Abstract
Nervous systems must adapt to shifts in behavioural ecology. One form of adaptation is neural exaptation, in which neural circuits are co-opted to perform additional novel functions. Here, we describe the co-option of a motor-to-somatosensory circuit into an olfactory network. Many moths beat their wings during odour-tracking, whether walking or flying, causing strong oscillations of airflow around the antennae, altering odour plume structure. This self-induced sensory stimulation could impose selective pressures that influence neural circuit evolution, specifically fostering the emergence of corollary discharge circuits. In Manduca sexta, a pair of mesothoracic to deutocerebral histaminergic neurons (MDHns), project from the mesothoracic neuromere to both antennal lobes (ALs), the first olfactory neuropil. Consistent with a hypothetical role in providing the olfactory system with a corollary discharge, we demonstrate that the MDHns innervate the ALs of advanced and basal moths, but not butterflies, which differ in wing beat and flight pattern. The MDHns probably arose in crustaceans and in many arthropods innervate mechanosensory areas, but not the olfactory system. The MDHns, therefore, represent an example of architectural exaptation, in which neurons that provide motor output information to mechanosensory regions have been co-opted to provide information to the olfactory system in moths.
Keywords: exaptation, histamine, evolution, arthropod, olfaction
1. Introduction
Exaptation is a core feature in the development of new phenotypic traits, allowing pre-existing traits to be co-opted to take on new or additional roles. There are numerous examples of exaptations involving the co-option of genes [1], body appendages [2] and behaviours [3]. For instance, the co-option of the teleost gas bladder into lungs, and lobe fins into limbs supported the conquest of land by tetrapods [4]. Feathers are another commonly cited example of exaptation; originally thought to support thermoregulation, they were exapted to produce thrust during flight [5]. While there are several examples of exaptation in the nervous system in general [6], the cellular and neural circuit basis for nervous system exaptation is poorly understood. For example, primitive insect wings originated from tracheal gills present on all body segments [7], yet the specific changes that occurred within motor networks to allow flight motor control in present-day insects remain to be identified. However, as traits such as appendages are co-opted to take on additional functions the neural networks associated with these structures are probably also co-opted for use in this new function.
Often, adaptations in nervous system function manifest as changes in biophysical and synaptic properties, which have been extensively described in networks that produce rhythmic output such as central pattern generators [8,9]. In addition to changes in biophysical and synaptic physiology, exaptations can also involve changes in neural architecture, such that neurons can be co-opted to innervate neural networks to which they did not project in the ancestral state. Architectural changes have the potential to modify existing brain regions to perform new functions [6]. In theory, the exaptation of circuitry could provide an existing network with additional information to enhance processing. Although there is evidence for neural exaptation within the context of entire brain regions [10], very little is known about the potential for neural exaptation at the level of individual neurons. In this study, we used comparative neuroanatomy to describe an example of architectural neural exaptation at the level of a pair of identified neurons, the mesothoracic to deutocerebral histaminergic neurons (MDHns). We demonstrate that the MDHns form a motor-to-mechanosensory circuit throughout the arthropods and were potentially co-opted to serve additional olfactory function in moths. This trait was subsequently lost in butterflies in correlation with changes in flight biomechanics and sensory dominance from olfaction to vision for locating food sources. This suggests that co-option of neural circuits at the level of individual neurons can result as a by-product of behaviour-specific natural selection. Furthermore, the conservation of the MDHn morphology across the arthropods suggests that interganglionic communication between limb motor control and mechanosensory centres in the brain is a fundamental feature of sensory processing.
2. Material and methods
(a). Animals
Manduca sexta were raised and maintained on a standard artificial diet [11]. Bombyx mori were purchased from Mulberry farms (Fallbrook, CA, USA), and raised on standard artificial diet. Idia aemula, Papilio appalachiensis and Limenitis archippus were collected in Morgantown, WV. Pieris rapae and Theatops californiensis were purchased from Carolina Biological Supply Co. (Burlington, NC). Grapholita molesta were provided by Dr Mark Willis (Case Western Reserve University). Galleria mellonella, Gyna lurida and Tenebrio molitor were provided by Dr. George Keeney (The Ohio State University). Caddisflies were provided by Kathy Kyle Stout (Wildscape Inc.). Drosophila melanogaster were raised at West Virginia University (WVU). Amblyomma americanum were provided by Dr Timothy Driscoll (WVU). At least six individuals were used for each species.
(b). Immunocytochemistry
Histamine (HA) labelling was performed as previously described [12]. Post-dissection, tissue was fixed in a 4% solution of N-3-dimethylaminopropyl-N′-ethylcarbodiimide (Sigma-Aldrich, 03449) in 0.01 M phosphate buffered saline (PBS, pH 6.9; Sigma-Aldrich, P-5368) at 4°C between 2 and 6 h depending on tissue volume (e.g. 2 h for D. melanogaster, 6 h for M. sexta). Tissue was then fixed in 4% paraformaldehyde (Electron Microscopy Sciences, 15710) in PBS overnight. Post-fixation, brains were washed in PBS. For sectioned tissue, brains were embedded in 5% agarose (Sigma-Aldrich, SLBJ3744 V) and sectioned between 100 and 150 µm using a Leica VT 1000S vibratome. The tissue was washed in PBS with 0.5% TritonTM-X 100 (PBST; Sigma-Aldrich, 110M0009 V), and blocked in 2% bovine serum albumin (BSA; Jackson Laboratory, 001-000-162) for 1 h. Brains were then incubated in 1 : 50 mouse anti-bruchpilot (Developmental Studies Hybridoma Bank, nc82) with 2% BSA in PBST at 4°C for 5 days before adding 1 : 500 rabbit anti-histamine, and incubating for another two days. The histamine antibody was raised against histamine conjugated to succinylated keyhole limpet haemocyanin via carbodiimide and this antibody shows no cross-reactivity to keyhole limpet haemocyanin alone [12]. Preadsorption with histamine also eliminates labelling [13]. Finally, in D. melanogaster, histidine decarboxylase mutants lack histamine immunolabelling using this antibody [14]. Following primary antibody application, tissue was washed in PBST, then blocked (as above), and incubated in 1 : 1000 Alexa 488, or 546 (Alexa Flour; Thermo Fisher Scientific A-11008, A-11030). Tissue was washed in PBST and PBS. For sectioned brains, tissue was run through an ascending glycerol (Sigma-Aldrich, BCBN3647 V) series (40%, 60% and 80%) and mounted in Vectashield® (Vector Laboratories, Za1222). For whole mounts, tissue was run through an ascending ethanol (Sigma-Aldrich, SHBF6704 V) series (30%, 50%, 70%, 95% and 100%) for 10 min; tissue was placed in a 1 : 1 solution of ethanol and methyl salicylate for 15 min, then 100% methyl salicylate for 15 min, then mounted in Permount® (Fisher Scientific, SP15-500).
(c). Optical imaging acquisition and analysis
Fluorescent tissue was viewed with a laser scanning confocal microscope (Olympus FV1000) equipped with red/green HeNe and argon lasers. Images were acquired using either a 20× or 40× magnification optical objective. Distance between confocal planes was optimized for the objective (1.79 μm for 20× and 0.54 μm for 40×) using Fluoview software (FV10-ASW v. 04.00.02.09). Pixel resolution was adjusted to compensate for the size of each specimen between 1024 × 1024 and 2048 × 2048 pixels. Images were only modified for contrast enhancement. All optical stacks were rendered with a maximum intensity projection across either the whole-mount or sectioned tissue. Figures were organized in CorelDraw (v. X4).
3. Results
In M. sexta, the pterothoracic ganglion is a fused structure that includes the prothoracic, mesothoracic, metathoracic and first two abdominal neuromeres. The MDHns branch extensively within the mesothoracic neuromere (MsN) and project ascending axons to innervate the suboesophageal zone (SEZ), antennal mechanosensory and motor centre (AMMC) and antennal lobe (AL) [13,15] (figure 1a). Excluding the optic lobes, there are 11 pairs of histaminergic neurons in the brain of M. sexta [15], however ablation experiments have demonstrated that the MDHns are the sole source of histamine in the AL [13]. Insects possess only two histamine receptors, both of which are histamine-gated chloride channels [16,17]. In the AL of M. sexta, the HisClB receptor is expressed by a subset of GABAergic local interneurons that innervate every glomerulus [13], and although histamine immunoreactivity (HA-ir) itself is constrained to several ventral glomeruli, this it is likely that the MDHns provide fast inhibitory input to a subset of neurons that themselves exert network-wide inhibition. However, while histaminergic neurons in the MsN of crickets [18], locusts [19] and Drosophila [14] project ascending axons into the AMMC (which also receives input from antennal mechanosensory neurons), they do not innervate the AL. This suggests that while the MDHns may be present in many insect taxa, they do not necessarily innervate the olfactory system, which may reflect differences in the impact of species-specific flight mechanics on odour plumes [20,21]. The olfactory system of M. sexta is able to track odours pulsed at the wing-beat frequency [22,23], so we, therefore, hypothesized that MDHn innervation of the AL arose because of selective pressures associated with a need to process odours carried by flight-induced air flow oscillations during plume tracking. We used a comparative approach to determine when over evolutionary time the MDHns began to innervate the AL and if this trait was lost with the evolution of different flight biomechanics within the Lepidoptera.
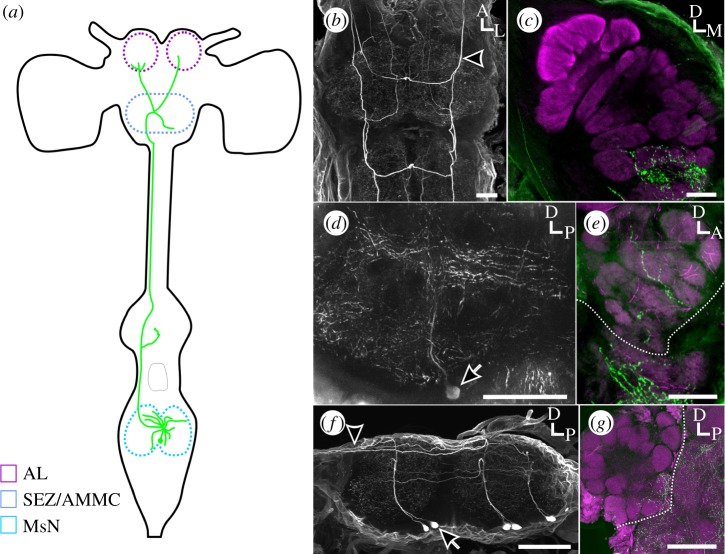
Figure 1.
MDHns in macrolepidopteran moths innervate the ALs. (a) Schematic of MDHns (green) in M. sexta. Each MDHn innervates the SEZ and AMMC before projecting to both ALs. (b) Whole-mount M. sexta pterothoracic ganglion immunolabelled for HA. MDHns are the most anterior pair of histaminergic neurons (arrow heads). Depth: 166.6 µm. (c) Frontal section of M. sexta AL immunolabelled for HA (green). Bruchpilot immunolabelling delineates neuropil (magenta). Depth: 52.36 µm. (d) Sagittal section of B. mori MsN immunolabelled for HA. Depth: 133 µm. (e) Sagittal section of B. mori AL immunolabelled for HA (green). Bruchpilot immunolabelling delineates neuropil (magenta). Depth: 58.8 µm. (f) Whole-mount sagittal view of I. aemula pterothoracic ganglion. Depth: 164.64 µm. (g) Sagittal section of I. aemula AL immunolabelled for HA (green). Bruchpilot immunolabelling delineates neuropil (magenta). Bruchpilot (magenta) is used to delineate neuropil. HA-ir (green). Depth: 124 µm. Scale bars, 100 µm. Arrows, cell bodies; arrow heads, ascending axons; hatched white lines in (e) and (g) delineates the boundary of the AL.
(a). Mesothoracic to deutocerebral histaminergic neuron innervation of the antennal lobe is specific to caddisflies and moths
To determine whether MDHn AL innervation was specific to M. sexta (Sphingidae), we examined the MDHns in B. mori (Bombycidae), a closely related species with similar wing-beating frequency and mechanics [24,25]. Both moths belong to the superfamily Bombycoidea and B. mori, although flightless, must beat their wings while walking to successfully track odour plumes [26]. The MDHns have a distinct, consistent morphology that, in combination with HA-immunolabelling allow their identification between species. In M. sexta, MDHn somata were located ventrally and send primary neurites dorsally where they project radially throughout the MsN (figure 1b). In addition, the MDHns project a single axon that ascends via the ventral nerve cord to the brain. HA-ir was present in the ALs of M. sexta in several ventral glomeruli (figure 1c). The MDHns in B. mori possessed nearly identical morphology with ventrally located cell bodies, dorsal radial MsN projections and axons that ascend to the brain (figure 1d). Similar to M. sexta, HA-ir was present in the AL of B. mori in several ventral glomeruli (figure 1e). To determine the phylogenetic distribution of AL innervation by the MDHns in the Macrolepidoptera further, we examined I. aemula (Erebidae), the powdered snout, which belongs to the superfamily Noctuoidea. The MsN of I. aemula contains histaminergic neurons with nearly identical morphology to the MDHns in M. sexta and B. mori, (figure 1f), including ascending projections to the brain and bilateral innervation of both ALs (figure 1g). Our results together indicate that histaminergic neurons that project from the MsN to the olfactory system are conserved within macrolepidopteran moths.
Butterflies also belong to the Macrolepidoptera, but primarily use vision to locate mates and food [27]. The flight patterns of butterflies are also much more heterogeneous than moths owing to non-periodic wing flapping, gliding and turn unpredictability [28]. These characteristics lower predation risk [28], but would theoretically reduce plume tracking ability. Butterflies are relatively closely related to the Bombycoidea and thus make great candidates for studying the emergence of MDHn innervation of the AL. Owing to these differences between butterfly and moth flight behaviour, we hypothesized that diurnal, and visually guided butterflies would have no AL MDHn innervation. We examined the ALs and MsNs of representative species from three of the five total families of butterflies (Nymphalidae, Papilionidae and Pieridae). In P. rapae (Pieridae), ventrally located MDHns in the MsN project ascending axons along the ventral nerve cord to the brain, and have a general architecture similar to M. sexta (figure 2a,b). However, in P. rapae and L. archippus (Nymphalidae) there were no HA-ir processes detected in the ALs (figure 2c,d, respectively). Finally, the MDHns of P. appalachiensis (Papilionidae) also branch radially throughout the MsN and project to the brain via the ventral nerve cord, but again HA-ir processes were absent within the AL (figure 2e,f). These results together suggest that MDHn innervation of the AL was either lost in butterflies or arose in the macrolepidopteran moths.
Figure 2.
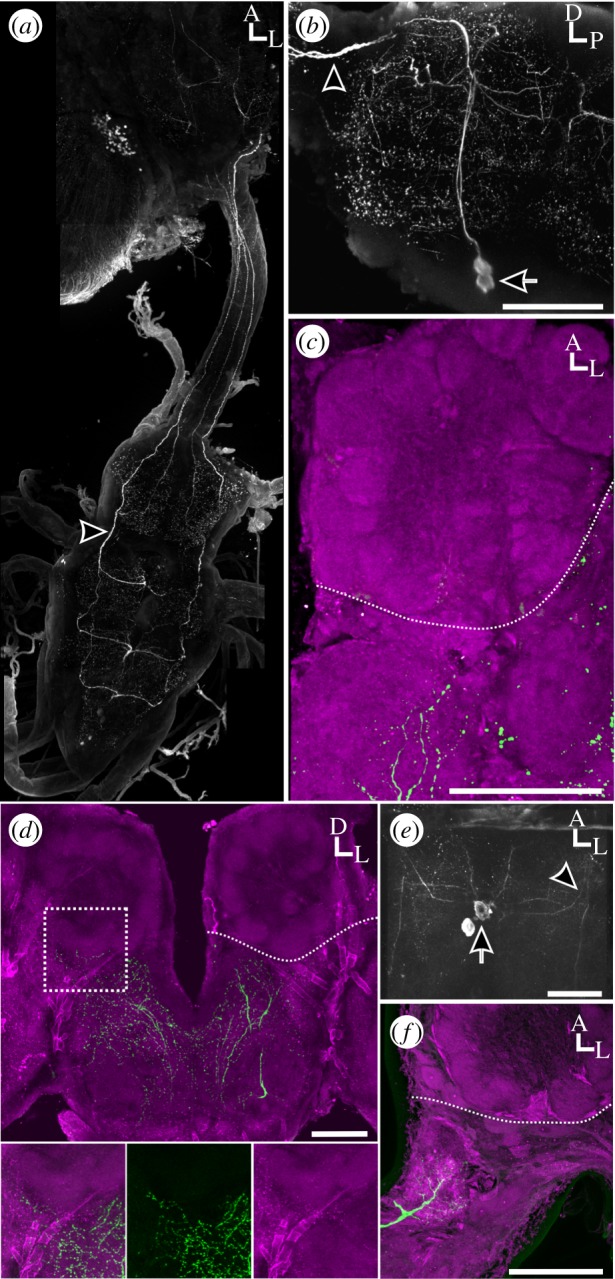
MDHns do not innervate ALs in butterflies. (a) Full central nervous system montage of HA-ir in P. rapae. Depth: 239.86 µm. (b) Whole-mount sagittal view of P. rapae MsN. Depth: 157.36 µm. (c) P. rapae AL showing absence of HA-ir (green). Bruchpilot immunolabelling delineates neuropil (magenta). Depth: 29.68 µm. (d) Whole-mount frontal view of L. archippus of brain showing no AL HA-ir. HA-ir can be seen directly posterior of the left AL in (d) (depth: 39.38 µm) however optical stacks restricted to the depth of tissue including only the AL (depth: 21.48 µm) demonstrate that these HA-ir processes do not enter the AL. (e) Horizontal view of MsN of P. appalachiensis. Depth: 170.05 µm. (f) Horizontal section of P. appalachiensis brain showing no HA-ir (green) in the AL. Bruchpilot (magenta) delineates neuropil. Depth: 25.76 µm. Scale bars, 100 µm. Arrows, cell bodies; arrow heads, ascending axons; hatched white lines in (c,d,f) delineates the boundary of the AL.
The Microlepidoptera are the most basal lepidopterans and are, therefore, ideally situated to determine whether AL HA innervation by the MDHns had been lost in butterflies, or arose in the macroplepidopteran moths. To this end, we examined the MDHns of two microlepidopterans, G. molesta (Tortricidae) and G. mellonella (Pyralidae), both of which walk and fan their wings during their final approach to an odour source [29,30]. Similar to the macrolepidopteran moths, the MDHn axons of G. molesta ascend from the MsN (figure 3a) via the ventral nerve cord to innervate the ALs (figure 3b). This was also the case for G. mellonella (Pyralidae) (figure 3c,d). We next examined the MDHns of one species of caddisfly (Limnephilidae) as Trichoptera is the sister taxon to the Lepidoptera. Although the wing kinematics of caddisflies has not been studied, caddisflies rely on sex pheromones as long distance communication cues [31], suggesting that they may be under similar behavioural and ecological constraints as moths. Similar to moths, the MDHns of caddisflies have ventrally located cell bodies that project ascending fibres to the brain (figure 3e) that innervate the ALs (figure 3f). These results suggest that MDHn innervation of the ALs was present in a common ancestor of the Lepidoptera and caddisflies, but subsequently lost in the butterflies.
Figure 3.
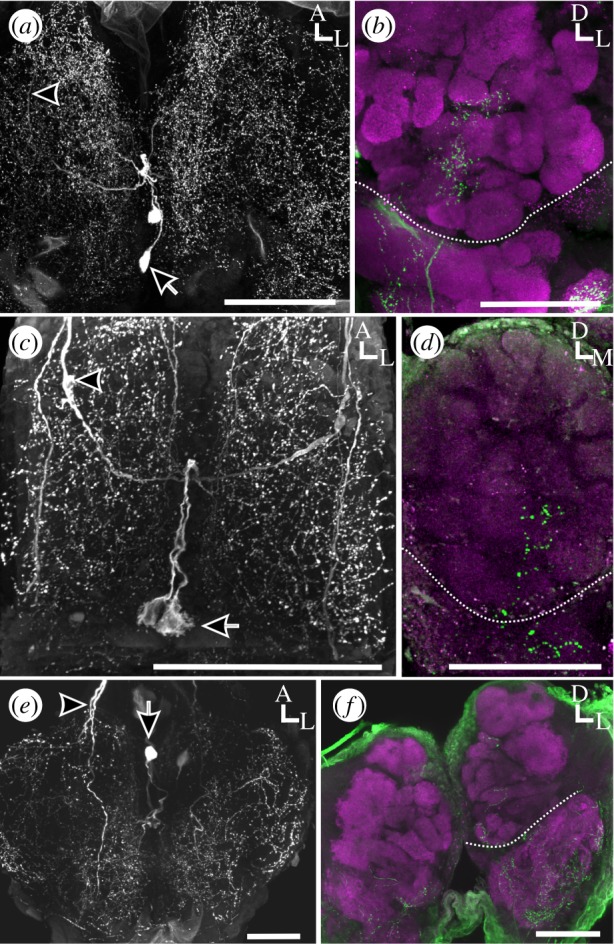
The MDHns in Microlepidoptera and Trichoptera innervate the ALs. (a) Whole-mount horizontal view of G. mellonella MsN. Depth: 123.3 µm. (b) Whole-mount frontal view of G. mellonella brain showing HA-ir (green) in the AL. Bruchpilot (magenta) delineates neuropil. Depth: 46.77 µm. (c) Whole-mount horizontal view of G. molesta MsN. Depth: 147.11 µm. (d) Whole-mount frontal view of G. molesta brain. Depth: 40.8 µm. (d) Whole-mount horizontal view of caddisfly (Limnephilidae) MsN. Depth: 103.82 µm. (f) Whole-mount frontal view of caddisfly (Limnephilidae) brain showing HA-ir (green) processes within the AL (brackets). Bruchpilot (magenta) delineates neuropil. Depth: 144.99 µm. Scale bars, 100 µm. Arrows, cell bodies; arrow heads, ascending axons; hatched white lines in (b,d,f) delineates the boundary of the AL.
(b). Mesothoracic to deutocerebral histaminergic neuron innervations are present throughout Arthropods
The olfactory systems of many arthropods species, including insects, are innervated by HA-ir processes from sources other than MDHns [12,32–37], while the olfactory systems of other species lack HA-ir altogether [12,14,38,39]. To determine when the characteristic morphology of the MDHns arose, we examined the MsN of several insect species and the second leg neuromeres of several more arthropod species (the equivalent neuromere to the mesothoracic neuromere in insects). Drosophila melanogaster (Drosophilidae) possess MDHns with the characteristic radial planar projections within the MsN and ascending axonal projections (figure 4a). However, while these ascending projections innervate the SEZ and AMMC, they do not enter the ALs (figure 4b). In T. molitor (Coleoptera), Oncopeltus fasciatus (Hemiptera) and G. lurida (Blattodea), ventrally located cell bodies with ascending HA fibres were also observed in the MsN (figure 4c,d,e), as is also the case for the maxillulary cephalic neuromere of the copepod Calanus finmarchicus (Crustaceae; [34]) and in the thoracic ganglia of the lobster Homarus americanus (Crustaceae; [40]). In the centipede T. californiensis, at least two pairs of histaminergic neurons were located in the ganglion corresponding to the segment bearing the second pair of legs (figure 4f). One pair of midline cells possessed ventral cell bodies and ascending axons. The extent of branching of these cells within the ganglion was minimal, but the axons were located dorsally, consistent with all other species observed. Finally, in the tick A. americanum (Chelicerata; Ixodidae), dorsally and laterally located cell bodies were observed, and there were no ascending projections (figure 4g), rather these cells projected diffusely in most neuromeres of the synganglion. In particular, we observed dense histaminergic innervation of the pedal, and cheliceral neuromeres, areas that process leg and mouthpart sensorimotor information [41]. This distribution of histaminergic neurons was similar to that observed in the synganglia of spiders [42]. It is unclear, however, whether these neurons are homologues of the MDHns as their cell bodies are dorsally located and reside along the lateral margin of the synganglion. Thus, MDHns appear to be widely distributed within the arthropods, and while homologous neurons are not apparent in ticks, histaminergic neurons that interconnect limb control and somatosensory regions appear to be a common feature of the arthropod nervous system.
Figure 4.
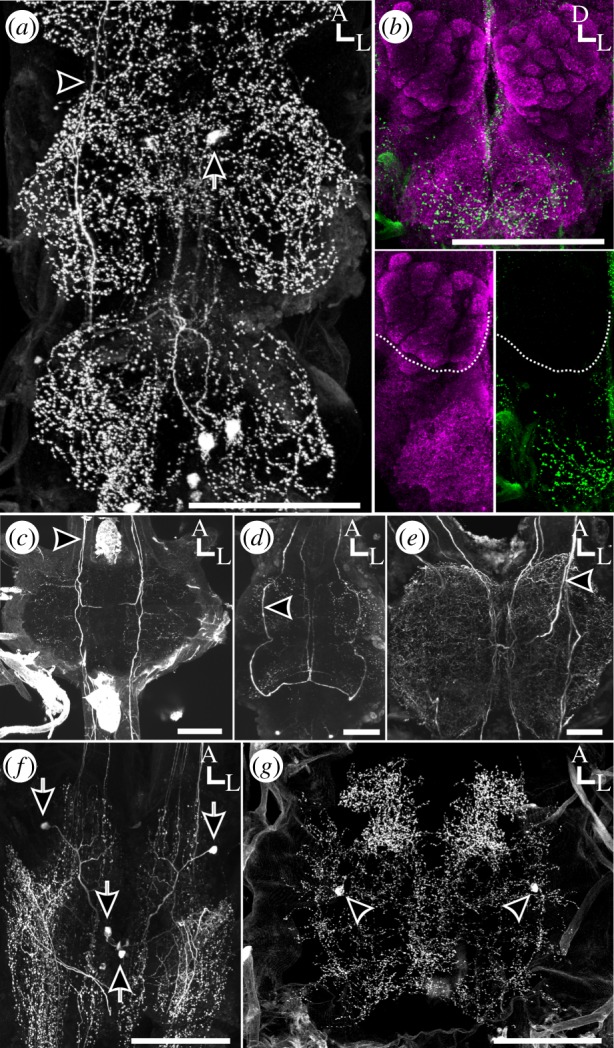
MDHns are present in the majority of arthropods. (a) Whole-mount horizontal view of the ventral nervous system of D. melanogaster. Depth: 132 µm. (b) Whole-mount frontal view of the brain of D. melanogaster. No HA-ir is observed in the ALs (insets). Bruchpilot (magenta) delineates neuropil. Depth: 132 µm. (c) Whole-mount horizontal view of the MsN of T. molitor immunolabelled for HA. Depth: 156 µm. (d) Whole-mount horizontal view of thoracic ganglia of O. fasciatus immunolabelled for HA. Depth: 211.22 µm. (e) Whole-mount horizontal view of the MsN of G. lurida immunolabelled for HA. Depth: 140 µm. (f) Whole-mount horizontal view of the first post-cephalic ganglion in T. californiensis immunolabelled for HA. Depth: 97.29 µm. (g) Whole-mount horizontal view of the synganglion in A. americanum. Depth: 119 µm. Scale bars, 100 µm. Arrows, cell bodies; arrow heads, ascending axons; hatched white lines in (b) delineates the boundary of the AL.
4. Discussion
Using a comparative approach to study specific neural circuits provides insight into how circuits are co-opted to perform new functions within a relatively short evolutionary time. Here, we hypothesized that the presence of a circuit interconnecting the flight motor and olfactory systems would correlate with flight mechanics that impact the sensory field. In this study, we found that a morphologically distinct neuron that ascends from the MsN to innervate the AL arose after the last common ancestor of the Diptera and Lepidoptera (figure 5). This circuit was conserved across much of the Lepidoptera, however this trait was lost in diurnal butterflies which differ dramatically from nocturnal moths in their behavioural ecology (figure 5). Thus, the MDHns are the sole source of histamine to the olfactory system in moths and the loss of their presence in the ALs resulted in a complete lack of histamine at this olfactory processing stage in butterflies. Furthermore, paired, histaminergic neurons that ascend from motor centres in the ventral nerve cord to the brain appear to be conserved within the insects and crustaceans. However, in ticks (figure 4) and spiders [42] the palpal/pedipalpal neuropil receive dense innervation from HA-ir neurons with dorsolaterally located somata, suggesting that the MDHns (which have ventromedial somata) probably arose after the Chelicerates. Regardless of origin, all arthropods appear to possess histaminergic neurons that interconnect ganglia representing different body segments.
Figure 5.
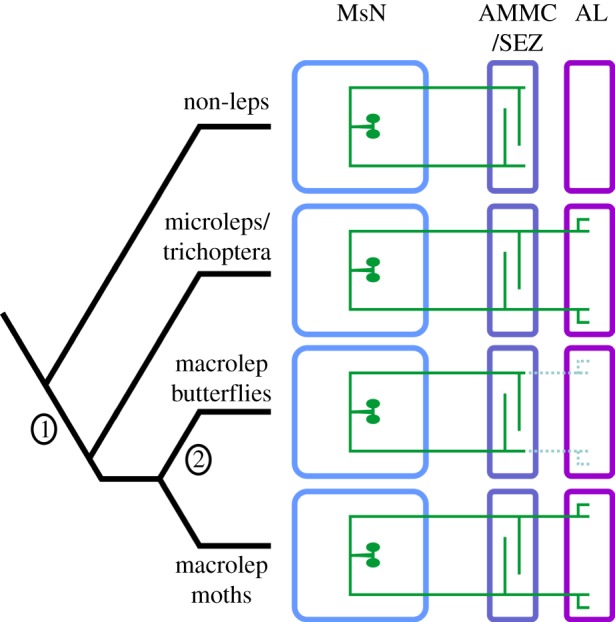
Schematic representation of the proposed evolutionary history of the MDHns. In this representation, the MDHns originally projected from the mesothoracic neuromere (MsN, blue) to the sub-oesophageal zone and antennal mechanosensory and motor centre (AMMC/SEZ, lavender). In the last common ancestor of the Lepidoptera and Trichoptera, the MDHns were co-opted (1; Dashed MDHn branches) to innervate the antennal lobes (ALs, magenta). The innervation of the ALs was lost in the butterflies (2), but maintained in macrolepidoteran moths.
Why would the olfactory systems of specific groups of insects receive input from flight motor centres, but not others? One potential explanation is that differences in MDHn structure arose in response to the effects of wing beating on odour plume structure. In M. sexta, wing beating in tethered flight creates strong oscillatory flow of air over the antennae that is tightly coupled to the wing-beat frequency [21]. Moths use odour plumes to locate mates, food and oviposition sites. Furthermore, wing-beating biomechanics in B. mori while walking [24] and M. sexta while hovering during odour-guided flight [25] are similar with respect to stable frequency and wing kinematics, suggesting that wing movement has similar impacts on odour plume structure and odour–antenna interactions. These wing beat-induced oscillations in airflow, therefore, create a periodic temporal structure that appears to be ecologically important. Butterflies, however, have strikingly different flight mechanics from moths. While moths have a consistent wing-beat frequency and stroke during odour-guided flight [25,43,44], butterflies have a more stochastic wing beat, and flight path [28]. Indeed, many butterflies incorporate protean behaviour into their flight patterns which ultimately creates a flight pattern with wing-beat frequencies that are not as stable as in moths, which may be a means to avoid predation [28]. While the distribution of turning angles in moths is either bimodal or normal [45], the distribution in butterflies is uniform across all angles [46] and butterflies have been shown to prioritize flower colour over scent [47]. Furthermore, although male and female butterflies produce pheromones, they are used as short-range cues (1–2 m) to determine mate quality after a potential mate has been located visually [27,48], whereas male moths locate female moths via pheromones over distances of several tens of meters [49]. Although the path of the wing tip during each wing stroke is similar between D. melanogaster and moths [50], the wing-beat frequency of D. melanogaster is approximately 190–230 Hz [51], much higher than the approximately 27–28 Hz wing-beat frequency of M. sexta [21] and much higher than the upper limit of what antennal responses in D. melanogaster can track [52]. Furthermore, antennal responses across several insect species can track rapid odour concentration fluctuations [22,53,54], in some cases exceeding 100 Hz [52]. Local field potentials within the AL have also been shown to respond to fluctuations at least up to approximately 70 Hz [22], well within the range of Lepidopteran wing-beat frequencies. In addition, neural population responses from the AL of M. sexta track and represent olfactory stimuli optimally when odours are presented at the wing-beat frequency [23]. This finding also corresponds to enhanced olfactory acuity as measured behaviourally [55], supporting the conclusion that their olfactory system has adapted to encode information that is embedded within a temporal structure induced by their own active sampling behaviour. The disturbances caused by the very high frequency wing beating in D. melanogaster on the other hand, are unlikely to be tracked by the AL, although there is clear evidence that the mechanoacoustic signature of the wing beat is detected by the arista and processed in the AMMC [56,57]. On the other hand, the lower frequency and relatively large amplitude disturbances in flow induced by wing beating in M. sexta, alter the structure of odour plumes in a manner that affects odour evoked activity in the AL [22,23]. There are potentially two ways in which the MDHns could communicate information about motor output to the AL of M. sexta. It is possible that MDHn activity is controlled by the overall level of motor activity in the MsN, which would suggest that the MDHns simply inform the olfactory system that the moth is moving. The other possibility is that the MDHns provide a precise efference copy to the olfactory system, thus informing this sensory network of the timing of motor output that will disrupt the structure of the olfactory stimulus. Future experiments that determine the context for MDHn activation will shed insight on the role of the MDHns in olfactory coding.
Typically, across more moderate periods of evolutionary time, neural circuits change by dedicating more space and resources to processing stimuli that are most important for an ecological niche. For instance, a third of the male M. sexta AL is devoted to processing female sex pheromone [58], the size of the mushroom body calyces is tightly correlated with ant and bee worker caste [59] and cortical expansion in star-nosed moles, hedgehogs and moles reflect species-specific changes in ecological niches and sensory appendages [60]. While many examples exist of the expansion and reduction of brain areas over time, very few examples exist of the invasion of new brain regions by identified neurons that are conserved across a broad range of species. Rather than an expansion within the context of a pre-existing function, the innervation of the ALs by the MDHns represent an example of co-option of a circuit into an additional network. The appearance and subsequent loss of MDHn innervation of the ALs within the Lepidoptera suggests that individual neurons can be co-opted into existing neural networks in a relatively short period of evolutionary time.
We observed ascending histaminergic neurons that innervated mechanosensory regions for head appendages in the brains of arthropods that span approximately 250 Myr of evolution. In D. melanogaster, as well as all moths and butterflies, MDHns innervate the AMMC, and even in ticks, which may lack MDHn homologues, there was dense histaminergic innervation of the dorsal anterior portion of the synganglion which receives sensory input from the mouthparts [41]. The conservation of this trait suggests that information about limb motor output is a critical component of mechanosensory network activity. The presence of interganglionic histaminergic neurons in the AMMC could also reflect the co-option of head appendages themselves from a locomotory function, to mechanosensory, and then olfactory function [61,62]. Our data suggest that behavioural and morphological specializations in moths resulted in the co-option of this circuit that provides input to a mechanosensory network in the ancestral state to also provide additional input to the olfactory system.
Supplementary Material
Acknowledgements
We thank John Boback, Mark Willis, George Keeney, Kathy Kyle Stout and Timothy Driscoll for providing various arthropod species. We would like to thank Kristyn Lizbinski, Tyler Sizemore, Kaylynn Coates and Kate Allen for comments on earlier drafts.
Data accessibility
This article has no additional data.
Authors' contributions
P.C., S.B., M.H., E.H., K.R. and A.D. collected data. P.C., S.B., K.D. and A.D. designed the project. All authors contributed to writing the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by NIH 1 R03 DC013997-01 to A.M.D. and USAFOSR FA9550-17-1-0117 to K.C.D. and A.M.D., as well as WVU Center for Neuroscience Summer Undergraduate Research Internships (NIGMS 5P30 GM103503) to M.M.H., E.J.H. and K.E.R.
References
- 1.True JR, Carroll SB. 2002. Gene co-option in physiological and morphological evolution. Annu. Rev. Cell Dev. Bi 18, 53–80. ( 10.1146/annurev.cellbio.18.020402.140619) [DOI] [PubMed] [Google Scholar]
- 2.Shubin N, Tabin C, Carroll S. 2009. Deep homology and the origins of evolutionary novelty. Nature 457, 818–823. ( 10.1038/nature07891) [DOI] [PubMed] [Google Scholar]
- 3.Borgia G, Coleman SW. 2000. Co-option of male courtship signals from aggressive display in bowerbirds. Proc. R. Soc. Lond. B 267, 1735–1740. ( 10.1098/rspb.2000.1203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahlberg PE, Clack JA. 2006. Palaeontology: a firm step from water to land. Nature 440, 747–749. ( 10.1038/440747a) [DOI] [PubMed] [Google Scholar]
- 5.Ostrom JH. 1974. Archaeopteryx and origin of flight. Q Rev. Biol. 49, 27–47. ( 10.1086/407902) [DOI] [Google Scholar]
- 6.Anderson ML. 2010. Neural reuse: a fundamental organizational principle of the brain. Behav. Brain Sci. 33, 245– 266 () [DOI] [PubMed] [Google Scholar]
- 7.Dumont JPC, Robertson RM. 1986. Neuronal circuits: an evolutionary perspective. Science 233, 849–853. ( 10.1126/science.233.4766.849) [DOI] [PubMed] [Google Scholar]
- 8.Dickinson PS. 2006. Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr. Opin Neurobiol. 16, 604–614. ( 10.1016/j.conb.2006.10.007) [DOI] [PubMed] [Google Scholar]
- 9.Katz PS, Harris-Warrick RM. 1999. The evolution of neuronal circuits underlying species-specific behavior. Curr. Opin Neurobiol. 9, 628–633. ( 10.1016/S0959-4388(99)00012-4) [DOI] [PubMed] [Google Scholar]
- 10.Anderson ML. 2007. Evolution of cognitive function via redeployment of brain areas. Neuroscientist 13, 13–21. ( 10.1177/1073858406294706) [DOI] [PubMed] [Google Scholar]
- 11.Bell RA, Joachim FG. 1976. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms Lepidoptera-Sphingidae-Gelechiidae. Ann. Entomol. Soc. Am. 69, 365–373. ( 10.1093/aesa/69.2.365) [DOI] [Google Scholar]
- 12.Dacks AM, Reisenman CE, Paulk AC, Nighorn AJ. 2010. Histamine-immunoreactive local neurons in the antennal lobes of the hymenoptera. J. Comp. Neurol. 518, 2917–2933. ( 10.1002/cne.22371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley SP, Chapman PD, Lizbinski KM, Daly KC, Dacks AM. 2016. A flight sensory-motor to olfactory processing circuit in the moth Manduca sexta. Front. Neural Circuits 10, 5 ( 10.3389/fncir.2016.00005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melzig J, Buchner S, Wiebel F, Wolf R, Burg M, Pak WL, Buchner E. 1996. Genetic depletion of histamine from the nervous system of Drosophila eliminates specific visual and mechanosensory behavior. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 179, 763–773. ( 10.1007/BF00207355) [DOI] [PubMed] [Google Scholar]
- 15.Homberg U, Hildebrand JG. 1991. Histamine-immunoreactive neurons in the midbrain and suboesophageal ganglion of sphinx moth Manduca sexta. J. Comp. Neurol. 307, 647–657. ( 10.1002/cne.903070410) [DOI] [PubMed] [Google Scholar]
- 16.Gisselmann G, Pusch H, Hovemann BT, Hatt H. 2002. Two cDNAs coding for histamine-gated ion channels in D. melanogaster. Nat. Neurosci. 5, 11–12. ( 10.1038/nn787) [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y, Hirschberg B, Yuan J, Wang AP, Hunt DC, Ludmerer SW, Schmatz DM, Cully DF. 2002. Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J. Biol. Chem. 277, 2000–2005. ( 10.1074/jbc.M107635200) [DOI] [PubMed] [Google Scholar]
- 18.Horner M, Helle J, Schurmann FW. 1996. The distribution of histamine-immunoreactive neurons in the ventral nerve cord of the cricket, Gryllus bimaculatus. Cell Tissue Res. 286, 393–405. ( 10.1007/s004410050709) [DOI] [PubMed] [Google Scholar]
- 19.Patschke A, Bicker G. 2011. Development of histamine-immunoreactivity in the central nervous system of the two locust species Schistocerca gregaria and Locusta migratoria. Microsc. Res. Tech. 74, 946–956. ( 10.1002/jemt.20980) [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Marin A, Duistermars BJ, Frye MA, Louis M. 2010. Mechanisms of odor-tracking: multiple sensors for enhanced perception and behavior. Front. Cell Neurosci 4, 6 ( 10.3389/fncel.2010.00006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sane SP, Jacobson NP. 2006. Induced airflow in flying insects II. Measurement of induced flow. J. Exp. Biol. 209, 43–56. ( 10.1242/jeb.01958) [DOI] [PubMed] [Google Scholar]
- 22.Tripathy SJ, Peters OJ, Staudacher EM, Kalwar FR, Hatfield MN, Daly KC. 2010. Odors pulsed at wing beat frequencies are tracked by primary olfactory networks and enhance odor detection. Front. Cell Neurosci. 4, 1 ( 10.3389/neuro.03.001.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houot B, Burkland R, Tripathy S, Daly KC. 2014. Antennal lobe representations are optimized when olfactory stimuli are periodically structured to simulate natural wing beat effects. Front. Cell Neurosci. 8, 159 ( 10.3389/fncel.2014.00159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishima T, Kanzaki R. 1998. Coordination of flipflopping neural signals and head turning during pheromone-mediated walking in a male silkworm moth Bombyx mori. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 183, 273–282. ( 10.1007/s003590050255) [DOI] [Google Scholar]
- 25.Willmott AP, Ellington CP. 1997. The mechanics of flight in the hawkmoth Manduca sexta. I. Kinematics of hovering and forward flight. J. Exp. Biol. 200, 2705–2722. [DOI] [PubMed] [Google Scholar]
- 26.Obara Y. 1979. Bombyx mori mating dance: essential in locating the female. Appl. Entomol. Zool. 14, 130–132. [Google Scholar]
- 27.Scott JA. 1974. Mate-locating behavior of butterflies. Am. Midl. Nat. 91, 103–117. ( 10.2307/2424514) [DOI] [Google Scholar]
- 28.Srygley RB, Chai P. 1990. Flight morphology of neotropical butterflies: palatability and distribution of mass to the thorax and abdomen. Oecologia 84, 491–499. ( 10.1007/BF00328165) [DOI] [PubMed] [Google Scholar]
- 29.Giner M, Balcells M, Avilla J. 2012. Insecticidal action of five allyl esters on eggs and larvae of three tortricid fruit pests: laboratory tests. Bull. Insectol 65, 63–70. [Google Scholar]
- 30.Roller H, Biemann K, Bjerke JS, Norgard DW, Mcshan WH. 1968. Sex pheromones of Pyralid moths. I. Isolation and identification of sex-attractant of Galleria mellonella L (greater waxmoth). Acta Entomol. Bohemos 65, 208. [Google Scholar]
- 31.Wood JR, Resh VH. 1984. Demonstration of sex pheromones in caddisflies (Trichoptera). J. Chem. Ecol. 10, 171–175. ( 10.1007/BF00987654) [DOI] [PubMed] [Google Scholar]
- 32.Bornhauser BC, Meyer EP. 1997. Histamine-like immunoreactivity in the visual system and brain of an orthopteran and a hymenopteran insect. Cell Tissue Res. 287, 211–221. ( 10.1007/s004410050747) [DOI] [PubMed] [Google Scholar]
- 33.Gebhardt S, Homberg U. 2004. Immunocytochemistry of histamine in the brain of the locust Schistocerca gregaria. Cell Tissue Res. 317, 195–205. ( 10.1007/s00441-003-0841-y) [DOI] [PubMed] [Google Scholar]
- 34.Hartline DK, Christie AE. 2010. Immunohistochemical mapping of histamine, dopamine, and serotonin in the central nervous system of the copepod Calanus finmarchicus (Crustacea; Maxillopoda; Copepoda). Cell Tissue Res. 341, 49–71. ( 10.1007/s00441-010-0974-8) [DOI] [PubMed] [Google Scholar]
- 35.Ignell R. 2001. Monoamines and neuropeptides in antennal lobe interneurons of the desert locust, Schistocerca gregana: an immunocytochemical study. Cell Tissue Res. 306, 143–156. ( 10.1007/s004410100434) [DOI] [PubMed] [Google Scholar]
- 36.Loesel R, Homberg U. 1999. Histamine-immunoreactive neurons in the brain of the cockroach Leucophaea maderae. Brain Res. 842, 408–418. ( 10.1016/S0006-8993(99)01864-8) [DOI] [PubMed] [Google Scholar]
- 37.Callaway JC, Stuart AE. 1999. The distribution of histamine and serotonin in the barnacle's nervous system. Microsc. Res. Tech. 44, 94–104. ( 10.1002/(SICI)1097-0029(19990115/01)44:2/3%3C94::AID-JEMT4%3E3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 38.Liu WW, Wilson RI. 2013. Transient and specific inactivation of Drosophila neurons in vivo using a native ligand-gated ion channel. Curr. Biol. 23, 1202–1208. ( 10.1016/j.cub.2013.05.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dacks AM, Nighorn AJ. 2011. The organization of the antennal lobe correlates not only with phylogenetic relationship, but also life history: a basal hymenopteran as exemplar. Chem. Senses 36, 209–220. ( 10.1093/chemse/bjq121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulloney B, Hall WM. 1991. Neurons with histamine-like immunoreactivity in the segmental and stomatogastric nervous systems of the crayfish Pacifastacus leniusculus and the lobster Homarus americanus. Cell Tissue Res. 266, 197–207. ( 10.1007/Bf00678725) [DOI] [PubMed] [Google Scholar]
- 41.Hummel NA, Li AY, Witt CM. 2007. Serotonin-like immunoreactivity in the central nervous system of two ixodid tick species. Exp. Appl. Acarol 43, 265–278. ( 10.1007/s10493-007-9120-z) [DOI] [PubMed] [Google Scholar]
- 42.Schmid A, Becherer C. 1999. Distribution of histamine in the CNS of different spiders. Microsc. Res. Tech. 44, 81–93. ( 10.1002/(SICI)1097-0029(19990115/01)44:2/3%3C81::AID-JEMT3%3E3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- 43.Liu H, Ellington C, Kawachi K. 1998. A computational fluid dynamic study of hawkmoth hovering. J. Exp. Biol. 201, 461–477. [DOI] [PubMed] [Google Scholar]
- 44.Usherwood JR, Ellington CP. 2002. The aerodynamics of revolving wings. I. Model hawkmoth wings. J. Exp. Biol. 205, 1547–1564. [DOI] [PubMed] [Google Scholar]
- 45.Kuenen LPS, Carde RT. 1994. Strategies for recontacting a lost pheromone plume: casting and upwind flight in the male gypsy-moth. Physiol. Entomol. 19, 15–29. ( 10.1111/j.1365-3032.1994.tb01069.x) [DOI] [Google Scholar]
- 46.Root RB, Kareiva PM. 1984. The search for resources by cabbage butterflies (Pieris rapae): ecological consequences and adaptive significance of Markovian movements in a patchy environment. Ecology 65, 147–165. ( 10.2307/1939467) [DOI] [Google Scholar]
- 47.Omura H, Honda K. 2005. Priority of color over scent during flower visitation by adult Vanessa indica butterflies. Oecologia 142, 588–596. ( 10.1007/s00442-004-1761-6) [DOI] [PubMed] [Google Scholar]
- 48.Andersson J, Borg-Karlson AK, Vongvanich N, Wiklund C. 2007. Male sex pheromone release and female mate choice in a butterfly. J. Exp. Biol. 210, 964–970. ( 10.1242/jeb.02726) [DOI] [PubMed] [Google Scholar]
- 49.Elkinton JS, Schal C, Ono T, Carde RT. 1987. Pheromone puff trajectory and upwind flight of male gypsy moths in a forest. Physiol. Entomol. 12, 399–406. ( 10.1111/j.1365-3032.1987.tb00766.x) [DOI] [Google Scholar]
- 50.Zanker JM. 1988. How does lateral abdomen deflection contribute to flight control of Drosophila melanogaster. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 162, 581–588. ( 10.1007/Bf01342633) [DOI] [Google Scholar]
- 51.Lehmann FO, Dickinson MH. 1997. The changes in power requirements and muscle efficiency during elevated force production in the fruit fly Drosophila melanogaster. J. Exp. Biol. 200, 1133–1143. [DOI] [PubMed] [Google Scholar]
- 52.Szyszka P, Gerkin RC, Galizia CG, Smith BH. 2014. High-speed odor transduction and pulse tracking by insect olfactory receptor neurons. Proc. Natl Acad. Sci. USA 111, 16 925–16 930. ( 10.1073/pnas.1412051111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemon W, Getz W. 1997. Temporal resolution of general odor pulses by olfactory sensory neurons in American cockroaches. J. Exp. Biol. 200, 1809–1819. [DOI] [PubMed] [Google Scholar]
- 54.Bau J, Justus KA, Carde RT. 2002. Antennal resolution of pulsed pheromone plumes in three moth species. J. Insect. Physiol. 48, 433–442. ( 10.1016/S0022-1910(02)00062-8) [DOI] [PubMed] [Google Scholar]
- 55.Daly KC, Kalwar F, Hatfield M, Staudacher E, Bradley SP. 2013. Odor detection in Manduca sexta is optimized when odor stimuli are pulsed at a frequency matching the wing beat during flight. PLoS ONE 8, e81863 ( 10.1371/journal.pone.0081863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai JS, Lo SJ, Dickson BJ, Chiang AS. 2012. Auditory circuit in the Drosophila brain. Proc. Natl Acad. Sci. USA 109, 2607–2612. ( 10.1073/pnas.1117307109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tootoonian S, Coen P, Kawai R, Murthy M. 2012. Neural representations of courtship song in the Drosophila brain. J. Neurosci. 32, 787–798. ( 10.1523/JNEUROSCI.5104-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rospars JP, Hildebrand JG. 2000. Sexually dimorphic and isomorphic glomeruli in the antennal lobes of the sphinx moth Manduca sexta. Chem. Senses 25, 119–129. ( 10.1093/chemse/25.2.119) [DOI] [PubMed] [Google Scholar]
- 59.Ehmer B, Gronenberg W. 2004. Mushroom body volumes and visual interneurons in ants: comparison between sexes and castes. J. Comp. Neurol. 469, 198–213. ( 10.1002/cne.11014) [DOI] [PubMed] [Google Scholar]
- 60.Catania KC. 2000. Cortical organization in insectivora: the parallel evolution of the sensory periphery and the brain. Brain Behav. Evol. 55, 311–321. ( 10.1159/000006666) [DOI] [PubMed] [Google Scholar]
- 61.Hansson BS, Stensmyr MC. 2011. Evolution of insect olfaction. Neuron 72, 698–711. ( 10.1016/j.neuron.2011.11.003) [DOI] [PubMed] [Google Scholar]
- 62.Jockusch EL, Williams TA, Nagy LM. 2004. The evolution of patterning of serially homologous appendages in insects. Dev. Genes Evol. 214, 324–338. ( 10.1007/s00427-004-0412-6) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.