Abstract
Prehistoric human impacts on megafaunal populations have dramatically reshaped ecosystems worldwide. However, the effects of human exploitation on smaller species, such as anatids (ducks, geese, and swans) are less clear. In this study we apply ancient DNA and osteological approaches to reassess the history of Australasia's iconic black swans (Cygnus atratus) including the palaeo-behaviour of prehistoric populations. Our study shows that at the time of human colonization, New Zealand housed a genetically, morphologically, and potentially ecologically distinct swan lineage (C. sumnerensis, Poūwa), divergent from modern (Australian) C. atratus. Morphological analyses indicate C. sumnerensis exhibited classic signs of the ‘island rule’ effect, being larger, and likely flight-reduced compared to C. atratus. Our research reveals sudden extinction and replacement events within this anatid species complex, coinciding with recent human colonization of New Zealand. This research highlights the role of anthropogenic processes in rapidly reshaping island ecosystems and raises new questions for avian conservation, ecosystem re-wilding, and de-extinction.
Keywords: ancient DNA, black swan, extinction, island rule, New Zealand, recolonization
1. Introduction
Prehistoric human harvesting of megafaunal populations has underpinned biodiversity declines in many parts of the world [1–3], but the effects of human exploitation of smaller animal species are less clear. The Anatidae (ducks, geese, and swans) are a cosmopolitan avian family (150 species, 40 genera; [4]) with a long history of exploitation by humans. Indeed, anatid remains are found in archaeological midden sites worldwide [5–7], reflecting their ongoing importance both culturally and economically [8]. Anthropogenic exploitation is thought to explain some prehistoric Holocene waterfowl extinction events, particularly involving large, flight-reduced or flightless island taxa (e.g. Hawaii [9], Reunion and Mauritius [10]). Such impacts, however, have yet to be well characterized.
In contrast to regions of the world with long histories of human–wildlife interaction (e.g. North America; [6]), New Zealand's (NZ) native biota (including 27 waterfowl species in 12 genera; [11–13]) has been subjected to human impacts only in the last millennium. Notably, one third of the region's anatid fauna has apparently become extinct since human colonization in the late thirteenth century [13,14]. Additionally, many of the region's surviving endemic waterfowl taxa are threatened by predation, habitat degradation, and hybridization with introduced waterfowl [11].
Among the extinct anatids, the fate of NZ's original indigenous swans is particularly enigmatic. The extant black swan (Cygnus atratus) is a highly distinctive component of modern-day Australasian wetland ecosystems. Archaeological data indicate that at the time of first human contact, black swans were distributed throughout NZ, including the isolated Chatham Islands (CI) [15]. However, Cygnus was absent from the region by the time of European settlement in the late eighteenth century, implying anthropogenic extirpation during NZ's megafaunal hunting period (ca. 1280–1450 AD; [11,15]). NZ's extant population of approximately 50 000 black swans is thought to be derived from Australian birds that were deliberately introduced during the mid-late nineteenth century [16], although natural self-introductions (see [17]) have also been hypothesized [11,18–20]. While only a single species of black swan is currently recognized (C. atratus) and seen as a rare example of extirpation and recolonization of NZ by the same species [11,21], some researchers have suggested from morphological evidence that the pre-human black swan may have represented a species complex rather than a single taxon [22,23].
New Zealand's rich archaeological record [24] presents intriguing opportunities to unravel the often complex dynamics between human populations and indigenous wildlife [25–31]. Recent analyses of ancient DNA (aDNA) have led to paradigmatic shifts in our understanding of the evolution of NZ's biota [32]. In particular, genetic studies of prehistoric remains have revealed a number of ‘cryptic’ biological turnover events, highlighting the role of density-dependent processes constraining lineage distributions [33] and leading to reappraisals of which lineages are truly ‘native’ [17]. In the current study we apply aDNA and osteological approaches to reassess the history of Australasia's iconic black swans including palaeo-behaviour of prehistoric populations, and to test for dynamic biological responses to anthropogenic change.
2. Material and methods
(a). Source of specimens
Modern and historic Cygnus atratus tissue, blood, and bone samples, covering the species' contemporary range in Australasia, were obtained from a variety of locations (n = 47; electronic supplementary material, figure S1 and table S1). Well-preserved pre-human Holocene fossil and archaeological Cygnus remains (AD 1280–1800), across the species prehistoric range in NZ and CI (including the holotype of Cygnus chathamicus Oliver, 1955), were sourced from well-constrained, radiocarbon-dated deposits, housed in museum and university collections (n = 39; electronic supplementary material, figure S1 and table S1). To ensure independence of samples, only common elements of the left or right orientation were sampled from an individual deposit.
(b). DNA extraction, amplification, and sequencing
DNA was extracted from modern tissue and blood samples following a modified Chelex protocol with 5% Chelex solution, 5 µl proteinase K (20 mg ml−1), and an overnight incubation at 56°C [34]. DNA was extracted from bone samples using the Qiagen DNeasy Tissue Kit following the manufacturer's instructions with an overnight incubation at 55°C. A 335 bp portion of the mitochondrial Control Region (CR) was amplified using the primer pair Cygn-1F (5′ GGTTATGCATATTCGTGCATAGAT 3′)/ Cygn-3R (5′ ACGTATGGGCCTGAAGCTAGT 3′) [35]. Each PCR reaction (10 µl) consisted of: 0.4 mg ml−1 BSA, 0.75 mM MgCl2 (Bioline), 1 × PCR buffer (Bioline), 2.5 mM dNTPs (Bioline), 0.5 µM of each primer, 0.05 U Taq Polymerase (Bioline), and 1 µl DNA. PCR thermocycling conditions were: 94°C 9 min, 35 cycles of 94°C 30 s, 50°C 45 s and 72°C 1 min, followed by a final extension step of 72°C 10 min. PCR products were run on a 2% 1 × TAE agarose gel and purified using ExoSap (1 U SAP, 1.5 U Exo1 (GE Healthcare); 30 min 37°C, 15 min 80°C) and sequenced bi-directionally using Big Dye terminator technology on an ABI 3730xl DNA analyser.
Ancient DNA extraction and PCR set-up was carried out at the University of Otago in a purpose-built aDNA laboratory (Otago Palaeogenetics Laboratory) physically isolated from other molecular laboratories. Strict aDNA procedures were followed to minimize the risk of contamination of samples with exogenous DNA and to authenticate aDNA sequences, including the use of negative extraction and PCR controls [36]. No swan specimens had been analysed in this laboratory prior to this study. DNA was extracted from historic museum specimens using the Qiagen DNeasy Tissue Kit following the manufacturer's instructions with the following modifications: addition of 20 µl of DTT (20 mg ml−1) prior to overnight incubation at 55°C, followed by the addition of 20 µl proteinase K (20 mg ml−1) and a second overnight incubation. DNA was extracted from 100–300 mg of bone powder following Rohland et al. [37]. The same 335 bp portion of CR as for modern specimens was amplified in three overlapping fragments using the primer pairs: Cygn-1F/Cygn-1R (5′ CATTCATGTTGGTYGGTTGGT 3′) (120 bp); Cygn-2F (5′ TACCATGYACACGGACATCAAA 3′)/Cygn-2R (5′ TATGTCCTGGGAGCATTCATT 3′) (101 bp); and Cygn-3F (5′ CCCAAGYACACAACAAGGCCA 3′)/Cygn-3R (138 bp). PCR reactions (20 μl) consisted of: 1 M Betaine (Sigma), 4 mM MgCl2 (Life Technologies), 1 × Gold Buffer II (Life Technologies), 0.625 mM dNTPs (Bioline), 0.25 μM of each primer, 1.25 U of AmpliTaq Gold Polymerase (Life Technologies), and 2 μl DNA. PCR thermocycling conditions were the same as for modern specimens except amplification was conducted over 60 cycles. Each PCR was replicated twice and unsuccessful PCRs were replicated with 2 µl 1:10 DNA or 4 μl DNA. Downstream post-PCR procedures were the same as for modern specimens. When ambiguous sites were observed due to DNA damage (C-T and G-A transitions), additional PCRs and sequencing were conducted, and a majority-rule consensus was applied [38]. DNA extraction and PCR were replicated if geographical location and genetic lineage conflicted.
(c). Phylogeographic analysis
Cygnus CR sequences were obtained from 47 modern and 39 ancient specimens (electronic supplementary material, table S1). Contiguous CR sequences (GenBank accessions MF455379-MF455462) were constructed using Sequencher (Genecodes) from high-quality sequence reads and aligned in MEGA 4.0 [39]. Parsimony-based phylogeographic network analysis in TempNet [40] was conducted on the complete CR dataset, in contrast to phylogenetic analysis due to the small fragment size (335 bp) and the evolutionary distant northern hemisphere outgroup—mute swan, C. olor (results not shown). Gene diversity (HE) was calculated for modern Australian and modern NZ/CI populations using the formula 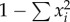 , where xi is haplotype frequency.
, where xi is haplotype frequency.
(d). Morphometric and osteological analysis
We recorded 24 length and width measurements of postcranial elements, and 11 cranial measurements (defined by von den Driesch [41]), from fully grown (i.e. osteologically mature) Cygnus specimens, covering the geographical and temporal range of the genus in Australasia (electronic supplementary material, table S2). Measurements were taken using Vernier calipers to the nearest 0.1 mm, using techniques defined by von den Driesch [41]. Only non-weathered bones where accurate length measurements could be obtained were analysed to avoid taphonomic biases influencing the results [21].
Morphological differentiation and diagnosibility between modern (Australian) and prehistoric (NZ/CI) genetic lineages (figure 1) were tested using principal component analysis (PCA) and discriminant function analysis (DFA) in R [42]. A Simpson's log ratio diagram was used to visualize differences in mean postcranial element lengths (i.e. ‘island rule’ effects) among modern and prehistoric lineages [43]. Relative flight ability of modern and prehistoric lineages was assessed using the multivariate (PCA) approach of Watanabe [44]. Mean body mass of Cygnus individuals was reconstructed using tibiotarsus mid-shaft width measurements, and the anatid body mass reference dataset of Dickison [45]. See electronic supplementary material for full methodology.
Figure 1.
Parsimony-based network reconstruction of Cygnus mtDNA CR sequences. Haplotypes are represented by circles. Circle size is proportional to haplotype frequency. Black circles represent undetected intermediate haplotypes. Lineage colouring: red, modern (Australia); light red, modern (NZ/CI); blue, prehistoric (NZ); light blue, prehistoric (CI). (Online version in colour.)
3. Results
(a). Temporal phylogeographic analysis
Cygnus CR sequences were recovered from all 47 modern and 39 prehistoric black swans, covering the geographical and temporal distribution of the genus in Australasia. Of the 335 characters in this dataset, 300 were constant, while 25 out of 35 variable characters were parsimony informative.
Black swan sequences cluster genetically into two distinct major lineages: ‘Australia’ and ‘NZ’ (figure 1). Within the prehistoric NZ lineage we detected geographical substructure, with distinct mainland NZ and CI haplogroups. The NZ haplogroup consists of 11 haplotypes (older than 1450 AD), while the CI haplogroup, separated from NZ by one fixed single nucleotide polymorphism (SNP), consists of three haplotypes (older than 1650 AD). A single prehistoric CI individual (NMNZ S.32994; electronic supplementary material, table S1) exhibits a NZ haplotype. We interpret this genetic outlier as a likely vagrant (see [27–29,32]). The most genetically diverse lineage (19 haplotypes) comprises individuals from Australia, and prehistoric, historic, and modern black swans from NZ (younger than 1450 AD) and CI (younger than 1864 AD). This widespread Australian lineage is distinguished from the NZ lineage by five fixed SNPs—indeed, phylogenetic analysis (data not shown) indicates that CR sequences from prehistoric (NZ and CI) swans form a monophyletic group, to the exclusion of modern (Australian) sequences. Gene diversity of modern Australian swan samples (HE = 0.88) was higher than that of modern NZ/CI samples (HE = 0.73).
(b). Osteological analyses
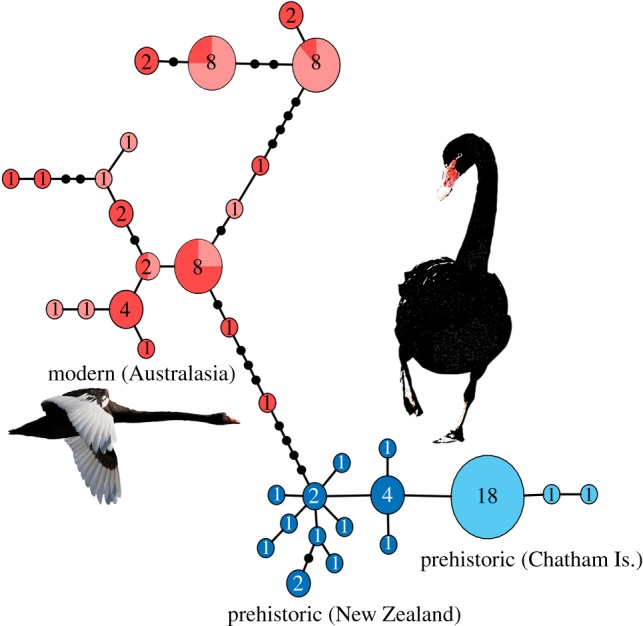
Statistical analysis of osteological datasets comprising modern and prehistoric specimens revealed significant morphological differentiation between modern (Australian) and prehistoric (NZ) lineages (figure 2a; electronic supplementary material). We also detected pronounced morphological substructure among ancient NZ and CI Cygnus populations, with more robust humeri and femora detected in prehistoric mainland NZ black swans (figure 2a; electronic supplementary material). Prehistoric individuals were overall larger and more robust (across all cranial and postcranial elements) than modern (Australian) black swans (figure 2a; electronic supplementary material). Size differences were particularly pronounced for hind limb elements (i.e. femur, tibiotarsus, and tarsometatarsus), with bones from prehistoric Cygnus highly elongated relative to those from modern black swans (figure 2b; electronic supplementary material). Prehistoric NZ Cygnus likely had reduced flight ability, as indicated by elongated hind limbs, and proportionally shorter/robust wings in relation to hind limbs [44], compared to modern (Australian) volant black swans (electronic supplementary material). Body mass reconstructions (mean weights) suggest that prehistoric NZ Cygnus were 20–32% heavier than modern specimens (figure 2c), with weights ranging from 6–10 kg, compared to 4–9 kg for modern (Australian) individuals (electronic supplementary material).
Figure 2.
(a) Principal Component Analysis of pooled postcranial measurements of modern (Australia) and prehistoric (NZ/CI) Cygnus swans. (b) A Simpson's log-ratio diagram, showing the logarithmic differences of mean lengths of the postcranial elements of prehistoric Cygnus from the CI (proxy for NZ cf. Corvus spp.; [46]) relative to the modern (Australia) Cygnus. Abbreviations: COR, coracoid; HUM, humerus; ULN, ulna; CMC, carpometacarpus; FEM, femur, TBT, tibiotarsus; TMT, tarsometatarsus. (c) Relationship between mean body mass (in kg) and tibiotarsus circumference in anatids. Data are from Dickison [44]. (Online version in colour.)
4. Discussion
Ancient DNA analyses of prehistoric Cygnus remains reveal that, at the time of human colonization, NZ had a swan lineage genetically distinct from the modern (Australian) C. atratus (figures 1 and 3). Similarly, osteological analyses indicate substantial phenotypic differentiation between prehistoric (NZ) versus modern (Australian) populations (figure 2). Specifically, the extinct NZ lineage exhibited classic morphological signs of the ‘island rule’ effect [43,46–49] (figure 2b,c), suggesting a relatively terrestrial life history compared to that of the extant Australian lineage. The distinctive large body size, elongated legs, proportionally short robust wings relative to legs, and substantially increased body mass of the extinct prehistoric swans suggest that this lineage was on an evolutionary pathway towards flightlessness [44,48].
Figure 3.
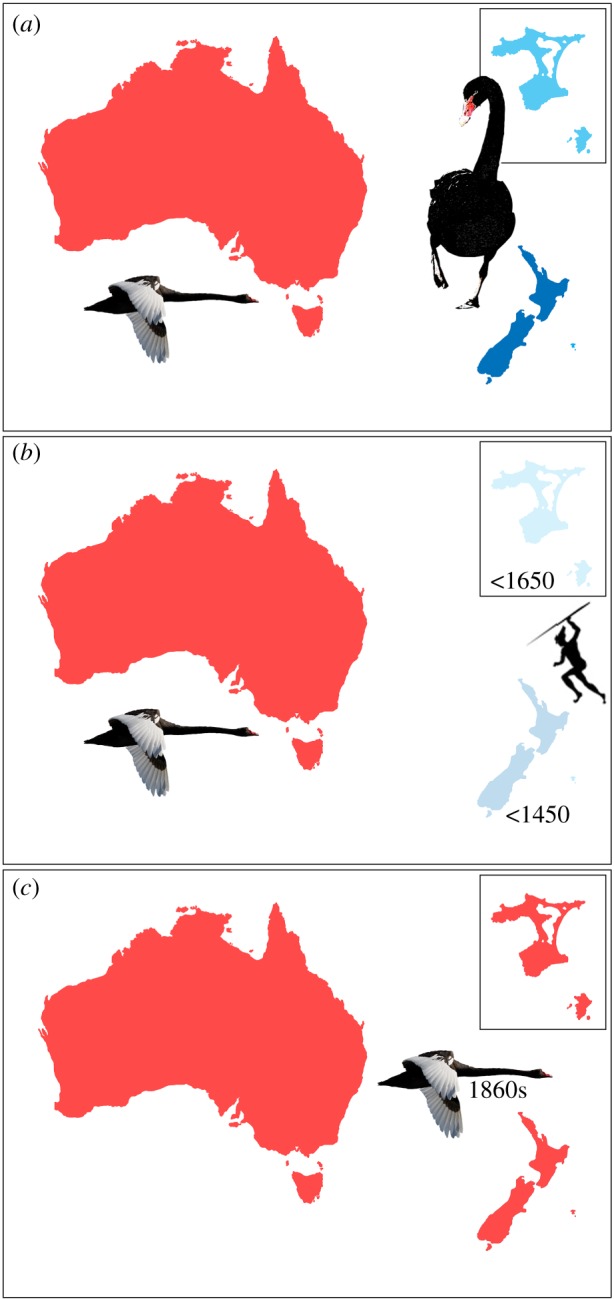
Extinction and replacement of Cygnus swans following human colonization of NZ and CI. At the time of human arrival in the NZ region (a) C. atratus was restricted to Australia, while C. sumnerensis was restricted to NZ. Within 200 years of human settlement (b), C. sumnerensis was extinct. During the late nineteenth century (c) C. atratus was deliberately introduced to NZ, though there is evidence for self-introduction around this time (and possibly prior to this). Red, modern (Australia) C. atratus; blue, prehistoric (NZ) C. s. sumnerensis; light blue, prehistoric (CI) C. s. chathamicus. (Online version in colour.)
The genetic (figure 1) and morphological (figure 2; electronic supplementary material) findings of the current study support recognition of distinct Australian Cygnus atratus (Latham, 1790) and NZ Cygnus sumnerensis (Forbes, 1890) swan species under the Diagnosable Species Concept [11,50,51], a derivative of the Phylogenetic Species Concept [52], designed to avoid taxonomic over-inflation [53] (see electronic supplementary material for further discussion). Based on additional morphological and genetic substructure (figures 1 and 2; electronic supplementary material), we also recommend recognition of two subspecies within C. sumnerensis: the prehistoric CI lineage C. s. chathamicus Oliver, 1955 and the prehistoric NZ lineage C. s. sumnerensis (Forbes, 1890). The common name of Poūwa for C. sumnerensis is appropriate. The Poūwa is a bird in CI Moriori legend thought to be the prehistoric (NZ/CI) swan [54–56].
Our study provides a rare example of a putatively flight-reduced and perhaps semi-terrestrial swan lineage. Indeed, only two suggested flightless swan taxa (both extinct), one of uncertain affinity within Anatidae, have previously been recorded in the fossil record [44,57,58] compared to numerous flight-reduced or flightless ducks and geese [44,49,59]. While substantial changes in relative body size are commonplace in insular island species [10,49,59–61], they have not previously been clearly characterized within Cygnus (e.g. [44,57]). Similarly, convergent reductions in flight ability—via increased body size, shortened wings in relation to legs, and elongation of hind limbs—are common features of island avifaunas [44,46,48,61]. The pronounced changes in body size and flight ability in C. sumnerensis no doubt evolved rapidly, given probable colonization of NZ within the past one to two million years as the climate cooled and the landscape became more open with onset of Pleistocene glacial cycles [47]. The increased body mass no doubt led to more robust wing bones, especially humeri, to offset this weight gain. The ‘island rule’ effect is characterized here for the first time in insular C. sumnerensis, which had a large body size, reduced flight ability, and elongated hind limbs (especially tarsometatarsus to femur ratio) relative to its ‘continental’ sister taxon C. atratus (figure 2). Notably, the elongated hind limbs of C. sumnerensis imply a relatively terrestrial lifestyle (figure 2b), favouring more terrestrial locomotion [43,62] and ground foraging [46], and leading to increased force required for take-off [48] or defence against large-bodied aerial predators [49]. This transition towards flightlessness likely reflects the absence of terrestrial mammalian predators from prehistoric NZ and CI, and also the relatively small number of prehistoric large-bodied aerial raptors (two species: Haast's Eagle and Eyles' Harrier) [14,48].
Following human settlement of the NZ region, this once diverse Cygnus species complex experienced a rapid loss of diversity, with extinction in NZ by the mid-fifteenth century and CI by the mid-seventeenth century (figure 3). The extinct lineages were subsequently replaced by Australian C. atratus (figure 3). This newly characterized ‘turnover’ event highlights that extinction can potentially facilitate major phylogeographic shifts, and parallels similar extinction-colonization events in costal and marine megafauna [25–27,29,32]. Anthropogenic impact and predation by introduced mammals no doubt caused the rapid extinction of C. sumnerensis—indeed, the presence of Cygnus remains in archaeological midden deposits attests to their use as food [24]. While there is some evidence to suggest that ‘vagrant’ C. atratus were present in NZ shortly after the extinction of C. sumnerensis (AM LB216, NMNZ S.46032; electronic supplementary material, table S1), ultimately successful ‘replacement’ did not occur until the mid to late nineteenth century [11,15,54,55]. The marginally reduced gene diversity detected in modern NZ/CI C. atratus compared to modern Australian C. atratus suggests only a modest founder effect (and thus a substantial founding size) relative to the findings of previous anatid translocation studies [63].
Our findings may raise a dilemma for species conservation [17], ecosystem re-wilding [64,65], and de-extinction [66]—new arrivals (or ‘native invaders’ [14]) do not represent the original endemic fauna, so could be seen as alien and unwanted pests, especially in anthropogenically modified landscapes. Cygnus atratus may not be an ecological replacement of C. sumnerensis despite their close genetic affinity. The public perception of the newly arrived C. atratus as alien or unwanted is no doubt influenced by their deliberate introduction, despite additional evidence of possible self-introduction events by modern black swans. Alternatively, one could argue for the conservation of C. atratus given congeneric status to C. sumnerensis akin to extinction-replacement events on mainland NZ within Megadyptes and Eudyptula penguins, and Phocarctos sea lions [25,26,31]. As shown by our study, aDNA can provide an evidence-based assessment of these often values-driven philosophical debates.
While numerous studies have revealed anthropogenic changes to prehistoric ecosystems [1,67], effects on taxa such as anatids have remained poorly understood, despite the broad cultural and economic importance of these birds [8]. The current study reveals sudden extinction events within an anatid species complex, coinciding with recent human colonization of an isolated archipelago. This research highlights the role of anthropogenic processes in rapidly reshaping island ecosystems [68] and raises new questions for avian conservation [17], ecosystem re-wilding [64,65], and de-extinction [66].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Western Australian Museum, South Australian Museum, Melbourne Museum, Australian National Wildlife Collection (CSIRO), Australian Museum, Queensland Museum, Auckland Museum (Matt Rayner), Museum of New Zealand Te Papa Tongarewa, Canterbury Museum, Otago Museum (Emma Burns), Justin Maxwell, Hokotihi (Chatham Island) Moriori, Pip Keen, Jill Hamell, and Chris Lalas for providing samples. We also thank the Erasmus Mundus International Masters in Applied Ecology programme for providing A.K. the opportunity of a research exchange at the University of Otago.
Ethics
As all Cygnus material examined was collected prior to this study and not by the researchers, no ethics approval was required.
Data accessibility
All DNA sequences have been deposited in GenBank under accessions MF455379-MF455462. Electronic supplementary material is available with the electronic version of this paper.
Authors' contributions
N.J.R. and J.M.W. conceptualized the study; N.J.R., A.K., L.J.E., A.J.D.T., and R.P.S. carried out the research and analysed the data; J.M.W. provided substantial funding towards this research. All authors contributed to the writing of the manuscript and gave final approval for publication. We thank Trevor Worthy and one anonymous reviewer for comments that greatly improved this manuscript.
Competing interests
The authors have no competing interests
Funding
This research was supported by the Allan Wilson Centre, Royal Society of New Zealand Marsden Fund, and the University of Otago.
References
- 1.Sandom C, Faurby S, Sandel B, Svenning JC. 2014. Global late Quaternary megafauna extinction linked to humans, not climate change. Proc. R. Soc. B 281, 20133254 ( 10.1098/rspb.2013.3254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saltre F, et al. 2016. Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat. Commun. 7, 10511 ( 10.1038/ncomms10511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Kaars S, Miller GH, Turney CSM, Cook EJ, Nurnberg D, Schonfeld J, Kershaw AP, Lehman SJ. 2017. Humans rather than climate the primary cause of Pleistocene megafaunal extinction in Australia. Nat. Commun. 8, 14142 ( 10.1038/ncomms14142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livezey BC. 1986. Phylogeny and historical biogeography of Steamer-Ducks (Anatidae: Tachyeres). Syst. Biol. 35, 458–469. ( 10.2307/2413109) [DOI] [Google Scholar]
- 5.Albarella U. 2005. Alternate fortunes? The role of domestic ducks and geese from Roman to medieval times in Britain In Feathers, grit and symbolism (eds G Grupe, J Peters), Documenta Archaeobiologiae3, 249–258.
- 6.Jones TL, Procasi JF, Erlandson JM, Dallas H, Wake TA, Schwaderer R. 2007. The protracted Holocene extinction of California's flightless sea duck (Chendytes lawi) and its implications for the Pleistocene overkill hypothesis. Proc. Natl Acad. Sci. USA 105, 4105–4108. ( 10.1073/pnas.0711140105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zangrando AFJ, Tivoli AM. 2015. Human use of birds and fish in marine settings of southern Patagonia and Tierra del Fuego in the Holocene: a first macro-regional approach. Quat. Int. 373, 82–95. ( 10.1016/j.quaint.2014.11.047) [DOI] [Google Scholar]
- 8.Kear J. 1990. Man and wildfowl. London, UK: Poyser. [Google Scholar]
- 9.Walther M, Hume JP. 2016. Extinct birds of Hawaii. Honolulu, HI: Mutual Publishing. [Google Scholar]
- 10.Hume JP, Waters M. 2012. Extinct birds. London, UK: Bloomsbury Publishing. [Google Scholar]
- 11.Gill BJ, Bell BD, Chambers GK, Medway DG, Palma RL, Scofield RP, Tennyson AJD, Worthy TH. 2010. Checklist of the birds in New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency Antarctica, 4th edn Wellington, New Zealand: Te Papa Press in association with the Ornithological Society of New Zealand. [Google Scholar]
- 12.Mitchell KJ, Wood JR, Scofield RP, Llamas B, Cooper A. 2014. Ancient mitochondrial genome reveals unsuspected taxonomic affinity of the extinct Chatham duck (Pachyanas chathamica) and resolves divergence times for New Zealand and sub-Antarctic brown teals. Mol. Phylogenet. Evol. 70, 420–428. ( 10.1016/j.ympev.2013.08.017) [DOI] [PubMed] [Google Scholar]
- 13.Williams M, Tennyson AJD, Sim D. 2014. Island differentiation of New Zealand's extinct mergansers (Anatidae: Mergini), with description of a new species from Chatham Island. Wildfowl 64, 3–34. [Google Scholar]
- 14.Tennyson AJD, Martinson P. 2007. (Revised edition) Extinct birds of New Zealand. Wellington, New Zealand: Te Papa Press. [Google Scholar]
- 15.Worthy TH, Holdaway RN. 2002. Lost world of the moa. Christchurch, New Zealand: Canterbury University Press. [Google Scholar]
- 16.Miers KH, Williams M. 1969. Nesting of the black swan at Lake Ellesmere, New Zealand. Wildfowl 20, 23–32. [Google Scholar]
- 17.Waters JM, Grosser S. 2016. Managing shifting species: ancient DNA reveals conservation conundrums in a dynamic world. Bioessays 38, 1177–1184. ( 10.1002/bies.201600044) [DOI] [PubMed] [Google Scholar]
- 18.Kirk T. 1896. The displacement of species in New Zealand. Trans. N.Z. Inst. 28, 1–27. [Google Scholar]
- 19.Richards EC. 1950. Diary of E.R. Chudleigh 1862–1921 Chatham Islands. Christchurch, New Zealand: Simpson and Williams Ltd. [Google Scholar]
- 20.Holdaway RN, Worthy TH, Tennyson AJD. 2001. A working list of breeding bird species of the New Zealand region at first human contact. N.Z. J. Zool. 28, 119–187. ( 10.1080/03014223.2001.9518262) [DOI] [Google Scholar]
- 21.Worthy TH. 1998. A remarkable fossil and archaeological avifauna from Marfells Beach, Lake Grassmere, South Island, New Zealand. Rec. Cant. Mus. 12, 79–176. [Google Scholar]
- 22.Forbes HO. 1892. Nature 41, 209. [Google Scholar]
- 23.Oliver WRB. 1955. New Zealand birds, 2 edn Wellington, New Zealand: AH and AW Reed. [Google Scholar]
- 24.Smith IWG, James-Lee T. 2010. Data for an archaeozoological analysis of marine resource use in two New Zealand study areas. Otago. Archaeological Laboratory Report No. 7. Anthropology Department, University of Otago, Dunedin. [Google Scholar]
- 25.Boessenkool S, Austin JJ, Worthy TH, Scofield RP, Cooper A, Seddon PJ, Waters JM. 2009. Relict or colonizer? Extinction and range expansion of penguins in southern New Zealand. Proc. R. Soc. B 276, 815–821. ( 10.1098/rspb.2008.1246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins CJ, et al. 2014. Extinction and recolonization of coastal megafauna following human arrival in New Zealand. Proc. R. Soc. B 281, 20140097 ( 10.1098/rspb.2014.0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rawlence NJ, et al. 2015. Geographically contrasting biodiversity reductions in a widespread New Zealand seabird. Mol. Ecol. 24, 4605–4616. ( 10.1111/mec.13338) [DOI] [PubMed] [Google Scholar]
- 28.Rawlence NJ, Perry GLW, Smith IWG, Scofield RP, Tennyson AJD, Matisoo-Smith EA, Boessenkool S, Austin JJ, Waters JM. 2015. Radiocarbon-dating and ancient DNA reveal rapid replacement of extinct prehistoric penguins. Quat. Sci. Rev. 112, 59–65. ( 10.1016/j.quascirev.2015.01.011) [DOI] [Google Scholar]
- 29.Rawlence NJ, et al. 2016. Genetic and morphological evidence for two species of Leucocarbo shag (Aves, Pelecaniformes, Phalacrocoracidae) from southern South Island of New Zealand. Zool. J. Linn. Soc. 177, 676–694. ( 10.1111/zoj.12376) [DOI] [Google Scholar]
- 30.Rawlence NJ, et al. 2017. Speciation, range contraction and extinction in the endemic New Zealand King Shag complex. Mol. Phylogenet. Evol. In Review. [DOI] [PubMed] [Google Scholar]
- 31.Grosser S, Rawlence NJ, Anderson CNK, Smith IWG, Scofield RP, Waters JM. 2016. Invader or resident? Ancient-DNA reveals rapid species turnover in New Zealand little penguins. Proc. R. Soc. B 283, 101098 ( 10.1098/rspb.2015.2879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters JM, Fraser CI, Maxwell JJ, Rawlence NJ. 2017. Did interaction between human pressure and Little Ice Age drive biological turnover in New Zealand. J. Biogeogr. 44, 1481–1490 ( 10.1111/jbi.12991) [DOI] [Google Scholar]
- 33.Waters JM, Fraser CI, Hewitt GM. 2013. Founder takes all: density-dependant processes structure biodiversity. Trends Ecol. Evol. 28, 78–85. ( 10.1016/j.tree.2012.08.024) [DOI] [PubMed] [Google Scholar]
- 34.Walsh PS, Metzger DA, Higuchi R. 2013. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 54, 134–139. ( 10.2144/000114018) [DOI] [PubMed] [Google Scholar]
- 35.Butkauskas D, Svazas S, Tubelyte V, Morkunas J, Sruoga A, Boiko D, Paulauskas A, Stanevicius V, Baublys V. 2012. Coexistence and population genetic structure of the whooper swan Cygnus cygnus and mute swan Cygnus olor in Lithuania and Latvia. Cent. Eur. J. Biol. 7, 886–984. ( 10.2478/s11535-012-0065-9) [DOI] [Google Scholar]
- 36.Knapp M, Clarke AC, Horsburgh KA, Matisoo-Smith EA. 2012. Setting the stage—building and working in an ancient DNA laboratory. Ann. Anat. 194, 3–6. ( 10.1016/j.aanat.2011.03.008) [DOI] [PubMed] [Google Scholar]
- 37.Rohland N, Siedel H, Hofreiter M. 2010. A rapid column-based ancient DNA extraction method for increased sample throughput. Mol. Ecol. Res. 10, 677–683. ( 10.1111/j.1755-0998.2009.02824.x) [DOI] [PubMed] [Google Scholar]
- 38.Brotherton P, Endicott P, Sanchez JJ, Beaumont M, Barnett R, Austin J, Cooper A. 2007. Novel high-resolution characterization of ancient DNA reveals C-U type base modification events as the sole cause of post mortem miscoding lesions. Nucleic Acids Res. 35, 5717–5728. ( 10.1093/nar/gkm588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Tamura K, Nei M. 2006. MEGA4: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5, 150–163. ( 10.1093/bib/5.2.150) [DOI] [PubMed] [Google Scholar]
- 40.Prost S, Anderson CNK. 2011. TempNet: a method to display statistical parsimony network for heterochronous DNA sequence data. Methods Ecol. Evol. 2, 663–667. ( 10.1111/j.2041-210X.2011.00129.x) [DOI] [Google Scholar]
- 41.von den Driesch A. 1976. A guide to the measurements of animal bones from archaeological sites Peabody Museum Bulletin 1.
- 42.R Development Core Team. 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 43.Wood JR, Mitchell KJ, Scofield RP, de Pietri VL, Rawlence NJ, Cooper A. 2017. Phylogenetic relationships and terrestrial adaptations of the extinct laughing owl, Sceloglaux albifacies (Aves: Strigidae). Zool. J. Linn. Soc. 179, 907–918. ( 10.1111/zoj.12483) [DOI] [Google Scholar]
- 44.Watanabe J. 2017. Quantitative discrimination of flightlessness in fossil Anatidae from skeletal proportions. Auk 134, 672–695. ( 10.1642/AUK-17-23.1) [DOI] [Google Scholar]
- 45.Dickison MR. 2007. The allometry of giant flightless birds. Unpublished PhD thesis, Duke University. [Google Scholar]
- 46.Grant PR. 1965. The adaptive significance of some size trends in island birds. Evolution 19, 355–367. ( 10.1111/j.1558-5646.1965.tb01727.x) [DOI] [Google Scholar]
- 47.Scofield RP, Mitchell KJ, Wood JR, de Pietri VL, Jarvie S, Llamas B, Cooper A. 2017. The origin and phylogenetic relationships of the New Zealand ravens. Mol. Phylogenet. Evol. 106, 136–143. ( 10.1016/j.ympev.2016.09.022) [DOI] [PubMed] [Google Scholar]
- 48.Wright NA, Steadman DW, Witt CC. 2016. Predictable evolution toward flightlessness in volant island birds. Proc. Natl Acad. Sci. USA 113, 4765–4770. ( 10.1073/pnas.1522931113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pavia M, Meijer HJM, Rossi MA, Gohlich UB. 2017. The extreme insular adaptation of Garganornis ballmanni Meijer, 2014: a giant Anseriformes of the Neogene of the Mediterranean Basin. R. Soc. Open Sci. 4, 160722 ( 10.1098/rsos.160722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baum DA, Donoghue MJ. 1995. Choosing among alternative ‘phylogenetic’ species concepts. Syst. Bot. 20, 560–573. ( 10.2307/2419810) [DOI] [Google Scholar]
- 51.Cicero C, Johnson NK. 2006. Diagnosability of subspecies: lessons from sage sparrows (Amphispiza belli) for analysis of geographic variation in birds. Auk 123, 266–274. ( 10.1642/0004-8038(2006)123%5B0266:DOSLFS%5D2.0.CO;2) [DOI] [Google Scholar]
- 52.Cracraft J. 1983. Species concepts and speciation analysis. Curr. Ornithol. 1, 159–187. ( 10.1007/978-1-4615-6781-3_6) [DOI] [Google Scholar]
- 53.Issac NJB, Mallet J, Mace GM. 2004. Taxonomic inflation: its influence on macroecology and conservation. Trends Ecol. Evol. 19, 464–469. ( 10.1016/j.tree.2004.06.004) [DOI] [PubMed] [Google Scholar]
- 54.Forbes HO. 1893. Exhibition and remarks on the osteological remains of birds from the Chatham Islands, and a description of the new genera Diaphorapteryx and Paleocorax. Ibis 6, 253–254. [Google Scholar]
- 55.Forbes HO. 1893. A list of birds inhabiting the Chatham Islands. Ibis 5, 232–237. ( 10.1111/j.1474-919x.1893.tb01240.x) [DOI] [Google Scholar]
- 56.Forbes HO. 1893. The Chatham Islands and their story. In The fortnightly review, vol. 53 (ed. Harris F.), pp. 669–690. London, UK: Chapman and Hall. [Google Scholar]
- 57.Northcote EM. 1982. Size, form and habit of the extinct Maltese swan Cygnus falconeri. Ibis 124, 148–158. ( 10.1111/j.1474-919X.1982.tb03753.x) [DOI] [Google Scholar]
- 58.Matsuoka H, Nakajima H, Takakuwa Y, Hasegawa Y. 2001. Preliminary note on the Miocene flightless swan from the Haraichi Formation, Tomioka Group of Annaka, Gunma, Japan . Bull. Gunma Mus. Nat. Hist. 3, 1–8. [Google Scholar]
- 59.Van der Greer AAE, Lomolino MV, Lyras GA. 2017. ‘Island life’ before man: Biogeography of palaeo-insular mammals. J. Biogeogr. 44, 995–1006 ( 10.1111/jbi.12857) [DOI] [Google Scholar]
- 60.Van den Bergh GD, Kaifu Y, Kurniawan I, Kono RT, Brumm A, Setiyabudi E, Aziz F, Morwood MJ. 2016. Homo floresiensis-like fossils from the early Middle Pleistocene of Flores. Nature 534, 245–248. ( 10.1038/nature17999) [DOI] [PubMed] [Google Scholar]
- 61.Wright NA, Steadman DW. 2012. Insular avian adaptions on two Neotropical continental islands. J. Biogeogr. 39, 1891–1899. ( 10.1111/j.1365-2699.2012.02754.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gould J. 1865. Handbook to the birds of Australia, vol. 2 London, UK: J. Gould. [Google Scholar]
- 63.Guay PJ, Williams M, Robinson RW. 2015. Lingering genetic evidence of North American mallards (Anas platyrhynchos) introduced to New Zealand. N. Z. J. Ecol. 39, 103–109. [Google Scholar]
- 64.Corlett RT. 2016. Restoration, reintroduction, and rewilding in a changing world. Trends Ecol. Evol. 31, 453–462. ( 10.1016/j.tree.2016.02.017) [DOI] [PubMed] [Google Scholar]
- 65.Nogues-Bravo D, Simberloff D, Rahbek C, Sanders NJ. 2016. Rewilding is the new Pandora's box in conservation. Curr. Biol. 26, R87–R91. ( 10.1016/j.cub.2015.12.044) [DOI] [PubMed] [Google Scholar]
- 66.Seddon PJ, Griffiths CJ, Soorae PS, Armstrong DP. 2014. Reversing defaunation: restoring species in a changing world. Science 345, 406–412. ( 10.1126/science.1251818) [DOI] [PubMed] [Google Scholar]
- 67.Lorenzen ED, et al. 2011. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359–364. ( 10.1038/nature10574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boivin NL, Zeder MA, Fuller DQ, Crowther A, Larson G, Erlandson JM, Denham T, Petraglia MD. 2016. Ecological consequences of human niche construction: examining long-term anthropogenic shaping of global species distributions. Proc. Natl Acad. Sci. USA 113, 6388–6396. ( 10.1073/pnas.1525200113) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All DNA sequences have been deposited in GenBank under accessions MF455379-MF455462. Electronic supplementary material is available with the electronic version of this paper.


