Abstract
Retinoic acid (RA) induces cell cycle arrest and differentiation of human neuroblastoma (NB) cells. Typically, NB cells differentiate along the neuronal lineage, but quiescent, “flat” cell types frequently have been described after treatment with differentiating agents. Two indistinguishable subclones of the cell line SK-N-SH, SK-N-SH-N (SH-N) and SK-N-SH-F (SH-F), display dramatically different responses to RA. In SH-N, RA induces neuronal differentiation, but in SH-F it transforms the small neuroblastic cells into large, flattened, epithelium-like cells. Here we analyze the mechanistic basis for the different effects of RA in the two NB subclones. First, we show that the flattened RA-treated SH-F expresses markers of cells undergoing replicative senescence. Inhibition of DNA synthesis by RA is significantly more rapid in SH-F than in SH-N. SH-F, which expresses basal amounts of p16INK4A, responds to RA with elevation of p18INK4C, marked down-regulation of cyclin D1, and swift inhibition of cyclin D-dependent kinases (cdks). Conversely, after addition of RA, SH-N retains cell cycling due to high expression of cyclin D1, the absence of Ink4 inhibitors, and accumulation of p21Cip1. These changes result in sustained cdk activity. Accordingly, overexpression of p21Cip1 but not p16INK4A induces neuronal differentiation of untreated NB cells. We propose that rapid inhibition of cdks by RA in NB leads to early cell cycle arrest, prevents neuronal differentiation, and results in a senescence-like state.
In mammalian cells, antimitogenic signals cause arrest in the G1 phase of the cell division cycle. Although growth arrest is the final outcome of antiproliferative signals, the cellular processes initiated by the cell cycle block depend on the nature of the extracellular signals and the cell type receiving them. Differentiation and senescence are two common cellular processes associated with cell cycle withdrawal by antimitogenic factors, and little is known about how the cell cycle engine converts the antiproliferative messages in the different cellular responses of differentiation and senescence (1, 2).
A cascade of consecutive waves of cyclin/cyclin-dependent kinase (cdk) activities is required for cell cycle progression during G1/S transition. Cyclin D-cdk4/6 complexes function early in G1 and act as the primary sensors of positive and negative environmental signals (3–5). We and others have shown that extracellular antiproliferative factors impinge primarily on cdks to arrest cells in the G1 phase of the cell cycle (6–10). Mechanisms for the inhibition of cyclin D-cdk4/6 by antimitogenic signals include regulation of cyclin production and binding to the cdks, subunit phosphorylation, and inhibition by cdk inhibitors (3, 11). In mammalian cells, the cooperation between two classes of cdk inhibitors, the cdk inhibitor protein/kinase inhibitor protein (Cip/Kip) and the cdk4 inhibitor (Ink4) family of inhibitors, is frequently responsible for withdrawal from the cell cycle (4, 6, 12).
Neuroblastoma (NB) is a highly malignant pediatric tumor derived from the neural crest (13). NB cells retain some features of neural crest progenitors, such as the ability to undergo neuronal differentiation in the presence of appropriate signals (14, 15). For this reason, when treated with retinoic acid (RA), human NB cells have been used as a model to study differentiation along the neuronal lineage (16, 17). However, as an alternative pathway to neuronal differentiation, a “flat, epithelioid or substrate-adherent” phenotype has been consistently described in NB cells in vitro and in vivo (18–23). Sometimes the two phenotypic variants coexist in the same parental NB cell line (24). Homogeneous sublines, displaying one or the other phenotype after treatment with differentiation factors, have been derived from parental NB cell lines (22, 23, 25). In this study, we have used two classical sublines from the SK-N-SH human NB cell line to investigate the nature of the primary set of events in the cell cycle machinery that occur during progression through their respective programs of neuronal differentiation and senescence.
Materials and Methods
NB cells were grown in DMEM supplemented with 10% FBS. For cells exposed to RA (Sigma), this agent was added at a concentration of 10 μM in 100% ethanol, and treatments were maintained for up to 14 days (when specified). Control cultures contained the same concentration of ethanol (<0.2%) and the medium was changed every 4 days. For senescence-associated β-galactosidase (SA-β-gal) staining, cells were fixed and stained at pH 6.0 with 5-bromo-4-chloro-3-indol β-d-galactopyranoside as described (26). Inhibition of DNA synthesis by RA was evaluated from the incorporation of [125I]iododeoxyuridine (Amersham Pharmacia) into DNA during the last 2 h of incubation.
For Western blot analysis, cell lysates were prepared in RIPA buffer (0.15 mM NaCl/0.05 mM Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) containing protease and phosphatase inhibitors, and proteins were separated on SDS/PAGE and finally visualized by enhanced chemiluminescence (Amersham Pharmacia). For immunoprecipitation/Western blot and immunoprecipitation/kinase assays, lysates were prepared and processed as described (6, 27). For retinoblastoma protein (Rb) kinase assays, the phosphorylated Rb (amino acids 773–928) was visualized by autoradiography and quantitated by densitometry. The antibodies used in this study have been described (6, 7).
SK-N-SH-N (SH-N) cells were transfected with pMEP vector, pMEPp21Cip1 or pMEPp16INK4a, by Lipofectamine Plus (GIBCO) according to the manufacturer's instructions, and resistant cells were selected in 200 μM hygromicin for 10 days. The percentage of cells with neurites was determined by counting ≈1,000 cells from randomly selected fields. A neurite was defined as a phase dark process with a clearly defined growth cone that was at least two cell diameters in length.
Results
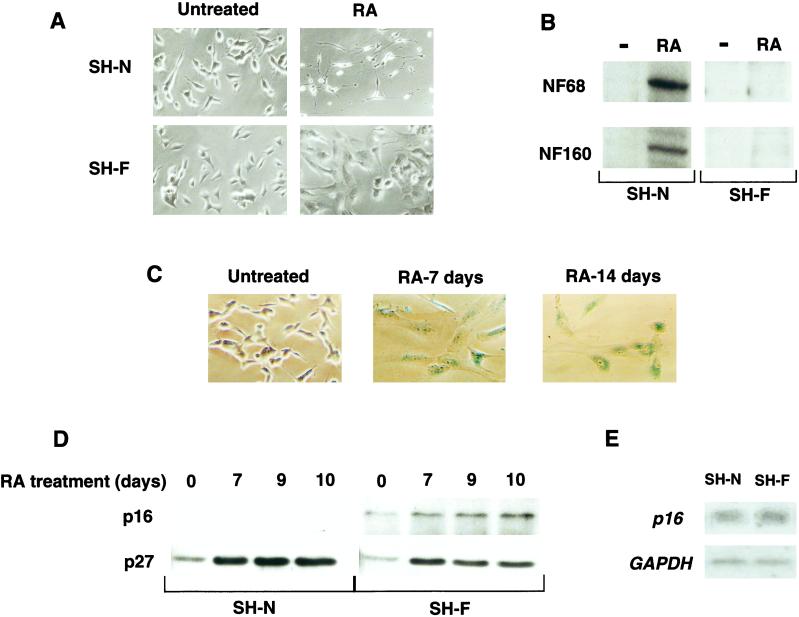
The two sublines of SK-N-SH, SH-N and SK-N-SH-F (SH-F), have an indistinguishable undifferentiated small-cell phenotype when left untreated, but display a strikingly different response to RA. After RA addition, SH-N responds with extensive neurite outgrowth and induction of markers such as neurofilament 68 and neurofilament 160, which are hallmarks of differentiation along the neuronal lineage (Fig. 1 A and B). Conversely, the addition of RA to SH-F results in a flattened, epithelium-like phenotype (Fig. 1A). The phenotypic changes induced by RA in SH-N and SH-F cells typically begin after 3 days, are fully manifested after 7 days, and are stable over the entire course of a 14-day experiment. Neuritic extension and expression of neuronal markers are consistently absent in RA-treated SH-F cells (Fig. 1 A and B). We noticed that SH-F cells treated with RA became much larger, flatter, and more granular than untreated cells, resembling primary cells that have attained senescence after extensive serial passage (28, 29). Senescent cells have increased volume, exhibit flattened morphology, and accumulate an endogenous β-gal activity at pH 6.0 (SA-β-gal) (26, 30). Staining of RA-treated SH-F cells for SA-β-gal showed progressive accumulation of this marker by 7 days of treatment. After 14 days, almost 100% of SH-F cells stained positive for SA-β-gal (Fig. 1C). In human cells, induction of the cdk inhibitor p16INK4a is also associated with the onset and maintenance of the senescent phenotype (2, 31, 32). SH-F cells expressed small but detectable amounts of p16INK4a in the untreated state and SH-N cells did not (Fig. 1D). Interestingly, SH-N and SH-F expressed similar amounts of p16INK4a mRNA, indicating differential posttranscriptional regulation of p16INK4a protein in the two NB sublines (Fig. 1E). In parallel with morphological changes and accumulation of SA-β-gal, treatment of SH-F with RA led to a significant increase in p16INK4a. Expression of p16INK4a was undetectable in SH-N treated with RA (Fig. 1D). These results indicate that RA treatment induces a phenotype typical of cells differentiating along the neuronal pathway in SH-N and a coordinated senescence-like phenotype in SH-F.
Figure 1.
Effects of RA on the NB cell subclones SH-N and SH-F. (A) Cellular morphology of SH-N and SH-F cells left untreated or treated with RA for 7 days. (B) Neurofilament 68 and neurofilament 160 Western immunoblot analysis of SH-N and SH-F left untreated (−) or treated with RA for 7 days (RA). (C) SH-F cells were treated with RA for the indicated times and stained with 5-bromo-4-chloro-3-indol β-d-galactopyranoside to measure SA-β-gal activity. (D) p16INK4a and p27Kip1 Western immunoblot analysis of SH-N and SH-F treated with RA for the indicated times. (E) Northern blot analysis for p16INK4a and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of untreated SH-N and SH-F.
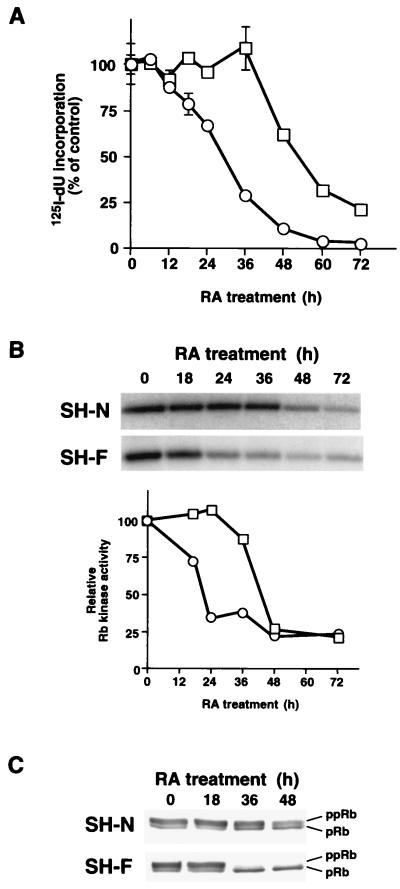
Differentiation and senescence are implemented by reprogramming of the cell cycle machinery, which leads to proliferative arrest. To identify the mechanisms of cell cycle arrest in SH-N and SH-F, we first determined the kinetics of S-phase inhibition by RA. The addition of RA impaired entry into the S phase of SH-F cells within 24 h, but it first reduced the S phase of SH-N cells only after 48 h (Fig. 2A). In both cell types, cell cycle arrest was in the G1 phase of the cell cycle, as determined by fluorescence-activated cell sorter analysis (data not shown). Thus the senescence-primed SH-F cell subclone responds to RA with early cell cycle arrest. In contrast, SH-N cells, predisposed to progress along the neuronal pathway, display a delayed antiproliferative response to RA.
Figure 2.
Kinetics of S-phase and cdk6 kinase inhibition by RA in SH-N and SH-F. (A) Inhibition of S phase in SH-N (□) and SH-F (○) treated with RA for the indicated times. The data shown are the average and standard deviations of triplicate assays. (B) SH-N and SH-F cells were treated with RA for the indicated times. Cdk6 immunoprecipitates were assayed for associated Rb kinase activity. The 32P autoradiographic signal of the Rb band (Upper) was quantified and is plotted relative to the value in cells without RA (□, SH-N; ○, SH-F). (C) Rb Western immunoblot from SH-N and SH-F treated with RA. ppRb, phosphorylated Rb; pRb, unphosphorylated Rb.
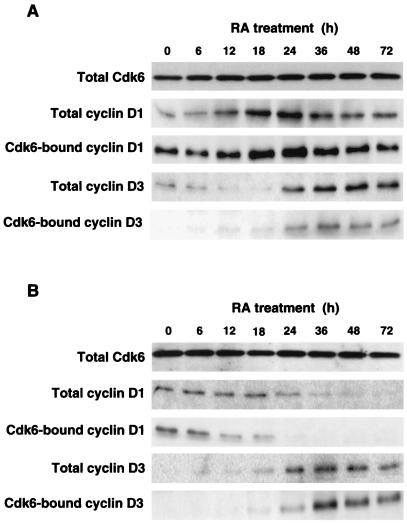
To test whether the differential cell cycle block after the addition of RA reflects a specific effect on the cell cycle machinery in the two cell types, we decided to assess the mechanism of inhibition by RA of cdk6 and cdk4, the first targets for G1 arrest by antimitogenic signals in mammalian cells (3, 11). Below, we will show our results on cdk6, but related experiments with cdk4 led to similar conclusions (data not shown). First, we determined the in vitro kinase activity toward exogenous Rb associated with cdk6 immunoprecipitates. The addition of RA to SH-F decreased cdk6-associated Rb kinase activity to nearly background levels by 24 h. Conversely, SH-N cells showed comparable inhibition of cdk6 only after 48–72 h of treatment with RA (Fig. 2B). The analysis of endogenous Rb showed similar differential kinetics of inhibition of phosphorylation among SH-N and SH-F, indicating that the delay of cdk6 inhibition in SH-N was physiologically relevant (Fig. 2C). Thus, inhibition of cdk6 kinase in vitro and in vivo paralleled inhibition of entry into the S phase by RA in SH-N and SH-F cells. Cdk6 kinase activity was inhibited by RA without any loss of the cdk6 subunit, but with changes in the abundance of D-type cyclins in both cell types (Fig. 3). Cyclin D1 was increased during the early phases of RA treatment in SH-N cells and was detectable throughout the entire period analyzed (72 h after the addition of RA). Accordingly, cyclin D1-cdk6 complexes were abundant in SH-N cells treated with RA. Interestingly, cyclin D1 and the corresponding cyclin D1-cdk6 complexes were strongly reduced in SH-F cells after 24 h of RA treatment (Fig. 3B). The other D-type cyclin expressed in the two NB cell types, cyclin D3 (cyclin D2 was not expressed in both cell lines), accumulated in SH-N and SH-F with similar kinetics and led to increased abundance of cyclin D3-cdk6 complexes after RA (Fig. 3). The accumulation of cyclin D3-cdk6 complexes was seen when cdk6 kinase activity was lost (SH-F) or would soon be lost (SH-N), indicating that during the antiproliferative response to antimitogenic signals these complexes lack associated Rb kinase activity (8, 33, 34).
Figure 3.
Cdk6, D-cyclins, and cyclin D-cdk6 complexes in SH-N and SH-F treated with RA. Total amounts of the indicated proteins were determined by Western immunoblotting of lysates from RA-treated SH-N (A) and SH-F (B). Cdk6-bound cyclins were determined by Western immunoblotting of cdk6 immunoprecipitates.
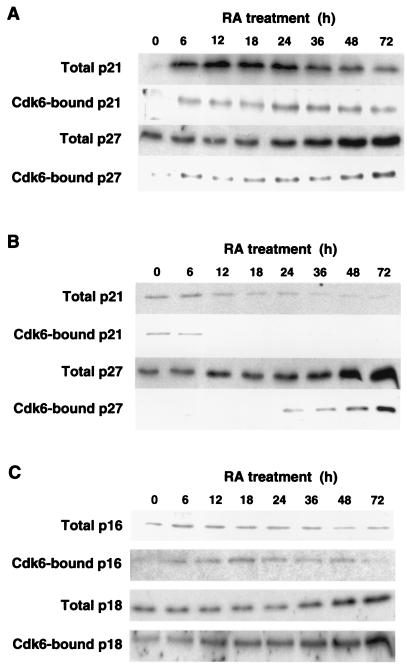
We then turned our attention to the effect of RA on cdk inhibitors and on their ability to form complexes with cdk6 in human NB cells. The inhibitors p57Kip2, p15Ink4b, and p19Ink4d were absent in untreated SH-N and SH-F and were not raised by RA (data not shown). p21Cip1 is known to accumulate in cells undergoing neuronal differentiation, including human neuroblastoma cells (35–37). RA rapidly increased p21Cip1 in SH-N, and this inhibitor remained high for at least 72 h of treatment (Fig. 4A). RA decreased p21Cip1 in SH-F cells (Fig. 4B). During RA treatment, cdk6-p21Cip1 complexes followed a kinetics that was related to the abundance of total p21Cip1, with accumulation of the complexes in SH-N and loss in SH-F (Fig. 4 A and B). The other Cip/Kip inhibitor, p27Kip1 (and the corresponding cdk6-p27Kip1 complexes), underwent progressive accumulation during treatment with RA in both cell types (Fig. 4 A and B). Expression of Ink4 inhibitors was dramatically different in the two NB cell types treated with RA. p16INK4a was constitutively expressed at low levels by SH-F, and an increase in this protein was found after 7 days of RA treatment (Fig. 1D). However, elevation of p16INK4a did not seem to play a role in the initiation of cell cycle arrest by RA because its abundance did not change, and cdk6-p16INK4a complexes, although consistently detected in cells exposed to RA, were not affected during the first 72 h of treatment (Fig. 4C). p16INK4a was never found in SH-N after short-term and long-term treatment with RA (Fig. 1D). Another Ink4 inhibitor, p18INK4c, was induced by RA in total extracts and in cdk6 immunoprecipitates from SH-F, but it was absent in SH-N cells before and after RA (Fig. 4C and data not shown). These results indicate that induction of the senescent phenotype by RA is associated with early cdk6 (and cdk4) inhibition and cell cycle arrest, in the context of high Ink4 inhibitory activity, whereas differentiation along the neuronal pathway is accompanied by delayed cdk6 (and cdk4) inhibition and cell cycle arrest, in the absence of Ink4 activity but with high Cip/Kip activity.
Figure 4.
Cdk inhibitors in SH-N and SH-F treated with RA. Total amounts of the indicated proteins were determined by Western immunoblotting of lysates from RA-treated SH-N (A) and SH-F (B and C). Cdk6-bound inhibitors were determined by Western immunoblotting of cdk6 immunoprecipitates.
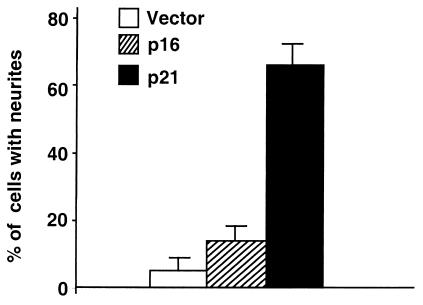
To test whether expression of Cip/Kip and Ink4 inhibitors resulted in different phenotypic changes by human NB cells, SH-N was transfected with vectors expressing p21Cip1 or p16INK4a or with empty plasmid. Cells were selected with hygromicin, and the resistant cells were analyzed for the presence of a neuronal phenotype by counting the number of cells with neurites. Introduction of p16INK4A and p21Cip1 in SH-N was associated with cell cycle arrest, as shown by lack of multicellular colonies in plates transfected with p16INK4a and p21Cip1 but not in those transfected with empty vector (data not shown). However, in three independent experiments, p21Cip1 expression induced significant neurite extension, but p16INK4a did not (Fig. 5).
Figure 5.
Expression of p21Cip1 induces neuronal differentiation in NB cells. SH-N were transfected with plasmids expressing the indicated proteins and selected with hygromicin. Shown is the percentage of hygromicin-resistant cells with neurites after each transfection. Bars represent means and standard deviations of triplicate samples.
In summary, our data suggest that a cycling phase after RA treatment, mediated by the combination of cyclin D1 and p21Cip1, is a permissive condition for neuronal differentiation of NB cells. Conversely, early inhibition of D-type cdk activity, implemented by a high threshold of Ink4 inhibitors, allows immediate and efficient cell cycle arrest followed by entry into a senescence-like phenotype.
Discussion
We have shown that, in response to RA, NB cells have the choice of following one of two alternative programs, each enabled by the ultimate extinction of D-type cdks and leading to G1 arrest. In the first program, enforced in cells undergoing neuronal differentiation (SH-N), cycling of cells during the first 48 h of RA treatment is allowed by persistent cyclin D-dependent kinase activity. Here, the main player in the cell cycle machinery recruited by RA appears to be the cdk inhibitor p21Cip1, which is rapidly elevated by RA. The increase in p21Cip1 takes place in the presence of abundant cyclin D1-cdk6 (and cyclin D1-cdk4) complexes. Indeed, RA promptly raises the expression of cyclin D1 in SH-N, in a manner that is very similar to that of the elevation of p21Cip1. The coordinated effects of RA on cyclin D1 and p21Cip1 in SH-N cells resemble the up-regulation of cyclin D1 and p21Cip1 observed in the course of nerve growth factor-induced neuronal differentiation of PC12 pheochromocytoma cells (36, 37). We recently have found that cyclin D1 can elevate p21Cip1 expression through an E2F site in the p21Cip1 promoter (38, 39). It is likely that the large availability of cyclin D1-cdk4/6 complexes in SH-N treated with RA for less than 48 h renders p21Cip1 a cofactor for cdk4/6 kinase activity, through its positive role on cyclin D1-cdk4/6 assembly (4, 40). The absence of Ink4 inhibitors in SH-N treated with RA allows continuous activity of cyclin D-dependent kinases. After prolonged treatment with RA (more than 48 h), cyclin D-dependent kinase activity finally disappears from SH-N cells, thus leading to G1 arrest. At this late time of RA treatment, two new coordinated sets of events modify the early response to RA, repression of cyclin D1 (and p21Cip1) and elevation of cyclin D3 (and p27Kip1). The final outcome is the substitution of active cyclin D1-cdk6- p21Cip1 complexes with inactive cyclin D3-cdk6- p27Kip1 complexes. The shift of cyclin D1 with cyclin D3 has been described during the response of different cell types to incoming antiproliferative signals (8, 33, 41). In one of the most studied systems, myogenic differentiation, elevation of cyclin D3 leads to sequestration of cdks and proliferating cell nuclear antigen into high-order structures, inhibition of kinase activity, and cell cycle arrest (42). The parallel elevation of cyclin D3 in SH-F cells treated with RA suggests that, rather than the implementation of specific differentiation events, accumulation of cyclin D3 is associated with cell cycle arrest by a wide array of antimitogenic pathways.
The cell cycle response of SH-F to RA is dictated by Ink4 inhibitors. First, basal p16INK4a expression is present throughout the early period of RA treatment, which leads to G1 arrest, and is elevated in parallel with the emergence of the senescence phenotype. Timely elevation of p18INK4c and a decrease in cyclin D1 quickly set the balance of cdk complexes and Ink4 inhibitors with the latter. The ability of Ink4 inhibitors to form highly stable binary inhibitor-cdk4/6 complexes, associated with rapid depletion of the intracellular cyclin D1 pool, allows immediate and efficient extinction of cyclin D-dependent kinase activity in SH-F treated with RA, which, in turn, leads to prompt exit from the cell cycle.
Thus there appears to be a good correlation between the timing and the degree of growth arrest, the kinetics of inhibition of cyclin D-dependent kinase activity, and the choices of NB cells treated with RA with respect to the alternative programs of neuronal differentiation or senescence. The idea that a proliferative phase, associated with elevation of p21Cip1, must precede overt differentiation was proposed for other cellular lineages (43–45). Our results suggest that neuronal differentiation of NB cells triggered by RA fulfills this model. However, if early and efficient inhibition of the cell cycle is attained by recruitment of cdk inhibitors of the Ink4 family, execution of the neuronal differentiation program is prevented and cells undergo a senescence-like phenotype. A previous study reported that RA inhibits protein kinase C in SH-N but enhances its activity in SH-F (46). It will be interesting to determine whether the differential inhibition of protein kinase C activity by RA in the two sublines is responsible for the alternative mechanisms of cell cycle arrest.
In conclusion, our results propose that NB cells may serve as a model with which to study the mechanisms of senescence of neural crest cells in vitro. We also suggest that determination of markers of senescence, such as SA-β-gal, in tumors of patients affected by NB and undergoing experimental treatment with RA, might provide a highly sensitive approach to monitoring of the antiproliferative tumor response to this form of therapy (47).
Abbreviations
- RA
retinoic acid
- NB
neuroblastoma
- SH-N
SK-N-SH-N
- SH-F
SK-N-SH-F
- cdk
cyclin-dependent kinase
- Cip
cdk inhibitor protein
- Kip
kinase inhibitor protein
- Ink4
inhibitor of cdk4
- SA-β-gal
senescence associated β-galactosidase
- Rb
retinoblastoma protein
References
- 1.Wright W E, Shay J W. Curr Opin Genet Dev. 2001;11:98–103. doi: 10.1016/s0959-437x(00)00163-5. [DOI] [PubMed] [Google Scholar]
- 2.Bringold F, Serrano M. Exp Gerontol. 2000;35:317–29. doi: 10.1016/s0531-5565(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 3.Sherr C J. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 4.Sherr C J, Roberts J M. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 5.Massague J, Blain S W, Lo R S. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 6.Reynisdottir I, Polyak K, Iavarone A, Massague J. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 7.Iavarone A, Massague J. Nature (London) 1997;387:417–422. doi: 10.1038/387417a0. [DOI] [PubMed] [Google Scholar]
- 8.Phelps D E, Xiong Y. Cell Growth Differ. 1998;9:595–610. [PubMed] [Google Scholar]
- 9.Morse L, Chen D, Franklin D, Xiong Y, Chen-Kiang S. Immunity. 1997;6:47–56. doi: 10.1016/s1074-7613(00)80241-1. [DOI] [PubMed] [Google Scholar]
- 10.Franklin D S, Xiong Y. Mol Biol Cell. 1996;7:1587–1599. doi: 10.1091/mbc.7.10.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherr C J, Roberts J M. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 12.Swarbrick A, Lee C S, Sutherland R L, Musgrove E A. Mol Cell Biol. 2000;20:2581–2591. doi: 10.1128/mcb.20.7.2581-2591.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maris J M, Matthay K K. J Clin Oncol. 1999;17:2264–2279. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 14.Abemayor E, Sidell N. Environ Health Perspect. 1989;80:3–15. doi: 10.1289/ehp.89803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeger R C, Siegel S E, Sidell N. Ann Intern Med. 1982;97:873–884. doi: 10.7326/0003-4819-97-6-873. [DOI] [PubMed] [Google Scholar]
- 16.Sidell N, Altman A, Haussler M R, Seeger R C. Exp Cell Res. 1983;148:21–30. doi: 10.1016/0014-4827(83)90184-2. [DOI] [PubMed] [Google Scholar]
- 17.Sidell N. J Natl Cancer Inst. 1982;68:589–596. [PubMed] [Google Scholar]
- 18.Piacentini M, Piredda L, Starace D T, Annicchiarico-Petruzzelli M, Mattei M, Oliverio S, Farrace M G, Melino G. J Pathol. 1996;180:415–422. doi: 10.1002/(SICI)1096-9896(199612)180:4<415::AID-PATH684>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Lombardo J M, Lanks K W. J Cell Physiol. 1981;108:221–230. doi: 10.1002/jcp.1041080212. [DOI] [PubMed] [Google Scholar]
- 20.Lanks K W, Lombardo J M. J Cell Physiol. 1981;109:45–51. doi: 10.1002/jcp.1041090106. [DOI] [PubMed] [Google Scholar]
- 21.Matsushima H, Bogenmann E. Int J Cancer. 1992;51:250–258. doi: 10.1002/ijc.2910510214. [DOI] [PubMed] [Google Scholar]
- 22.Ross R A, Spengler B A, Domenech C, Porubcin M, Rettig W J, Biedler J L. Cell Growth Differ. 1995;6:449–456. [PubMed] [Google Scholar]
- 23.Sidell N, Sarafian T, Kelly M, Tsuchida T, Haussler M. Exp Cell Biol. 1986;54:287–300. doi: 10.1159/000163368. [DOI] [PubMed] [Google Scholar]
- 24.Ciccarone V, Spengler B A, Meyers M B, Biedler J L, Ross R A. Cancer Res. 1989;49:219–225. [PubMed] [Google Scholar]
- 25.Gaitonde S V, Qi W, Falsey R R, Sidell N, Martinez J D. Cell Growth Differ. 2001;12:19–27. [PubMed] [Google Scholar]
- 26.Dimri G P, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E E, Linskens M, Rubelj I, Pereira-Smith O, et al. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasorella A, Iavarone A, Israel M A. Mol Cell Biol. 1996;16:2570–2578. doi: 10.1128/mcb.16.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayflick L. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 29.Sedivy J M. Proc Natl Acad Sci USA. 1998;95:9078–9081. doi: 10.1073/pnas.95.16.9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodwin E C, Yang E, Lee C J, Lee H W, DiMaio D, Hwang E S. Proc Natl Acad Sci USA. 2000;97:10978–10983. doi: 10.1073/pnas.97.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcorta D A, Xiong Y, Phelps D, Hannon G, Beach D, Barrett J C. Proc Natl Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Mol Cell Biol. 1996;16:859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiess M, Gill R M, Hamel P A. Oncogene. 1995;10:159–166. [PubMed] [Google Scholar]
- 34.Bartkova J, Lukas J, Strauss M, Bartek J. Oncogene. 1998;17:1027–1037. doi: 10.1038/sj.onc.1202016. [DOI] [PubMed] [Google Scholar]
- 35.Poluha W, Poluha D K, Chang B, Crosbie N E, Schonhoff C M, Kilpatrick D L, Ross A H. Mol Cell Biol. 1996;16:1335–1341. doi: 10.1128/mcb.16.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan G Z, Ziff E B. J Neurosci. 1997;17:6122–6132. doi: 10.1523/JNEUROSCI.17-16-06122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan G Z, Ziff E B. J Neurosci. 1995;15:6200–6212. doi: 10.1523/JNEUROSCI.15-09-06200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiyama H, Iavarone A, LaBaer J, Reeves S A. Oncogene. 1997;14:2533–2542. doi: 10.1038/sj.onc.1201080. [DOI] [PubMed] [Google Scholar]
- 39.Hiyama H, Iavarone A, Reeves S A. Oncogene. 1998;16:1513–1523. doi: 10.1038/sj.onc.1201667. [DOI] [PubMed] [Google Scholar]
- 40.Cheng M, Olivier P, Diehl J A, Fero M, Roussel M F, Roberts J M, Sherr C J. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siavoshian S, Segain J P, Kornprobst M, Bonnet C, Cherbut C, Galmiche J P, Blottiere H M. Gut. 2000;46:507–514. doi: 10.1136/gut.46.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cenciarelli C, De Santa F, Puri P L, Mattei E, Ricci L, Bucci F, Felsani A, Caruso M. Mol Cell Biol. 1999;19:5203–5217. doi: 10.1128/mcb.19.7.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth P K, Dotto G P. Science. 1998;280:1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- 44.Dai M S, Mantel C R, Xia Z B, Broxmeyer H E, Lu L. Blood. 2000;96:3985–3987. [PubMed] [Google Scholar]
- 45.Rots N Y, Iavarone A, Bromleigh V, Freedman L P. Blood. 1999;93:2721–2729. [PubMed] [Google Scholar]
- 46.Slack R S, Proulx P. Biochim Biophys Acta. 1990;1053:89–96. doi: 10.1016/0167-4889(90)90030-h. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds C P. Curr Oncol Rep. 2000;2:511–518. doi: 10.1007/s11912-000-0104-y. [DOI] [PubMed] [Google Scholar]