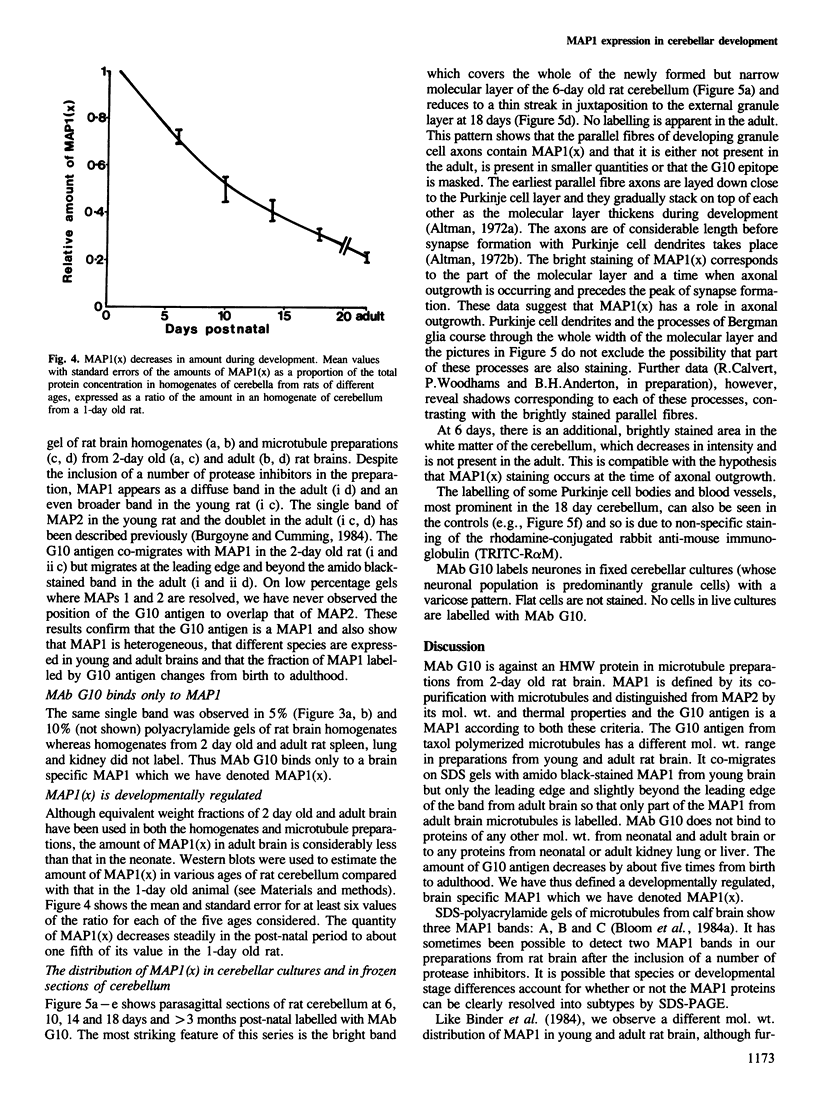
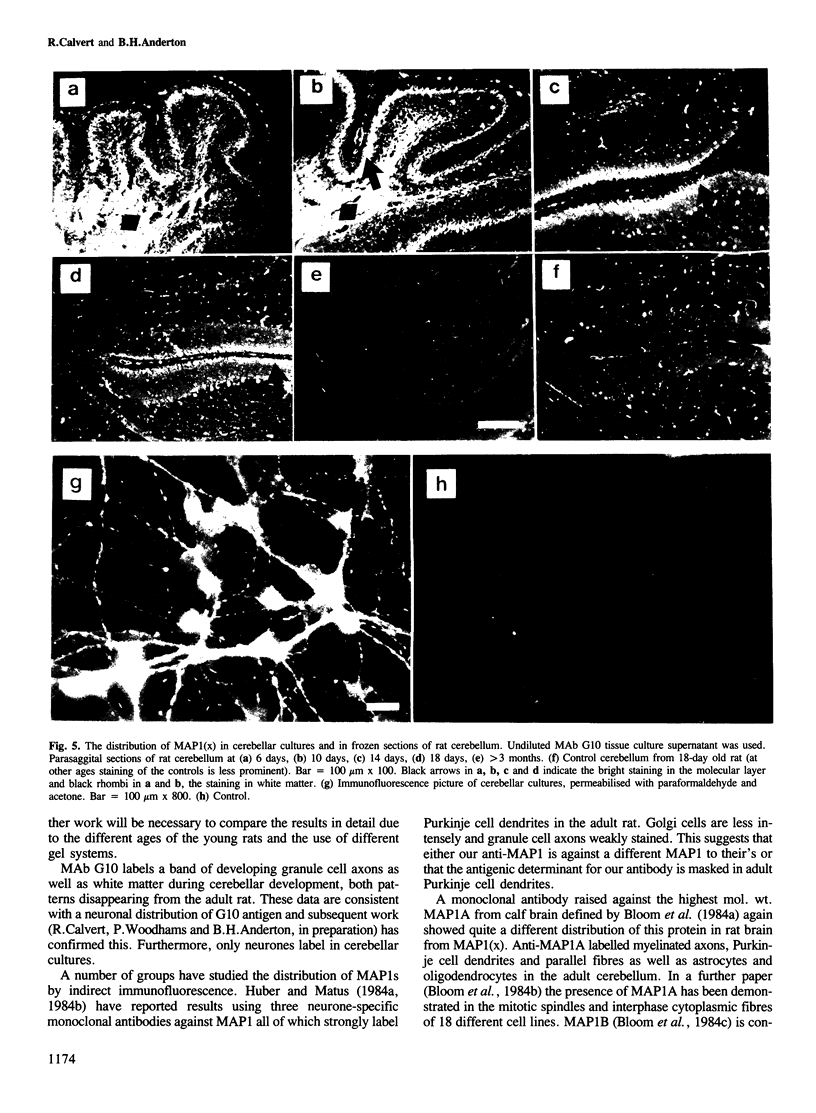
Abstract
Monoclonal antibody (MAb) G10 labels a single high mol. wt. (HMW) band on Western blots of microtubule preparations from 2 day old rat brain. The G10 antigen is thermolabile and co-migrates with microtubule-associated protein (MAP)1 from young rat brain on low percentage (5%) polyacrylamide-SDS gels. The G10 antigen decreases by about five times from birth to adulthood in the rat cerebellum. The same single band is labelled on Western blots of homogenates of whole neonatal rat brain but no labelling is found using neonatal or adult kidney, lung or liver. We have therefore identified a brain-specific MAP1, designated MAP1(x). Immunofluorescence microscopy using MAb G10 on parasagittal sections of rat cerebella shows labelling of the newly formed molecular layer in 6 day old rats. Only a narrow band close to the pial surface is labelled in 18 day old animals, which disappears in the adult. Labelling of the cerebellar white matter found in young rats also disappears. Neurones but not flat cells in cerebellar cultures label with MAb G10. All staining patterns are consistent with an axonal distribution of the antigen. MAP1(x) may be part of a developmentally regulated microtubule structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adoutte A., Claisse M., Cance J. Tubulin evolution: an electrophoretic and immunological analysis. Orig Life. 1984 Mar;13(3-4):177–182. doi: 10.1007/BF00927169. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972 Jul;145(3):353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972 Aug;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Bennett G. S., DiLullo C. Expression of a neurofilament protein by the precursors of a subpopulation of ventral spinal cord neurons. Dev Biol. 1985 Jan;107(1):94–106. doi: 10.1016/0012-1606(85)90379-3. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Kim H., Caceres A., Payne M. R., Rebhun L. I. Heterogeneity of microtubule-associated protein 2 during rat brain development. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5613–5617. doi: 10.1073/pnas.81.17.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Luca F. C., Vallee R. B. Widespread cellular distribution of MAP-1A (microtubule-associated protein 1A) in the mitotic spindle and on interphase microtubules. J Cell Biol. 1984 Jan;98(1):331–340. doi: 10.1083/jcb.98.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Schoenfeld T. A., Vallee R. B. Widespread distribution of the major polypeptide component of MAP 1 (microtubule-associated protein 1) in the nervous system. J Cell Biol. 1984 Jan;98(1):320–330. doi: 10.1083/jcb.98.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Cumming R. Ontogeny of microtubule-associated protein 2 in rat cerebellum: differential expression of the doublet polypeptides. Neuroscience. 1984 Jan;11(1):156–167. doi: 10.1016/0306-4522(84)90220-3. [DOI] [PubMed] [Google Scholar]
- Cumming R., Burgoyne R. D., Lytton N. A. Immunocytochemical demonstration of alpha-tubulin modification during axonal maturation in the cerebellar cortex. J Cell Biol. 1984 Jan;98(1):347–351. doi: 10.1083/jcb.98.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie D. N., Dutton G. R., Cohen J. Monolayer cultures of perikarya isolated from postnatal rat cerebellum. Experientia. 1979 Mar 15;35(3):345–347. doi: 10.1007/BF01964343. [DOI] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. Tubulin microheterogeneity increases with rat brain maturation. Nature. 1978 Nov 23;276(5686):411–413. doi: 10.1038/276411a0. [DOI] [PubMed] [Google Scholar]
- Huber G., Matus A. Differences in the cellular distributions of two microtubule-associated proteins, MAP1 and MAP2, in rat brain. J Neurosci. 1984 Jan;4(1):151–160. doi: 10.1523/JNEUROSCI.04-01-00151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber G., Matus A. Immunocytochemical localization of microtubule-associated protein 1 in rat cerebellum using monoclonal antibodies. J Cell Biol. 1984 Feb;98(2):777–781. doi: 10.1083/jcb.98.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Wright B., Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982 Jun;93(3):576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrla K. J., Goodman C. S. Transient expression of a surface antigen on a small subset of neurones during embryonic development. Nature. 1984 Sep 13;311(5982):151–153. doi: 10.1038/311151a0. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Rodionov V. I., Gelfand V. I., Rosenblat V. A. Microtubule-associated protein MAP1 promotes microtubule assembly in vitro. FEBS Lett. 1981 Dec 7;135(2):241–244. doi: 10.1016/0014-5793(81)80791-0. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Rodionov V. I., Gelfand V. I., Rosenblat V. A. Purification of high-Mr microtubule proteins MAP1 and MAP2. FEBS Lett. 1981 Dec 7;135(2):237–240. doi: 10.1016/0014-5793(81)80790-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mareck A., Fellous A., Francon J., Nunez J. Changes in composition and activity of microtubule-associated proteins during brain development. Nature. 1980 Mar 27;284(5754):353–355. doi: 10.1038/284353a0. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. The patterns of expression of two ankyrin isoforms demonstrate distinct steps in the assembly of the membrane skeleton in neuronal morphogenesis. Cell. 1984 Dec;39(2 Pt 1):309–320. doi: 10.1016/0092-8674(84)90009-6. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rothman T. P., Specht L. A., Gershon M. D., Joh T. H., Teitelman G., Pickel V. M., Reis D. J. Catecholamine biosynthetic enzymes are expressed in replicating cells of the peripheral but not the central nervous system. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6221–6225. doi: 10.1073/pnas.77.10.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U. Developmental biology of a neural cell adhesion molecule. Nature. 1984 Aug 16;310(5978):549–554. doi: 10.1038/310549a0. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Weber K. Differential expression of neurofilament triplet proteins in brain development. Nature. 1982 Jul 15;298(5871):277–279. doi: 10.1038/298277a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherline P., Mascardo R. Epidermal growth factor-induced centrosomal separation: mechanism and relationship to mitogenesis. J Cell Biol. 1982 Oct;95(1):316–322. doi: 10.1083/jcb.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Davis S. E. Low molecular weight microtubule-associated proteins are light chains of microtubule-associated protein 1 (MAP 1). Proc Natl Acad Sci U S A. 1983 Mar;80(5):1342–1346. doi: 10.1073/pnas.80.5.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland J., Willingham M. C., Sandoval I. V. A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. I. Biochemical characterization, effects on microtubule polymerization in vitro, and microtubule polymerization and organization in vivo. J Cell Biol. 1983 Nov;97(5 Pt 1):1467–1475. doi: 10.1083/jcb.97.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Briones E., Hirt H., Krepler R., Artlieb U., Denk H. Differential distribution of microtubule-associated proteins MAP-1 and MAP-2 in neurons of rat brain and association of MAP-1 with microtubules of neuroblastoma cells (clone N2A). EMBO J. 1983;2(11):1915–1920. doi: 10.1002/j.1460-2075.1983.tb01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Briones E., Koszka C., Artlieb U., Krepler R. Widespread occurrence of polypeptides related to neurotubule-associated proteins (MAP-1 and MAP-2) in non-neuronal cells and tissues. EMBO J. 1984 May;3(5):991–998. doi: 10.1002/j.1460-2075.1984.tb01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. N., Anderton B. H. Monoclonal antibodies to mammalian neurofilaments. Biosci Rep. 1981 Mar;1(3):263–268. doi: 10.1007/BF01114913. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Axon growth: roles of microfilaments and microtubules. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1206–1212. doi: 10.1073/pnas.66.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]






