Abstract
Lymphangiogenesis is essential for fluid homeostasis in vascularized tissues. In the normally avascular cornea, however, pathological lymphangiogenesis mediates diseases like corneal transplant rejection, dry eye disease, and allergy. So far, a physiological role for lymphangiogenesis in a primarily avascular site such as the cornea has not been described. Using a mouse model of perforating corneal injury that causes acute and severe fluid accumulation in the cornea, we show that lymphatics transiently and selectively invade the cornea and regulate the resolution of corneal edema. Pharmacological blockade of lymphangiogenesis via VEGFR-3 inhibition results in increased corneal thickness due to delayed drainage of corneal edema and a trend towards prolonged corneal opacification. Notably, lymphatics are also detectable in the cornea of a patient with acute edema due to spontaneous Descemet´s (basement) membrane rupture in keratoconus, mimicking this animal model and highlighting the clinical relevance of lymphangiogenesis in corneal fluid homeostasis. Together, our findings provide evidence that lymphangiogenesis plays an unexpectedly beneficial role in the regulation of corneal edema and transparency. This might open new treatment options in blinding diseases associated with corneal edema and transparency loss. Furthermore, we demonstrate for the first time that physiological lymphangiogenesis also occurs in primarily avascular sites.
Introduction
The lymphatic vascular system is essential for fluid and lipid homeostasis and is critically involved in the regulation of inflammation, immunity, and cancer dissemination1, 2. Therefore, lymphatic vessels are present in almost every tissue of the organism. One exception is the transparent cornea, which is physiologically devoid of both blood and lymphatic vessels3. This state, which is termed corneal “angiogenic privilege”, is essential for proper vision and is actively maintained by several antiangiogenic mechanisms4–10. However, this angiogenic privilege of the cornea is not invulnerable: whereas minor inflammatory vascular stimuli are buffered and do not induce an angiogenic response, severe tissue damage and inflammation can “overwhelm” the cornea’s antiangiogenic mechanisms and lead to a secondary ingrowth of blood and clinically invisible lymphatic vessels from the adjacent limbus towards the central cornea3, 11. Studies have demonstrated the presence of corneal lymphatic vessels in various injury models including chemical injury such as silver nitrate12 or alkali burn13, thermal injury14, corneal suture placement15, herpetic keratitis16, and cornea transplantation17. In these models, lymphatic vessels and blood vessels seem to grow in parallel into the cornea. In contrast, corneal lymphangiogenesis without concurrent hemangiogenesis has been detected in mouse models of dry eye and allergic disease18, 19. Although the knowledge on the specific function of corneal lymphatic vessels is growing, a functional role for these vessels has only been demonstrated in the context of corneal transplantation, dry eye and allergic disease20, 21. In these diseases, lymphatic vessels seem to facilitate the migration of antigen presenting cells from the cornea to the regional lymph nodes, where accelerated sensitization against allo- or autoantigens occurs5, 18, 19, 21–24. Thus, corneal lymphatic vessels seem to contribute to the induction and progression of corneal transplant rejection, dry eye and allergic disease and are therefore mostly considered undesirable. Importantly, blockade of corneal lymphangiogenesis has been shown to promote corneal transplant survival and ameliorate dry eye or allergic disease17, 19, 22, 23, 25.
Although a plethora of studies have demonstrated pathological and undesirable functions for corneal lymphangiogenesis, evidence for beneficial, physiological functions for corneal lymphangiogenesis in clinically relevant diseases was so far missing. In contrast, studies in extraocular tissues, such as the skin, demonstrate that lymphangiogenesis is important e.g. for appropriate wound repair. In this regard, lymphatic vessels regulate tissue pressure, prevent the development of edema and allow the drainage and removal of fluid and cell debris from the inflamed site1, 2, 26. Moreover, lymphangiogenesis is also important to terminate inflammatory responses in the skin, as recent studies indicate that the blockade of cutaneous lymphatic vessels can result in chronic inflammation and edema, whereas the specific activation of lymphatic vessels can ameliorate these conditions27–29.
It remains elusive, whether lymphatic vessels at the cornea might also have physiological, possibly beneficial functions after tissue damage, e.g. facilitating adequate wound healing or regulation of corneal edema. We therefore investigated whether a corneal injury that causes acute corneal edema is accompanied by the ingrowth of lymphatic vessels and whether corneal lymphangiogenesis contributes to the physiological healing response.
Results
Corneal perforating incision injury results in transient corneal edema and selective corneal lymphangiogenesis
We first analyzed whether a corneal injury that leads to a profound increase in corneal edema results in corneal lymphangiogenesis. For this purpose, we used an established mouse model of perforating corneal incision injury, which is known to induce transient edema formation and opacification30 (Fig. 1A to E). Immunohistochemical analysis of corneal whole mounts at various time points after injury revealed that corneal incision injury results in significant corneal lymph- but not hemangiogenesis. LYVE-1high/CD31low lymphatic vessels growing towards the central cornea were clearly detectable 1 week after injury (lymphvascularized area in uninjured: 2.09% versus after 1 week: 7.35%, p < 0.001; Fig. 1F,G) and persisted until 2 weeks after injury (after 2 weeks: 6.87%, p < 0.001; Fig. 1H). Afterwards, corneal lymphatic vessels regressed. After 4 weeks, the amount of corneal lymphangiogenesis was comparable to uninjured corneas (after 4 weeks: 3.45%, p > 0.05; Fig. 1I). In contrast to corneal lymphatic vessels, we could not detect any ingrowth of LYVE-1neg/CD31high blood vessels into the cornea after injury (Fig. 1F to I). In addition, we analyzed gene expression of the major prolymphangiogenic growth factors VEGF-C and VEGF-D and the corresponding receptor VEGFR-3 by real-time PCR. Incision injury led to a significant increase in corneal VEGF-C and VEGF-D expression: VEGF-C and VEGF-D mRNA expression levels peaked at 1 week after injury and declined afterwards (Supplemental Figure 1A and B). Similarly, VEGFR-3 mRNA expression was also strongly upregulated: expression levels were significantly increased after 1 week and decreased afterwards, but were still elevated when compared to uninjured corneas (Supplemental Figure 1C). Gene expression of VEGFR-2, which can in addition to VEGF-C also be activated by VEGF-A and induce corneal hemangiogenesis, was significantly reduced during the first 2 weeks after injury before returning to normal levels after 4 weeks (Supplemental Figure 1D). We also analyzed mRNA expression of the soluble forms of VEGFR-2 and VEGFR-3 (sVEGFR-2 and sVEGFR-3, respectively), which both have been shown to contribute to corneal alymphaticity5, 6. After incision injury, mRNA expression of sVEGFR-2 significantly decreased after 1 and 2 weeks and returned to normal values after 4 weeks (Supplemental Figure 1E), whereas mRNA expression of sVEGFR-3 was upregulated at later time points (Supplemental Figure 1F). Gene expression of PROX-1, a master regulator of lymphatic endothelial cell differentiation, was significantly elevated at all analyzed time points (Supplemental Figure 1G).
Figure 1.

Corneal perforating incision injury induces transient edema and results in isolated lymphangiogenesis without hemangiogenesis. (A) Mouse model of central perforating corneal incision injury. After application of atropine to dilate the pupil and avoid iris incarceration, a linear perforating corneal incision (1.0 mm length) is performed (dashed lines). The lens and iris are not touched. Ac: anterior chamber; co: cornea; ir: iris; le: lens; pu: pupil; sc: sclera. (B–E) In vivo optical coherence tomography scans of the anterior segment demonstrate a transient increase of corneal thickness as a measure of corneal edema; green bars: areas of corneal thickness measurements; green values: central corneal thickness. (F–I) Corneal whole mounts stained with LYVE-1 (red) and CD31 (green); dashed lines: area of incision injury. LYVE-1high/CD31low lymphatic vessels (arrows) growing towards the central cornea are detectable 1 week after injury and persist until 2 weeks after injury. Afterwards, lymphatic vessels regress and the area covered by lymphatic vessels after 4 weeks is comparable to uninjured corneas. In contrast to lymphatic vessels, no significant ingrowth of LYVE-1neg/CD31high blood vessels into the cornea is detectable; p.i.: post injury. (J–K) Quantification of corneal lymph- and hemangiogenesis; data are shown as mean + SD; n = 5–10 per time point; statistical analysis was performed using the unpaired 2-tailed Student’s t test: ***: p < 0.001; n.s.: not significant.
Thus, an incisional perforating corneal injury results in transient corneal edema and the transient and selective ingrowth of lymphatic, but not blood vessels into the cornea.
Blockade of corneal lymphangiogenesis results in delayed resolution of corneal edema
In extraocular tissues, lymphatic vessels are known to mediate fluid homeostasis and clearance of cells and debris after tissue damage1, 26. We hypothesized that lymphangiogenesis might also regulate corneal edema and therefore determine whether blockade of corneal lymphangiogenesis after incision injury has an impact on corneal edema and transparency. For this purpose, we treated mice with the anti-VEGFR-3 antibody mF4-31C1 after injury. Treatment with mF4-31C1 resulted in a significant inhibition of corneal lymphangiogenesis (lymphvascularized area after 1 week: controls: 8.87% versus mF4-31C1: 4.86%, p < 0.001; after 2 weeks: controls: 10.88% versus mF4-31C1: 4.93%, p < 0.001; Supplemental Figure 2). In vivo optical coherence tomography (OCT) scans of the anterior segment showed that injured corneas developed significant corneal edema noticeable in an increase of central corneal thickness (CCT). CCT peaked within the first 2 weeks and then gradually regressed. Importantly, blockade of lymphangiogenesis resulted in delayed regression of CCT (CCT in uninjured: 90.1 µm; after 1 week: controls: 146.3 µm versus mF4-31C1: 140.3 µm, p > 0.05; after 2 weeks: controls: 132.4 µm versus mF4-31C1: 131.7 µm, p > 0.05; after 3 weeks: controls: 100.6 µm versus mF4-31C1: 110.8 µm, p < 0.05; after 4 weeks: controls: 101.4 µm versus mF4-31C1: 102.3 µm, p < 0.05; Fig. 2A to I). In addition, we clinically scored corneal opacity after incision injury in a blinded fashion. Similar to CCT, corneal opacity peaked in the first 2 weeks and then gradually decreased until corneas became almost entirely clear after 4 weeks. Blockade of lymphangiogenesis by mF4-31C1 resulted in a trend towards higher opacity scores, although the differences did not reach statistical significance (opacity score in uninjured: 0; after 1 week: controls: 3.1 versus mF4-31C1: 3.1; after 2 weeks: controls: 2.5 versus mF4-31C1: 3.2; after 3 weeks: controls: 2.1 versus mF4-31C1: 3.0; after 4 weeks: controls: 1.7 versus mF4-31C1: 2.5; all p-values > 0.05; Fig. 2J to R). Therefore, our experiments show that blockade of corneal lymphangiogenesis after incision injury leads to an increase of CCT - arguably due to a delay in the resolution of corneal edema - and a trend towards prolonged opacification.
Figure 2.
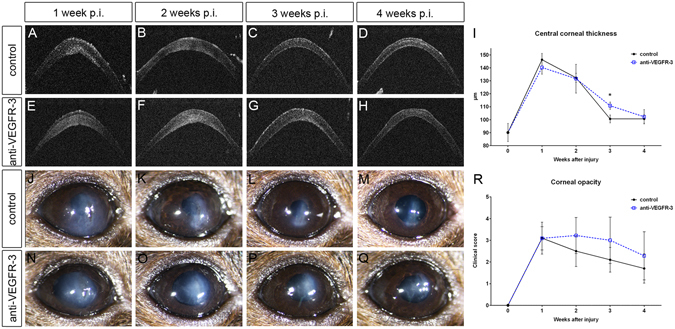
Blockade of lymphangiogenesis results in delayed resolution of corneal edema and prolonged opacification. Mice were treated with the anti-VEGFR-3 antibody mF4-31C1 (500 µg, intraperitoneally) directly before, on day 3, 6, 9, and 12 after incision injury or equal amounts of PBS. (A–H) In vivo optical coherence tomography scans of the anterior segment measuring central corneal thickness (CCT); (I) Blockade of corneal lymphangiogenesis resulted in delayed regression of CCT. (J–Q) Clinical images of injured corneas; (R) Blockade of lymphangiogenesis resulted in a trend towards higher opacity scores. p.i.: post injury; data are shown as mean + /− SD; n = 5–10 per group per time point; statistical analysis was performed using the unpaired 2-tailed Student’s t test *: p < 0.05.
Acute edema in a patient with keratoconus is associated with selective corneal lymphangiogenesis
As our animal experiments indicate that corneal lymphangiogenesis is involved in the regulation of at least acute corneal edema, we sought to determine whether lymphatic vessels are also detectable in patients with acute corneal edema (Fig. 3A). For this purpose, we analyzed the cornea of a patient with advanced keratoconus who had developed acute corneal edema due to a spontaneous rupture of the corneal basement membrane (Descemet´s membrane) and subsequent fluid accumulation (Fig. 3A,B). The patient’s cornea was obtained after corneal transplantation, which was performed 2 weeks after Descemet´s membrane rupture, and was evaluated for the presence of lymphatic vessels. Notably, LYVE-1 positive lymphatic vessels were clearly detectable in the corneal stroma (Fig. 3C). In contrast, blood vessels were not detectable. Thus, our results demonstrate that also in the human setting, acute corneal edema is accompanied by the selective ingrowth of corneal lymphatic vessels.
Figure 3.

Evidence of isolated corneal lymphangiogenesis in a patient with acute keratoconus. (A) Representative clinical image of a patient with keratoconus and acute corneal edema due to spontaneous rupture of the (Descemet´s) basement membrane and subsequent fluid accumulation (acute keratoconus). (B) Schematic drawing depicting acute keratokonus: due to rupture of the Descemet´s membrane (DM; red arrows), fluid from the anterior chamber (AC) rapidly accumulates in the cornea (CO), leading to loss of corneal transparency. (C) Histological analysis of the cornea from a patient with acute keratoconus obtained after corneal transplantation shows LYVE-1 positive lymphatic vessels in the corneal stroma (arrows) without evidence for blood vessels.
Discussion
A variety of studies demonstrate that pathological lymphatic vessels are critical for the development of graft rejection after corneal transplantation, dry eye disease, or ocular allergy, and targeting corneal lymphangiogenesis has emerged as a promising approach to treat these diseases5, 17–19, 22–25. However, we questioned whether lymphatic vessels at the cornea might also have beneficial functions, as in several extraocular tissues it was shown that lymphatic vessels mediate physiological wound repair and regulate edema1, 26. Recently, we could demonstrate that lymphatic vessels support the egress of macrophages form the inflamed cornea, facilitating the resolution of inflammation31. In the current study, we additionally analyzed whether lymphatic vessels are also involved in the physiological corneal wound healing response using a mouse model of perforating incision injury, which is known to induce transient corneal edema and opacification30. Importantly, our study indicates that in this injury model, selective growth of corneal lymphatic vessels (without concurrent blood vessel growth) seems to play a role in the regulation of corneal edema and the reestablishment of corneal transparency. Consistently, specific blockade of corneal lymphangiogenesis results in a slight but significant delay in the resolution of corneal edema and possibly prolonged corneal opacification. This is in line with previously observed findings after experimental corneal transplantation, where blockade of corneal lymphangiogenesis results in a slight delay of corneal clearing (unpublished, DH, FB, CC), although long-term graft survival is improved17, 22, 32.
Although the differences in CCT between the anti-VEGFR-3 treated corneas and controls reached statistical significance 3 weeks after injury, analysis of earlier time points showed no difference in CCT. Furthermore, corneal opacification was also similar between anti-VEGFR-3 treated eyes and controls. There might be several possible explanations for the rather small impact of VEGFR-3 blockade on the resolution of corneal edema and opacification. Although treatment with anti-VEGFR-3 significantly reduced corneal lymphangiogenesis after injury, inhibition was not complete. Therefore, it is possible that the remaining lymphatic vessels were still capable of draining large portions of corneal edema. Furthermore, it is also conceivable that other factors are involved in the resolution process. In this context, it has previously been shown that lymphatic drainage is also regulated by other vascular endothelium-specific factors33, the Angiopoetin-Tie system34, 35 or by several inflammatory chemo-/cytokines including CCL2136, TNF-α37, or IL-738. The carbonic anhydrase activity and pump function of the corneal endothelium is also capable to drain large amounts of intracorneal fluid and might also be responsible for the marginal impact of VEGFR-3 blockade on corneal edema resolution after incision injury.
Lymphatic vessels were also detectable in the cornea of a patient who had developed acute corneal swelling due spontaneous rupture of the Descemet´s membrane in acute keratoconus. This indicates that corneal lymphangiogenesis might be involved in the regulation of at least acute corneal edema also in the clinical setting. However, we could analyze only one patient with acute corneal edema, as human histological specimens with acute corneal edema (without concurrent inflammation) are extremely rare. This is because corneal transplantation is usually performed at much later time points (e.g. one year) after Descemet´s membrane rupture, when corneal edema has declined and an inert corneal scar has developed to ensure better suturing and stability of the graft. Nevertheless, further analyses of patient samples with acute corneal edema are necessary to verify our pilot results.
In summary, we demonstrate that a perforating corneal incision injury transiently and selectively induces corneal lymphangiogenesis. These lymphatic vessels seem to contribute to the regulation of acute corneal edema and transparency. To our knowledge, this is the first report that a primarily avascular site is transiently invaded by lymphatic vessels during the physiological healing course. Our results might lead to novel treatment strategies for several blinding eye diseases due to excessive corneal edema and swelling, such as acute keratoconus or corneal endothelial dystrophies. In fact, these diseases are the most common indications for corneal transplantation and are among the most common causes for blindness worldwide39–43.
Methods
Animals
Animal protocols were approved by the local animal care committee (Landesamt fuer Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen; AZ 2015.A487) and were in accordance with the Association for Research in Vision and Ophthalmology´s Statement for the Use of Animals in Ophthalmology and Vision Research. Mice were 8 to 12 weeks old female C57BL/6 mice. For corneal incision injury, mice were anaesthetized with an intraperitoneal injection of a combination of ketamine (100 mg/kg) and xylazine hydrochloride (10 mg/kg).
Corneal incision injury
A central perforating corneal incision injury was generated in the right eye of C57BL/6 mice as previously described30. 20 minutes before injury, one eye drop of atropine sulfate (1%, Ursapharm GmbH, Saarbrücken, Germany) was applied to avoid iris incarceration30. Afterwards, the central cornea was gently marked with a trephine (1.0 mm diameter) and a linear perforating incision was performed with a surgical blade (Fig. 1A,B). Mice received ofloxacin eye drops after injury (Floxal EDO, Dr. Mann Pharma GmbH, Berlin, Germany) twice daily for the first 3 days and were monitored daily during the first week and then weekly until the end of the experiment. In indicated experiments, mice received the anti-VEGFR3 antibody mF4-31C1 (Eli Lilly and Company, New York, NY, USA; 500 µg, intraperitoneally) directly before, on day 3, 6, 9, and 12 after surgery or equal amounts of PBS.
Analysis of corneal hem- and lymphangiogenesis after corneal incision injury
For the assessment of corneal hem- and lymphangiogenesis, corneas (n = 5–10 per analyzed time point) were excised at indicated time points after incision injury, fixed in acetone and stained with a rabbit anti-mouse LYVE-1 antibody (AngioBio, Del Mar, CA) and a rat anti-mouse CD31 antibody (Acris Antibodies, Herford, Germany). Whole mount pictures were assembled automatically from 9 to 12 pictures taken at 100x magnification. Afterwards, the area covered with blood and lymphatic vessels was detected with a semiautomatic algorithm established in the image analyzing program CellF (Soft Imaging System) as previously described44. Briefly, before analysis, gray value images of the whole mount pictures were modified using several filters, vessels were then detected by threshold setting including the bright vessels and excluding the dark background.
mRNA isolation and real-time PCR
Central corneas without the limbus were excised before, 1 week, 2 weeks and 4 weeks after incision injury and RNA was isolated using RNeasy Micro Kit (Qiagen, Valencia, CA). Complementary DNA (cDNA) was synthesized with random hexamers using reverse transcriptase (SuperScript III; Invitrogen, Carlsbad, CA). Primers (MWG Biotech, Ebersberg, Germany) were designed using Primer3 software and BLAST (Basic Local Alignment Search Tool, NCBI). PCR reactions (25 μl) contained 20 ng of cDNA, 0.4 µM of each forward and reverse primer, and master mix (SsoFast EvaGreen Supermix; Bio-Rad, Hercules, CA). Real-time PCR was performed under the following conditions: 95 °C for 2 minutes, 40 cycles at 95 °C for 5 seconds and 60 °C (for 15 seconds, followed by 95 °C for 60 seconds and a subsequent melt curve analysis to check amplification specificity. Real-time PCR results were analyzed by the comparative threshold cycle method with HPRT as the endogenous reference gene. The relative mRNA levels of uninjured corneas were chosen as the normalized controls for all analyzed time points after injury. All assays were performed in triplicate and a non-template control was included in all experiments to exclude DNA contamination. Primer sequences and product sizes are summarized in the Supplemental Table 1.
Assessment of central corneal thickness
Central corneal thickness (CCT) as a measure of corneal edema was analyzed in in vivo optical coherence tomography (OCT) scans of the anterior segment. OCT scans were obtained using an 840 nm Spectral Domain OCT device specifically developed for investigating mouse eyes (OCT-HSM, OptoMedical Technologies GmbH, Luebeck, Germany). The OCT device performs 21.000 A-Scans per second, delivering an axial scan resolution about 5.7μm in tissue. Volume scans of the central cornea were obtained after thorough adjustment ensuring a rectangular incidence of the light to avoid refractive aberrations. B-Scans parallel adjacent to the corneal incision were then analyzed and CCT was calculated as the mean value of corneal thickness within the central region of interest measured with 15 pixels lateral width (accords to approximately 75 µm on the corneal surface) using an open source image processing software (ImageJ, NIH, Bethesda, Maryland, USA). CCT was calculated by using intensity depth plots to determine corneal boundaries and under consideration of the refractive index of corneal tissue.
Assessment of corneal opacity
For the analysis of corneal opacity, slit-lamp photographs were taken every week after injury. To grade corneal opacity, following scoring system, which is commonly used in the assessment of corneal transplants, was used: 0: clear cornea; 1: minimal, superficial (non-stromal) opacity, pupil margin and iris vessels readily visible; 2: minimal, deep (stromal) opacity, pupil margins and iris vessels visible; 3: moderate stromal opacity, only pupil margin visible; 4: intense stromal opacity, only a portion of pupil margin visible; 5: maximum stromal opacity, anterior chamber not visible.
Immunohistological analysis of a cornea obtained from a patient with acute edema in keratoconus
A male patient (age 26 years) with advanced keratoconus developed acute corneal edema due a rupture of the corneal basement membrane (Descemet´s membrane) and subsequent fluid accumulation. 2 weeks later, penetrating keratoplasty was performed and the patient’s own cornea was immunohistologically analyzed for the presence of lymphatic vessels. For this purpose, the cornea was fixed in phosphate-buffered saline (PBS) containing 4% formaldehyde, rinsed in PBS, dehydrated via graded alcohol series and embedded in paraffin. Serial cross sections (3 µm) were prepared and stained with an antibody directed against LYVE-1 (rabbit anti-human, Zytomed, Berlin, Germany). Negative control staining was performed by omission of the primary antibody and resulted in no staining. Conjunctiva was taken as positive control and revealed specific staining.
Histological experiments were performed in accordance with all relevant guidelines and regulations and were approved by the institutional committee (Ethikkommission der Medizinischen Fakultaet der Universitaet zu Koeln). Informed consent was obtained from the patient.
Statistical Analyses
Statistical analyses were performed with Microsoft Excel (Microsoft Corp, Redmond, WA) and InStat Version 3.06 (GraphPad Software Inc., San Diego, CA). Statistical significance was determined using the student’s t-test for parametric and the Mann-Whitney-U test for non-parametric variables. P-values < 0.05 were considered statistically significant. Graphs were drawn using Prism4 version 4.03 (GraphPad Software Inc., San Diego, CA). All data are reported as the mean + standard deviation (SD) unless otherwise indicated.
Data availability statement
The datasets generated during and analyzed in this study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
We thank Dr. Bronislaw Pytowski (Eli Lilly and Company, New York, NY, USA) for the anti-VEGFR-3 antibody mF4-31C1 and Martina Becker for outstanding support in immunohistochemistry. Funding: German Research Foundation (DFG) FOR2240 “(Lymph)angiogenesis and Cellular Immunity in Inflammatory Diseases of the Eye”, HO 5556/1-1 (DH), HE 6743/2-1 (LMH), HE 6743/3-1 (LMH), STE 1928/4-1 (PS), Cu 47/4-2 (CC), Cu 47/6-1 (CC), Cu 47/9-1 (CC) (www.for2240.de); EU COST BM1302 (DH, FBo, CC); EU Horizon 2020 ARREST BLINDNESS (CC); GEROK Program, University of Cologne (DH, FBu, LMH); Center for Molecular Medicine Cologne, University of Cologne (DH, FBo, SAE, CC); NIH EY-012963 (RD); Bayer Graduate School Pharmacology, University of Cologne (CC).
Author Contributions
D.H. performed the experiments, analyzed the data and wrote the manuscript. A.B. performed whole mount stainings and vessel quantification. J.H., S.S. and P.S. performed O.C.T. measurements and quantifications. F.B.o. analyzed histological stainings and created parts of the figures. F.B.u. acquired images for opacity grading. L.M.H. performed opacity grading. R.D. and S.A.E. supervised the study. R.D. edited the manuscript. C.C. conceptualized and supervised the study and wrote the manuscript. All authors read and approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07806-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alitalo K. The lymphatic vasculature in disease. Nature medicine. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 2.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 3.Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chemical immunology and allergy. 2007;92:50–57. doi: 10.1159/000099253. [DOI] [PubMed] [Google Scholar]
- 4.Ambati BK, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albuquerque RJ, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nature medicine. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh, N. et al. Soluble vascular endothelial growth factor receptor-3 is essential for corneal alymphaticity. Blood, doi:10.1182/blood-2012-08-453043 (2013). [DOI] [PMC free article] [PubMed]
- 7.Cursiefen C, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11405–11410. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cursiefen C, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. The Journal of experimental medicine. 2011;208:1083–1092. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cursiefen C, et al. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Investigative ophthalmology & visual science. 2004;45:1117–1124. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- 10.Lin HC, et al. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Investigative ophthalmology & visual science. 2001;42:2517–2524. [PubMed] [Google Scholar]
- 11.Cursiefen C, et al. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and podoplanin. Investigative ophthalmology & visual science. 2002;43:2127–2135. [PubMed] [Google Scholar]
- 12.Mimura T, et al. Expression of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in corneal lymphangiogenesis. Experimental eye research. 2001;72:71–78. doi: 10.1006/exer.2000.0925. [DOI] [PubMed] [Google Scholar]
- 13.Ling S, et al. Development of new lymphatic vessels in alkali-burned corneas. Acta ophthalmologica. 2009;87:315–322. doi: 10.1111/j.1755-3768.2008.01349.x. [DOI] [PubMed] [Google Scholar]
- 14.Detry B, et al. Sunitinib inhibits inflammatory corneal lymphangiogenesis. Investigative ophthalmology & visual science. 2013;54:3082–3093. doi: 10.1167/iovs.12-10856. [DOI] [PubMed] [Google Scholar]
- 15.Cursiefen C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. The Journal of clinical investigation. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. The Journal of experimental medicine. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cursiefen C, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Investigative ophthalmology & visual science. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 18.Goyal S, et al. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Archives of ophthalmology. 2010;128:819–824. doi: 10.1001/archophthalmol.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HS, et al. Involvement of corneal lymphangiogenesis in a mouse model of allergic eye disease. Investigative ophthalmology & visual science. 2015;56:3140–3148. doi: 10.1167/iovs.14-16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hos, D., Schlereth, S. L., Bock, F., Heindl, L. M. & Cursiefen, C. Antilymphangiogenic therapy to promote transplant survival and to reduce cancer metastasis: what can we learn from the eye? Seminars in cell & developmental biology, doi:10.1016/j.semcdb.2014.11.003 (2014). [DOI] [PubMed]
- 21.Bock F, et al. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Progress in retinal and eye research. 2013;34:89–124. doi: 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich T, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. Journal of immunology. 2010;184:535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal S, Chauhan SK, Dana R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Archives of ophthalmology. 2012;130:84–89. doi: 10.1001/archophthalmol.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hos D, Cursiefen C. Lymphatic vessels in the development of tissue and organ rejection. Advances in anatomy, embryology, and cell biology. 2014;214:119–141. doi: 10.1007/978-3-7091-1646-3_10. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nature medicine. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 26.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes & development. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 27.Huggenberger R, Detmar M. The cutaneous vascular system in chronic skin inflammation. The journal of investigative dermatology. Symposium proceedings / the Society for Investigative Dermatology, Inc. [and] European Society for Dermatological Research. 2011;15:24–32. doi: 10.1038/jidsymp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huggenberger R, et al. An important role of lymphatic vessel activation in limiting acute inflammation. Blood. 2011;117:4667–4678. doi: 10.1182/blood-2010-10-316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huggenberger R, et al. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. The Journal of experimental medicine. 2010;207:2255–2269. doi: 10.1084/jem.20100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco-Mezquita JT, Hutcheon AE, Zieske JD. Role of thrombospondin-1 in repair of penetrating corneal wounds. Investigative ophthalmology & visual science. 2013;54:6262–6268. doi: 10.1167/iovs.13-11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hos D, et al. IL-10 Indirectly Regulates Corneal Lymphangiogenesis and Resolution of Inflammation via Macrophages. The American journal of pathology. 2016;186:159–171. doi: 10.1016/j.ajpath.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Hos D, et al. Inflammatory corneal (lymph)angiogenesis is blocked by VEGFR-tyrosine kinase inhibitor ZK 261991, resulting in improved graft survival after corneal transplantation. Investigative ophthalmology & visual science. 2008;49:1836–1842. doi: 10.1167/iovs.07-1314. [DOI] [PubMed] [Google Scholar]
- 33.Danussi C, et al. Emilin1 deficiency causes structural and functional defects of lymphatic vasculature. Molecular and cellular biology. 2008;28:4026–4039. doi: 10.1128/MCB.02062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kajiya K, et al. Promotion of lymphatic integrity by angiopoietin-1/Tie2 signaling during inflammation. The American journal of pathology. 2012;180:1273–1282. doi: 10.1016/j.ajpath.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Qu X, Tompkins K, Batts LE, Puri M, Baldwin HS. Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice. Development. 2010;137:1285–1295. doi: 10.1242/dev.043380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miteva DO, et al. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circulation research. 2010;106:920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang Q, et al. Lymphatic endothelial cells efferent to inflamed joints produce iNOS and inhibit lymphatic vessel contraction and drainage in TNF-induced arthritis in mice. Arthritis research & therapy. 2016;18:62. doi: 10.1186/s13075-016-0963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iolyeva M, et al. Interleukin-7 is produced by afferent lymphatic vessels and supports lymphatic drainage. Blood. 2013;122:2271–2281. doi: 10.1182/blood-2013-01-478073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg P, Krishna PV, Stratis AK, Gopinathan U. The value of corneal transplantation in reducing blindness. Eye. 2005;19:1106–1114. doi: 10.1038/sj.eye.6701968. [DOI] [PubMed] [Google Scholar]
- 40.Gain P, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA ophthalmology. 2016;134:167–173. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 41.Matthaei M, et al. Changing Indications in Penetrating Keratoplasty: A Systematic Review of 34 Years of Global Reporting. Transplantation. 2016 doi: 10.1097/TP.0000000000001281. [DOI] [PubMed] [Google Scholar]
- 42.Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA: the journal of the American Medical Association. 2003;290:2057–2060. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- 43.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bulletin of the World Health Organization. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 44.Bock F, et al. Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Experimental eye research. 2008;87:462–470. doi: 10.1016/j.exer.2008.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed in this study are available from the corresponding author on reasonable request.


