Timiryasova TM, Bonaparte MI, Luo P, Zedar R, Hu BT, Hildreth SW. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am J Trop Med Hyg (2013) 88(5):962–70. 10.4269/ajtmh.12-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lam SK. Challenges in reducing dengue burden; diagnostics, control measures and vaccines. Expert Rev Vaccines (2013) 12(9):995–1010. 10.1586/14760584.2013.824712 [DOI] [PubMed] [Google Scholar]
- 61.Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS. Dengue virus infection in Africa. Emerg Infect Dis (2011) 17(8):1349–54. 10.3201/eid1708.101515 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.