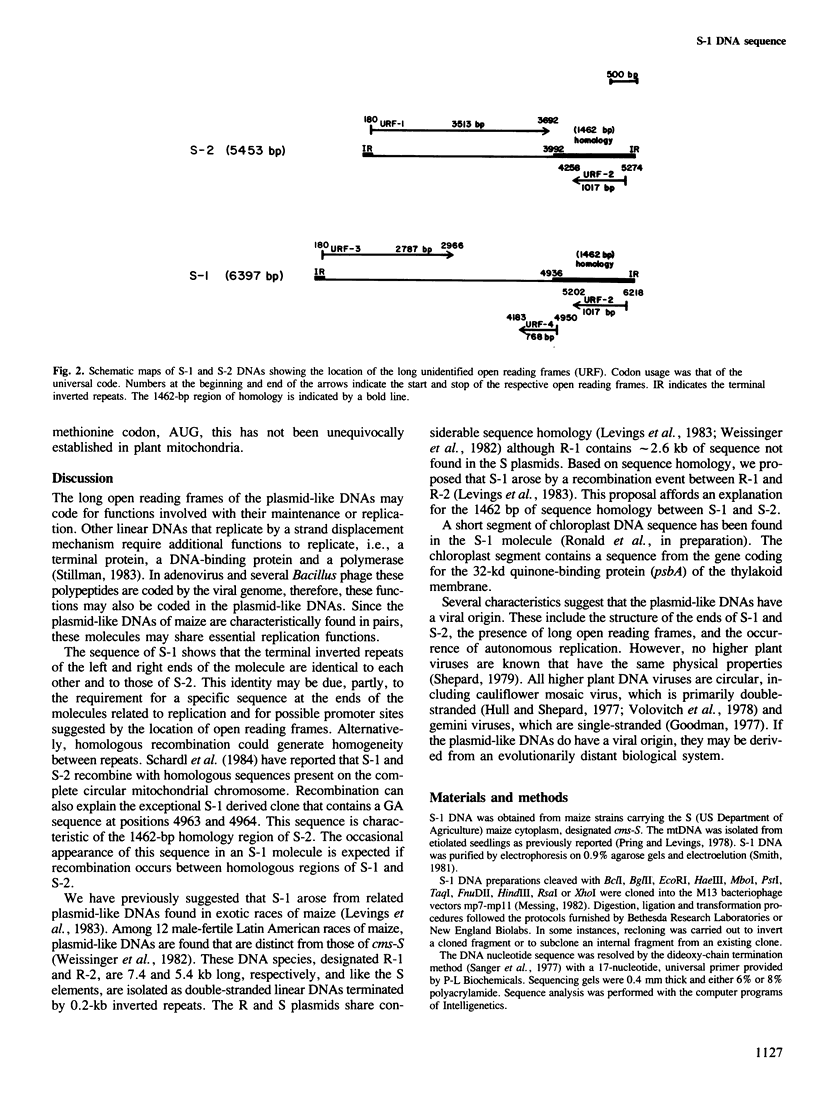
Abstract
Mitochondria from the S male-sterile cytoplasm of maize contain unique DNA-protein complexes, designated S-1 and S-2. These complexes consist of double-stranded linear DNAs with proteins covalently attached to the 5' termini. To learn more about these unusual DNAs we have determined the complete nucleotide sequence of the S-1 DNA molecule (6397 bp). The sequence of S-2 has been previously determined. S-1 and S-2 are structurally similar and contain ˜1.7kb of sequence homology. S-1 is terminated by exact 208-bp inverted repeats that are identical with the terminal inverted repeats of S-2. S-1 and S-2 also contain a 1462-bp region of nearly perfect homology, which includes one of the terminal inverted repeats. The homology between the two molecules may be maintained, in part, by homologous recombination. S-1 has three long unidentified open reading frames, URF2 (1017 bp), URF3 (2787 bp) and URF4 (768 bp). URF2 occurs in the 1462-bp region of homology and is identical in length and location in both S-1 and S-2. Based on their structural organization and their viral-like characteristics, we propose that S-1 and S-2 code for functions involved with their maintenance and replication.
Keywords: plasmid-like DNAs, terminal inverted repeats, cytoplasmic male sterility, nucleotide sequence
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Harding N. E., Ito J., David G. S. Identification of the protein firmly bound to the ends of bacteriophage phi 29 DNA. Virology. 1978 Feb;84(2):279–292. doi: 10.1016/0042-6822(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Hiesel R., Brennicke A. Cytochrome oxidase subunit II gene in mitochondria of Oenothera has no intron. EMBO J. 1983;2(12):2173–2178. doi: 10.1002/j.1460-2075.1983.tb01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R., Shepherd R. J. The structure of cauliflower mosaic virus genome. Virology. 1977 Jun 1;79(1):216–230. doi: 10.1016/0042-6822(77)90346-4. [DOI] [PubMed] [Google Scholar]
- Ito J. Bacteriophage phi29 terminal protein: its association with the 5' termini of the phi29 genome. J Virol. 1978 Dec;28(3):895–904. doi: 10.1128/jvi.28.3.895-904.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble R. J., Mans R. J. Examination of the mitochondrial genome of revertant progeny from S cms maize with cloned S-1 and S-2 hybridization probes. J Mol Appl Genet. 1983;2(2):161–171. [PubMed] [Google Scholar]
- Kemble R. J., Thompson R. D. S1 and S2, the linear mitochondrial DNAs present in a male sterile line of maize, possess terminally attached proteins. Nucleic Acids Res. 1982 Dec 20;10(24):8181–8190. doi: 10.1093/nar/10.24.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughnan J. R., Gabay-Laughnan S. Cytoplasmic male sterility in maize. Annu Rev Genet. 1983;17:27–48. doi: 10.1146/annurev.ge.17.120183.000331. [DOI] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Kim B. D., Pring D. R., Conde M. F., Mans R. J., Laughnan J. R., Gabay-Laughnan S. J. Cytoplasmic Reversion of cms-S in Maize: Association with a Transpositional Event. Science. 1980 Aug 29;209(4460):1021–1023. doi: 10.1126/science.209.4460.1021. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M., Thompson R. D., Hodge T. P. The integrated forms of the S1 and S2 DNA elements of maize male sterile mitochondrial DNA are flanked by a large repeated sequence. Nucleic Acids Res. 1981 Aug 11;9(15):3657–3669. doi: 10.1093/nar/9.15.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring D. R., Levings C. S. Heterogeneity of Maize Cytoplasmic Genomes among Male-Sterile Cytoplasms. Genetics. 1978 May;89(1):121–136. doi: 10.1093/genetics/89.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring D. R., Levings C. S., Hu W. W., Timothy D. H. Unique DNA associated with mitochondria in the "S"-type cytoplasm of male-sterile maize. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2904–2908. doi: 10.1073/pnas.74.7.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- Stillman B. W. The replication of adenovirus DNA with purified proteins. Cell. 1983 Nov;35(1):7–9. doi: 10.1016/0092-8674(83)90201-5. [DOI] [PubMed] [Google Scholar]
- Volovitch M., Drugeon C., Yot P. Studies on the single-stranded discontinuities of the cauliflower mosaic virus genome. Nucleic Acids Res. 1978 Aug;5(8):2913–2925. doi: 10.1093/nar/5.8.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O. Genome-linked protein associated with the 5' termini of bacteriophage phi29 DNA. J Virol. 1978 Sep;27(3):776–783. doi: 10.1128/jvi.27.3.776-783.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Ito J. Terminal proteins and short inverted terminal repeats of the small Bacillus bacteriophage genomes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2596–2600. doi: 10.1073/pnas.78.4.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]


