Abstract
Vertical transmission mode is predicted to decrease the virulence of symbionts. However, Wolbachia, a widespread vertically transmitted endosymbiont, exhibits both negative and beneficial effects on arthropod fitness. This ‘Jekyll and Hyde’ behaviour, as well as its ability to live transiently outside host cells and to establish new infections via horizontal transmission, may reflect the capacity of Wolbachia to exhibit various phenotypes depending on the prevailing environmental constraints. To study the ability of Wolbachia to readily cope with new constraints, we forced this endosymbiont to spread only via horizontal transmission. To achieve this, we performed serial horizontal transfers of haemolymph from Wolbachia-infected to naive individuals of the isopod Armadillidium vulgare. Across passages, we observed phenotypic changes in the symbiotic relationship: (i) The Wolbachia titre increased in both haemolymph and nerve cord but remained stable in ovaries; (ii) Wolbachia infection was benign at the beginning of the experiment, but highly virulent, killing most hosts after only a few passages. Such a phenotypic shift after recurrent horizontal passages demonstrates that Wolbachia can rapidly change its virulence when facing new environmental constraints. We thoroughly discuss the potential mechanism(s) underlying this phenotypic change, which are likely to be crucial for the ongoing radiation of Wolbachia in arthropods.
Keywords: symbionts, virulence, serial passage, transmission modes
1. Introduction
The transmission mode of symbionts is a key factor to predict their virulence [1–3]. In the case of vertical transmission, when symbionts pass from mother to offspring and the fitness of both host and symbiont are linked, low virulence levels and even mutualistic traits are favoured [4]. Contrariwise, in the case of horizontal transmission, high virulence levels can be maintained if a high multiplication rate promotes symbiont transmission [3,5–7]. The transmission mode is thus pre-eminent in the virulence expressed by a symbiont towards its host. However, it is not the only force that shapes the virulence phenotype of a symbiotic association. Indeed, some vertically transmitted endosymbionts have deleterious effects on host fitness. For instance, the α-proteobacterium Wolbachia, highly prevalent in arthropods, can reduce host fitness due to both (i) reproductive manipulations such as male-killing, feminization or cytoplasmic incompatibility [8–11] and (ii) negative impacts on life-history traits such as body size [12], male fertility [13], survival [14] and fecundity [15]. These negative impacts can be at least partially balanced by benefits on other traits. Indeed, Wolbachia can protect their hosts against pathogens such as viruses [16,17] and bacteria [18]. Wolbachia has thus more diverse impacts on host phenotype than its well-known reproductive manipulations. The fact that Wolbachia affects many life-history traits of their hosts is certainly linked to their wide distribution in host organs [19]: Wolbachia not only colonizes the gonads for vertical transmission but many somatic organs as well, resulting in a systemic infection [20].
In many cases, Wolbachia thus acts as ‘Jekyll and Hyde’, exhibiting both beneficial and parasitic traits [21,22]. The ability to express both types of traits may result from antagonistic constraints acting on the symbionts at different scales. For instance, an increased multiplication rate may confer a fitness advantage in the context of within-host competition for resources, but a disadvantage at the between-host level, as the cost due to symbiont proliferation may impede symbiont vertical transmission [1]. At the within-host level, vertically transmitted symbionts can therefore respond in a ‘short-sighted manner’ [23]: while vertical transmission ensured by mild oocyte colonization is the main distal ‘goal’, the proximal interests of Wolbachia can lead to an intense competition in somatic organs, especially in long-lived host species. Considering these aspects, we can predict that facultative vertically transmitted endosymbionts such as Wolbachia, by experiencing opposite constraints at different scales, can change their virulence in only a few generations, depending on the preeminent constraints. Such phenotypic changes could derive from phenotypic plasticity and/or a selection process leading to the emergence of new favourable phenotypes.
Phenotypic plasticity and/or a rapid adaptive ability are skills also required to successfully transfer between host species. Wolbachia is the most widely distributed facultative bacterial endosymbiont reported to date (more than 50% of arthropod species are estimated to be infected [24]). This wide host range relies on both efficient vertical transmission and occasional horizontal transfers, as revealed by non-congruent phylogenies between Wolbachia and their hosts [25–27]. In order to thrive and be vertically transmitted in a new host species (i.e. a new environment), Wolbachia needs (i) to tolerate environmental variations and/or (ii) to adapt rapidly (change tissue tropism, multiplication rate, etc.). Experimental demonstrations of Wolbachia's ability to cope with new environmental conditions result from transinfection experiments mimicking natural host switching [28]. Some experiments also demonstrated an unexpected tolerance of Wolbachia towards stressful conditions by performing successful plant-mediated horizontal transmissions [29] and by maintaining live Wolbachia in synthetic liquid medium outside of their host cells for at least a week [30].
To date, it remains challenging to determine whether the ability of Wolbachia to face novel environmental constraints derives from an inherent phenotypic plasticity and/or from genetic polymorphisms within Wolbachia populations that selection can act upon. Nonetheless, it is known that Wolbachia genomes evolve quickly [31–33] and recent selection experiments in Drosophila melanogaster infected with the pathogenic Wolbachia strain wMelpop demonstrated that (i) genetic polymorphism in a Wolbachia population within a single host does exist, and (ii) changes in the genetic composition of a Wolbachia population can arise quickly, depending on the nature of selection [34]. Fast-evolving Wolbachia have been reported in different host species, suggesting that Wolbachia strains may be considered as populations of diverse haplotypes with changing frequencies over time, which may lead to changes in the resulting extended phenotype [35–37].
In this paper, we study the changes in the extended phenotype expressed by Wolbachia in its host (including titre change in different organs and virulence) when being constrained to horizontal transmission, while vertical transmission is impaired. To achieve this, the association between the terrestrial isopod Armadillidium vulgare and its native feminizing Wolbachia (wVulC) constitutes an appropriate model for three reasons: (i) successful horizontal transfers of Wolbachia between adults are performed easily [28,38,39]; (ii) A. vulgare lives for years in symbiotic interaction with Wolbachia, thereby providing an opportunity for strong within-host competition, especially when considering the high Wolbachia titres reported in this host species [39,40]; and (iii) Wolbachia infects all tissues of A. vulgare, including haematopoietic organs and haemocytes [40–44]. This systemic Wolbachia infection, sustainable through years, could potentially result in specialized subpopulations of Wolbachia in the different tissues. Although such a specialization has not yet been formally demonstrated, tissue-specific Wolbachia titres have been revealed, indicating tissue-specific constraints that may lead to different Wolbachia phenotypes within a single host [39,40]. The higher transinfection efficiency of Wolbachia colonizing haemocytes compared with those isolated from oocytes further supports this hypothesis [42,43]. Several studies suggest that the haemolymph could therefore represent a privileged path for Wolbachia horizontal transmission between terrestrial isopods [42,43,45]. By isolating Wolbachia from ovaries (i.e. the subpopulation predicted to transmit vertically) and subjecting them to horizontal passages through serial haemolymph transfers between infected and uninfected individuals, we monitored the response of normally vertically transmitting Wolbachia when confronted with a new dominant and controlled transmission mode. We show that wVulC can be turned into a true pathogen against its native host after only a few horizontal passages. These results demonstrate the ability of Wolbachia to phenotypically respond to a new constraint by increasing its virulence in a situation where winning the within-host competition might be more beneficial for horizontal than for vertical transmission.
2. Material and methods
(a). Biological models
All A. vulgare used in this experiment were reared as previously described [15]. In these laboratory conditions, A. vulgare can live for up to 2 years. Recipient hosts used in the experiments were from a Wolbachia-free (i.e. ‘asymbiotic’) lineage derived from a population collected at Heraklion (Greece) in 1989. Individuals from a lineage naturally infected with the wVulC strain (i.e. ‘symbiotic’ lineage) derived from a population sampled at Helsingor (Denmark) in 1991 were used as Wolbachia donors to initiate the infection.
(b). Serial passages of Wolbachia
To initiate the serial passage experiment, we injected four independent batches of five 1-year-old asymbiotic A. vulgare females: three batches were injected with ovary suspension (i.e. tissue rich in Wolbachia for vertical transmission [46]) from five A. vulgare symbiotic females, in order to create three replicate experimental lines (A, B and C; electronic supplementary material, figure S1). The fourth batch was injected with ovary suspension from five asymbiotic individuals as a control line without Wolbachia. We independently prepared each of the four inoculate suspensions as previously described [47]. The three wVulC-positive inoculates were quantified by quantitative PCR (see below), and the respective doses injected to the animals were 1.86 × 105 ± 2.55 × 102 (mean ± s.e.) Wolbachia µl−1 for line A, 2.10 × 105 ± 4.12 × 102 Wolbachia µl−1 for line B and 2.03 × 105 ± 1.87 × 102 Wolbachia µl−1 for line C. B0-injected animals were then kept for 30 days under the laboratory rearing conditions. From passage P0 to P8, horizontal transmissions of wVulC were performed using 2 µl of haemolymph from Bn − 1 donors. To do so, we pierced the cuticle of each donor using a sterile needle and collected 10 µl of haemolymph infected with Wolbachia using a micropipette. For each experimental line, haemolymph collected from donor females was pooled in a 1.5 ml microtube and kept on ice to limit coagulation. We injected 2 µl of the collected haemolymph to each recipient individual of the next passage (Bn + 1) as previously described [47]. Newly infected individuals were then reared for a maximum of 45 days (electronic supplementary material, figure S1).
(c). Mortality of injected Armadillidium vulgare across serial passages
Mortality of injected individuals was recorded immediately before the next passage, at day 45, for B1–B4 (electronic supplementary material, figure S1). For B5 and B6, we recorded the mortality every week for 45 days post-injection (PI). Then, in B7 and B8, due to the earlier mortality for these batches and the necessity to keep some individuals alive to perform the next passage, we recorded mortality every week for 35 days PI (electronic supplementary material, figure S1).
(d). Tissue sampling, DNA extraction and Wolbachia quantification
We dissected each donor under a binocular microscope after 45 (P1–P6) or 35 days (P7 and P8) PI, immediately after having performed the next passage, to sample the ovaries (i.e. tissue for vertical transmission) and the nerve cord (i.e. nerve cells and neighbouring adipocytes: tissues known to be highly colonized by wVulC [40,47]). A subsample of the haemolymph (i.e. tissue used for next horizontal passage) was also kept for wVulC quantification. We extracted total DNA from each inoculate and tissue sample as described in [48], and assessed DNA concentration and quality (ratios OD 260/280 and 260/230 nm) using the Nanodrop 1000 spectrophotometer (Thermo).
We measured the Wolbachia titres in each inoculate and tissue of injected individuals by qPCR (LightCycler 480 Roche) using specific primers targeting 205 bp of the single-copy Wolbachia surface protein gene (wsp) as previously described [47]. The total DNA quantity (i.e. host + Wolbachia DNA) of each sample was used to normalize the wsp gene copy number (i.e. wsp copies per ng of total DNA).
(e). Visualization of Wolbachia in haemocytes of injected individuals by fluorescence in situ hybridization
For each experimental line (i.e. A, B and C), we collected and pooled haemolymph from donors after 45 (P1–P6) or 35 days (P7 and P8) PI. A 2 µl fraction of this pool of haemolymph was used to visualize Wolbachia in the haemocytes using fluorescence in situ hybridization (FisH), as previously described [49]. The number of haemocytes and their Wolbachia colonization status were counted on 10 random fluorescence microscopy images per experimental line and for each horizontal transfer, using ImageJ v. 1.45 software [50].
(f). Bacterial community profiling using temperature gradient gel electrophoresis
The haemolymph of A. vulgare is known to contain a high bacterial diversity, although Wolbachia is always the predominant taxon [40,44]. Therefore, we verified whether other bacteria might have been sustainably co-transferred with Wolbachia during the serial horizontal passages. To this end, we obtained microbial community profiles from the haemolymph samples of the donors at each passage (i.e. the same haemolymph samples also used to quantify the Wolbachia titre at each passage) using temperature gradient gel electrophoresis (TGGE). A 196 bp fragment of the variable region V3 of the bacterial 16S rRNA gene was amplified using a nested PCR approach: first, a 795 bp fragment was amplified using primers 27F and 786R [51], followed by a second amplification targeting the V3 region using primers 338F-GC and 520R [52,53]. Primer 338F contained a 42-nucleotide GC-clamp preventing the complete denaturation of the DNA strands during TGGE [52]. TGGE was performed using the DCode Universal Mutation Detection System (Bio-Rad) following a protocol modified from Dittmer et al. [53]. Briefly, 20 µl of the final PCR products were run across a temperature gradient from 38 to 70°C (ramping of 2°C h−1) at 95 V on 10% polyacrylamide gels. Three replicates of a standard containing V3 16S rRNA gene fragments from several reference bacteria (Bacillus megaterium, Escherichia coli, Listeria ivanovii, Micrococcus luteus, Salmonella typhimurium and Wolbachia spp.) were loaded onto every gel to allow the standardization of bands between gels and the visual screening of Wolbachia infection across all samples. After electrophoresis, gels were stained with ethidium bromide and photographed under UV. A total of 19 bands were cloned and sequenced (two replicates from different samples if a band occurred in more than one sample and four Wolbachia bands from different passages). These bands were excised from the TGGE gels and incubated in 70 µl of sterile water at 60°C for 1 h and at 4°C for 4 h to elute the DNA. Five microlitres of each eluate were then used as PCR template, using the same primers for the V3 region as above but without the GC-clamp for primer 338F. Purified PCR products were cloned into competent E. coli JM109 using the pGEM-T Easy Vector Systems kit (Promega). Sixty-two recombinant clones were sequenced on an ABI 310 sequencer (Applied Biosystems) and the 16S rRNA gene sequences were identified using BlastN searches against the non-redundant nucleotide database (http://blast.ncbi.nlm.nih.gov).
(g). Statistical analyses
All statistical analyses were performed using R v. 3.3.2 software. The Wolbachia titres in the different tissues as well as the proportion of infected haemocytes and the mortality rates for all injected individuals of each experimental line across horizontal transfers were analysed with a non-parametric Kruskal–Wallis rank-sum test followed by a Dunn post hoc test, proportional odds ordinal logistic model and parametric Welch t-test. Survival curves were compared using a general mixed-effects Cox model with randomized block effect (coxme function in kinship package).
3. Results
(a). Changes in Wolbachia titres in Armadillidium vulgare tissues across horizontal passages
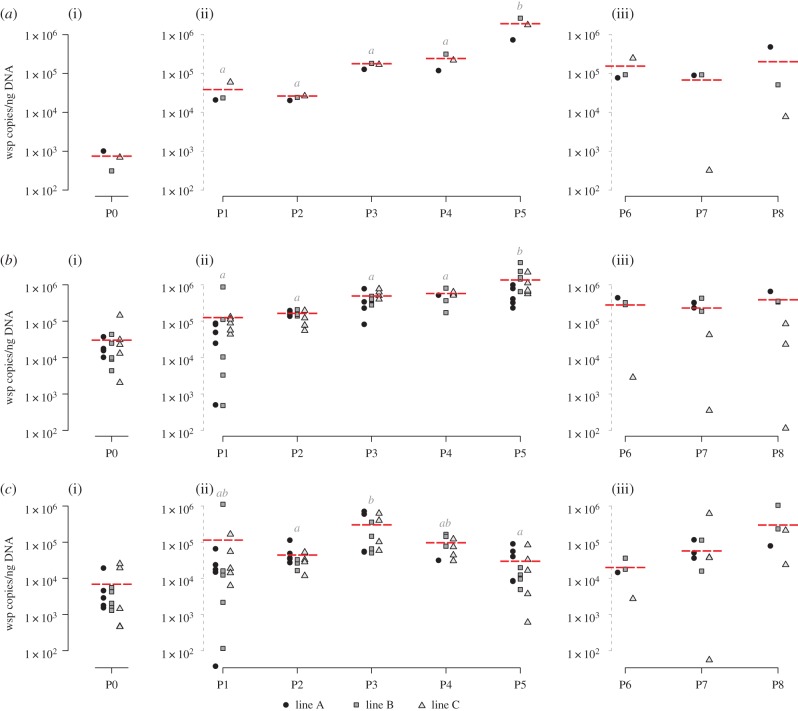
The average Wolbachia titre in the haemolymph of donor individuals globally increased around 50-fold across the first five passages (P1–P5) (Kruskal–Wallis test; W = 12.23, d.f.= 4, p = 0.0157; figure 1a(ii)). This increase was positively correlated with the number of horizontal passages (Spearman's test across P1–P5: S = 58.97, ρ = 89.46%, p ≤ 0.0001). Furthermore, across these first five passages, no difference in Wolbachia titre was detected between the three independent experimental lines for each passage (proportional odds ordinal logistic model with lines as covariate; W = 1.22, d.f. = 2, p = 0.5434). When considering only the last three passages (P6–P8) for which haemolymph was collected earlier (i.e. 35 days PI), this correlation between Wolbachia titre in the haemolymph and the number of passages was not significant (Spearman's test across P6–P8: S = 151.62, ρ = −26.35%, p = 0.4933; figure 1a(iii)) and the titres were generally lower compared with P5, reaching titres similar to those previously observed at P3 or P4 (Kruskal–Wallis test; W = 4.9333, d.f. = 4, p = 0.2942). However, as for P1–P5, no difference in Wolbachia titre was detected between the three experimental lines in P6–P8 (proportional odds ordinal logistic model with lines as covariate; W = 1.15, d.f. = 2, p = 0.5611).
Figure 1.
Quantification of Wolbachia titres by qPCR in (a) haemolymph, (b) nerve cord and (c) ovaries across the serial passages. Each symbol represents a measurement in a different experimental line (see legends below the figures). The horizontal dotted line represents the mean titre for each passage. (a(i)), (b(i)) and (c(i)) correspond to the quantification of Wolbachia titres at P0, the initiation of infection, in haemolymph, nerve cord and ovaries, respectively. (a(ii)), (b(ii)) and (c(ii)) correspond to the quantification of Wolbachia titres 45 days PI for P1–P5 in haemolymph, nerve cord and ovaries, respectively, and (a(iii)), (b(iii)) and (c(iii)) correspond to Wolbachia titres 35 days PI for P6–P8 in haemolymph, nerve cord and ovaries, respectively. Titres not connected by the same letter are significantly different.
In the nerve cord, a tissue known to be rapidly colonized by Wolbachia after injection [39,42,47], we also observed significant differences in Wolbachia titres across the first five passages (P1–P5) (Kruskal–Wallis test; W = 42.834, d.f. = 4, p ≤ 0.0001; figure 1b(ii)), but still no difference in Wolbachia titre between the three experimental lines for each passage (proportional odds ordinal logistic model with lines as covariate; W = 2.0493, d.f. = 2, p = 0.3589). Interestingly, while the average Wolbachia titre increased by 50-fold between P1 and P5 in the haemolymph, here the average Wolbachia titre only increased by 11-fold. However, we still observed a positive correlation with the number of horizontal passages (Spearman's test across P1–P5: S = 7388.9, ρ = 82.26%, p ≤ 0.0001). As observed for the haemolymph, no correlation between Wolbachia titre and the number of passages was found for the last three passages (P6–P8) (Spearman's test across P6–P8: S = 973.3, ρ = −0.443%, p = 0.9861; figure 1b(iii)). Moreover, as for the haemolymph, titres were generally lower compared with P5, reaching titres similar to those previously observed at P2 (Kruskal–Wallis test; W = 1.7099, d.f. = 3, p = 0.6347).
Unlike the haemolymph and the nerve cord, the ovaries of the donors exhibited a more random variation in Wolbachia titre across passages (figure 1c(i)–(iii)). Although we observed significant differences between the passages when considering only the first five passages (P1–P5) (Kruskal–Wallis test; W = 25.57, d.f. = 4, p ≤ 0.0001; figure 1c(ii)), there was no correlation between Wolbachia titre and the number of horizontal passages (Spearman's test across P1–P5: S = 39 314, ρ = 5.64%, p = 0.6606). This lack of correlation was also observed for the last three passages (P6–P8) (Spearman's test across P6–P8: S = 524.35, ρ = 45.88%, p = 0.05543), as well as no significant differences in Wolbachia titres (Kruskal–Wallis test; W = 3.8684, d.f.= 2, p = 0.1445; figure 1c(iii)). As observed for the two other tissues, we did not detect any differences in Wolbachia titre between the three experimental lines for each passage, neither for the first five passages (P1–P5) (proportional odds ordinal logistic model with lines as covariate; W = 0.58263, d.f. = 2, p = 0.7473) nor for the last three passages (P6–P8) (proportional odds ordinal logistic model with lines as covariate; W = 2.2845, d.f. = 2, p = 0.3191).
(b). Changes in proportion of infected haemocytes across horizontal passages
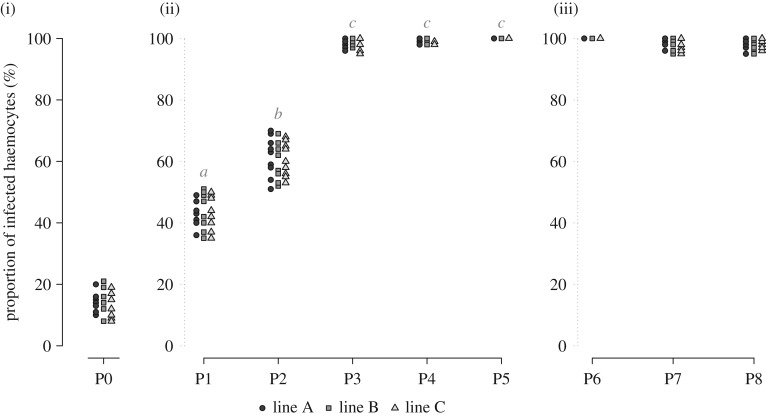
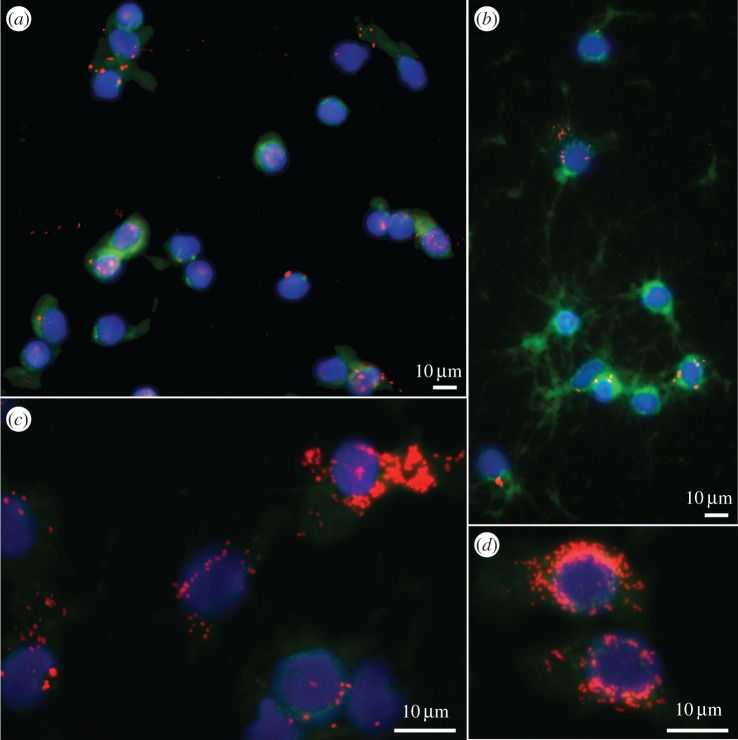
The global increase in Wolbachia titre in the haemolymph between P1 and P5 was also illustrated by FisH. We observed a strong increase and positive correlation in the proportion of Wolbachia-infected haemocytes across horizontal passages (Kruskal–Wallis test; W = 131.14, d.f. = 4, p ≤ 0.0001, Dunn's post hoc test; Spearman's test: S = 42 638, ρ = 92.41%, p ≤ 0.0001; figure 2(ii)), without any difference between the three independent experimental lines, for the first five passages (P1–P5) (proportional odds ordinal logistic model with lines as covariate; W = 0.69388, d.f. = 2, p = 0.7068; figure 2(ii)) as well as for the last three passages (P6–P8) (proportional odds ordinal logistic model with lines as covariate; W = 0.17517, d.f. = 2, p = 0.9161; figure 2(iii)). Specifically, the proportion of infected haemocytes was around 15% after the initiation of infection (P0) and increased to 43% after P1 (figure 3a), a proportion similar to that observed in vertically infected animals [41]. At P2, the percentage of infected haemocytes increased to 61% (figure 3b) and finally reached 98–100% from P3 to P8, with some haemocytes exhibiting a cytoplasm densely colonized by Wolbachia (figure 3c,d). Unlike the Wolbachia titre measurements, we did not observe a decrease in the proportion of infected haemocytes from P6 to P8 (always around 98–100%), although the infected cells seemed to be less densely colonized by Wolbachia than those observed at P5.
Figure 2.
Proportion of Wolbachia-infected haemocytes across the serial passages. FisH allowed us to detect and count haemocytes harbouring Wolbachia, at each passage and for each experimental line. Each symbol represents a measurement in a different experimental line (see legends below the figure). Proportions not connected by the same letter are significantly different. (i) P0, (ii) P1–P5 and (iii) P6–P8.
Figure 3.
Visualization of Wolbachia in A. vulgare haemocytes by FisH. Wolbachia are labelled in red, cytoskeleton in green (Phalloidin) and nucleus in blue (DAPI). Pictures illustrate the strong increase in infection intensity in haemocytes between (a) P1, (b) P2 and (c,d) P3. (Online version in colour.)
(c). Impact of Wolbachia serial horizontal passages on host survival
We observed a strong and global increase in mortality from batches B1 to B8 (Kruskal–Wallis test; W = 20.509, d.f. = 7, p = 0.0045, Dunn's post hoc test; figure 4a), with particularly high mortality for the last three batches B6–B8. As for the Wolbachia titres and the proportion of infected haemocytes, the three experimental lines A, B and C showed similar patterns of mortality (proportional odds ordinal logistic model with lines as covariate; W = 0.315, d.f. = 2, p = 0.8543). This increase in mortality was correlated with the increase in Wolbachia titre in the haemolymph (Spearman's test: S = 171.72, ρ = 82.27%, p ≤ 0.0001), the tissue used to perform the passages, for the batches B1–B6 (which received a Wolbachia dose corresponding to the titres measured from P0 to P5; electronic supplementary material, figure S1). However, this was no longer the case for B7 and B8 (Spearman's test: S = 44.131, ρ = −26.09%, p = 0.6175), where mortality was still very high while the injected ‘dose’ of Wolbachia was lower than those previously injected (i.e. similar to P3–P4; figure 1a(iii)). It should also be noted that the initial doses of Wolbachia received by B0 to initiate the experiment (i.e. inoculates from ovary suspensions) were similar to the Wolbachia titre in the haemolymph inoculate at P4 (and used to infect B5) (Welch t-test; t = −0.31562, d.f. = 2.0634, p = 0.7814), although the consequences in terms of mortality were clearly different: no mortality in B0 but an average of 46% of mortality in B5 (figure 4a).
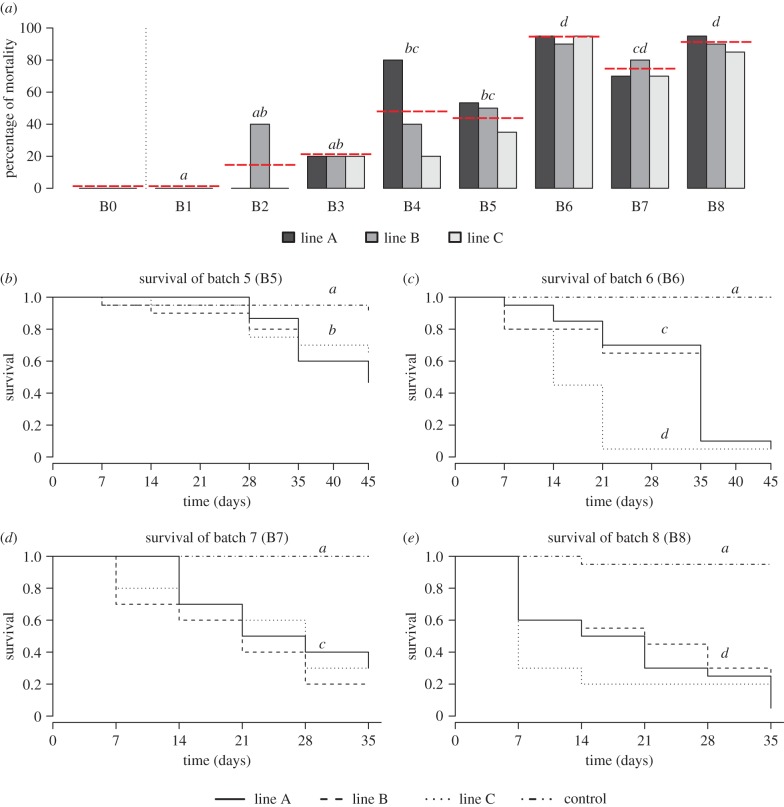
Figure 4.
Impact of the serial horizontal passages of Wolbachia on host survival. (a) Global proportions of mortality for each batch. (b–e) Survival curves for (b) B5, (c) B6, (d) B7 and (e) B8. The horizontal dotted line represents the mean for each passage. (Online version in colour.)
From B5 to B8, we monitored the survival of the injected individuals at shorter intervals (i.e. every week), and then compared the Kaplan–Meier survival curves for each experimental line within and between transfers. First, comparing the survival of individuals from the experimental lines and the control lines confirmed that the injection of Wolbachia significantly reduced host survival (P5: ANOVA after Cox's model; F = 10.0070, d.f. = 3, p = 0.0185; figure 4b; P6: ANOVA after Cox's model; F = 78.086, d.f. = 3, p ≤ 0.0001; figure 4c; P7: ANOVA after Cox's model; F = 20.4620, d.f. = 3, p = 0.0001; figure 4d; P8: ANOVA after Cox's model; F = 51.9370, d.f. = 3, p ≤ 0.0001; figure 4e). Second, no difference was observed between experimental lines or due to the dose of Wolbachia received for the same passage (P5: ANOVA after Cox's model; F = 0.9409, d.f. = 2, p = 0.6247; figure 4b; P7: ANOVA after Cox's model; F = 0.7193, d.f. = 2, p = 0.6980; figure 4d; P8: ANOVA after Cox's model; F = 0.8162, d.f. = 2, p = 0.6649; figure 4e), except for P6 (ANOVA after Cox's model; F = 11.2100, d.f. = 2, p = 0.0036; figure 4c), where injected individuals from line C died more rapidly than those from the two other lines, but the received Wolbachia dose did not explain this pattern. Our results demonstrate an increase in the negative impact of Wolbachia on host survival throughout the serial passages (figure 4b–e): (i) injected individuals from P6 died more rapidly than those injected from P5 (ANOVA after Cox's model; F = 40.658, d.f. = 1, p ≤ 0.0001); (ii) survival curves were similar for P6 and P7; but (iii) injected individuals from P8 died more rapidly than those from the other passages (ANOVA after Cox's model; F = 4.1506, d.f. = 1, p = 0.0416; figure 4b–e).
(d). Wolbachia was the only dominant bacterium throughout the serial horizontal passages
TGGE profiles of the V3 region of the bacterial 16S rRNA gene revealed eight different bands across the 26 haemolymph samples (electronic supplementary material, figure S2). The band corresponding to Wolbachia (according to its position on the gel compared with the Wolbachia band in the standard) appeared as the predominant bright band in all samples except for line C at P7 (electronic supplementary material, figure S2). All other bands were comparatively faint and none of them was observed in all samples (electronic supplementary material, figure S2). These results indicate that no other predominant taxa had been systematically co-transferred with Wolbachia. This result was further supported by Sanger sequencing of cloned 16S rRNA gene fragments from excised TGGE bands, because 86% (51 of 59) of high-quality sequences were most similar to Wolbachia (95–100% sequence identity; data not shown). The remaining sequences were similar to two other bacterial taxa: Pseudomonas sp. (97–100% sequence identity), a bacterium known to be naturally present in the haemolymph of A. vulgare [44], as well as Paracoccus sp. (98–100% sequence identity).
4. Discussion
Wolbachia is certainly the most widespread endosymbiont in arthropods, with more than 50% of species estimated to be infected [24]. Despite being mainly vertically inherited, we now have both experimental and phylogenetic evidence for its ability to extend its prevalence by switching horizontally from one host to another [25–27]. In this paper, we investigated the response of Wolbachia to the constraints of recurrent horizontal transmission events while vertical transmission was impaired. To this end, we performed serial passages of Wolbachia using haemolymph transfusion from Wolbachia-infected A. vulgare to uninfected individuals. Serial passages represent a classic method in the study of host–parasite systems originally used to establish new associations more experimentally tractable than natural ones. However, serial passage experiments performed with parasites such as viruses or fungi mainly resulted in a rapid increase in parasite virulence towards novel hosts, often preventing further utilization of the newly formed associations [54–57]. As all parasites used in these previous studies exhibit horizontal transmission as their dominant transmission route, this important increase in virulence may seem in discordance with theoretical predictions because these parasites should have already been selected for an optimal virulence under horizontal transmission pressures before serial passages [1,58]. However, the observed rapid increase in parasite virulence across serial passages can be linked to the protocol used to perform horizontal transmission that does not mimic the natural path of infection efficiently. Indeed, in serial passage experiments, the parasites are usually sampled during the exponential phase of infection, sometimes even before symptoms appear [58]. This experimental procedure places the parasites under a strong constraint (i) to reproduce rapidly and (ii) to increase their virulence at the same time. In this situation, serial passages induce a disruption of the trade-off between transmission and virulence, which maintained an optimal moderate virulence in the ancestral association [1].
The above illustrates that serial passage experiments are an appropriate means to assess Wolbachia's ability to cope with a sudden change of transmission mode. Wolbachia clearly differs from other ‘parasites’ previously investigated using similar experimental approaches. In our experiments, the symbionts (i) had not previously evolved under the dominant pressure of horizontal transmission because they are mainly vertically transmitted, (ii) are not strictly parasites of their native host (i.e. their virulence is low in the donors) because wVulC exhibits both negative and beneficial traits [14,18], and (iii) are not transferred to a novel host species but remain in their native host, A. vulgare.
After only a few horizontal passages, an increase in wVulC titre was observed in several tissues. Specifically, the Wolbachia titre increased in both the haemolymph (tissue used for the horizontal transmissions) and the nerve cord. In addition, both the proportion of infected haemocytes and the Wolbachia titre in the haemolymph were found to increase in correlation with the number of passages based on FisH and qPCR. This dramatic increase in Wolbachia in haemocytes suggests an ability of this endosymbiont to specifically target and multiply in the tissue responsible for its artificial transmission mode. The nerve cord also exhibited an increase in Wolbachia titre across the passages but at a lower magnitude compared with the haemolymph. In contrast with the haemolymph, this tissue is not directly involved in horizontal transmission of Wolbachia. However, haemocytes are frequently trafficking into the nerve cord, which may lead to coinciding infection patterns of both tissues [43]. The Wolbachia titre remained quite stable in the ovaries, the tissue for vertical transmission. As the reproduction of isopods takes at least a month, it was not possible to assess whether vertical transmission was impacted by serial horizontal passages.
Along with the increase in Wolbachia titre, host mortality also increased dramatically across experimental passages. From B1 to B6, host mortality gradually reached an experimental plateau at 80–90%. While mortality was clearly correlated with the Wolbachia titre in haemocytes from B1 to B6, this was no longer the case for the last two passages (B7 and B8), where mortality was still very high, although the injected ‘dose’ of Wolbachia was lower than before. These results demonstrate that Wolbachia virulence was clearly higher in the latter passages. Moreover, the weekly monitoring of mortality from B5 to B8 demonstrated obvious changes in the timing of mortality. For instance, the same injected dose caused no host mortality during the first week PI in B5, while at least 40% of hosts died during the first week PI in B8. These results clearly demonstrate that the increase in mortality observed throughout the serial passages was not only linked to a higher injected dose of Wolbachia, as previously reported in the case of interspecific transfers of wVulC from A. vulgare to Porcellio dilatatus [42], but to a change in virulence of wVulC towards its native host A. vulgare across serial passages. This dramatic change in virulence forced us to stop the experiment after 1 year due to the lack of survivors to perform further passages. Considering the diverse bacterial community known to infect A. vulgare [44,59], it is important to note that Wolbachia was the only stable dominant taxon present throughout the serial passages based on TGGE profiles and sequencing of cloned 16S rDNA gene fragments. Therefore, it can be concluded that no other dominant bacteria had been co-transferred with Wolbachia and could be responsible for the observed mortality of A. vulgare.
Our experiments demonstrate that Wolbachia can readily cope with new environmental constraints, as already suggested by their ability to experimentally switch from one host species to another [28]. The mechanisms allowing Wolbachia to respond rapidly to environmental changes have not been investigated here and we can only hypothesize on this matter. To manipulate the reproduction of their hosts, Wolbachia has to be able to communicate with its host's cells [19]. They might also be able to adjust their gene expression in response to cellular perturbations. Therefore, epigenetic changes could provide Wolbachia with enough plasticity to face the ‘stress’ of horizontal passage and may result in an increase in symbiont multiplication, thereby resulting in higher virulence. An alternative hypothesis would be that the changes in Wolbachia virulence through serial passages result from a selection of alleles conferring a better adaptation to horizontal transmission (e.g. higher multiplication rate in haemocytes). Different genotypes of Wolbachia in the population hosted by the donors could be the source for the observed phenotypic changes. In this scenario, the most competitive Wolbachia genotypes found in the haemolymph are those selected throughout the serial passages. These highly competitive genotypes might be virulent towards A. vulgare because, due to the serial passages experiment, the transmission of Wolbachia is less dependent on the long-term survival of the host, and virulence can thus increase dramatically.
Although the mechanism underlying the phenotypic change of wVulC has not been investigated in our study, this serial passages experiment provides a clear demonstration that Wolbachia is able to rapidly and deeply modify its virulence. This observation strengthens the important plasticity and/or adaptive ability of these bacteria and the importance of symbiont transmission mode in the virulence resulting from the interaction. Here, the versatile wVulC, which can have both negative (lower brood size, decreased survival [14,15]) and beneficial impacts (protection against pathogenic bacteria [18]) on its native host A. vulgare, exhibits a rapid increase in virulence when vertical transmission is disrupted and horizontal transmission becomes its exclusive transmission path. This ability of Wolbachia to rapidly modify the nature of its interaction with its host is probably a key feature explaining the impressive adaptive radiation of Wolbachia in arthropods through horizontal transfer.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank all the technical staff of EBI. We also thank Mine Altinli for her comments on an earlier version of this manuscript.
Data accessibility
The data supporting the analyses are deposited online on Dryad (http://dx.doi.org/10.5061/dryad.f6rc2) [60].
Authors' contributions
W.L.C., D.B. and M.S. designed the study. W.L.C. and M.R. carried out the laboratory work. W.L.C. performed statistical analyses. J.D. carried out TGGE experiments. All the authors wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
W.L.C. and J.D. were supported by a grant from Région Poitou-Charentes. This work was supported by the Agence Nationale de la Recherche (ANR-09-JCJC-0109-01).
References
- 1.Alizon S, Hurford A, Mideo N, Van Baalen M. 2009. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J. Evol. Biol. 22, 245–259. ( 10.1111/j.1420-9101.2008.01658.x) [DOI] [PubMed] [Google Scholar]
- 2.Ewald PW. 1987. Transmission modes and evolution of the parasitism–mutualism continuum. Ann. N. Y. Acad. Sci. 503, 295–306. ( 10.1007/BF02692179) [DOI] [PubMed] [Google Scholar]
- 3.Stewart AD, Logsdon JM, Kelley SE. 2005. An empirical study of the evolution of virulence under both horizontal and vertical transmission. Evolution 59, 730–739. ( 10.1554/03-330) [DOI] [PubMed] [Google Scholar]
- 4.Kakehashi M. 1996. Populations and infectious diseases: dynamics and evolution. Res. Popul. Ecol. (Kyoto) 38, 203–210. ( 10.1007/BF02515728) [DOI] [Google Scholar]
- 5.Lipsitch M, Siller S, Nowak M. 1996. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution (N.Y.). 50, 1729–1741. ( 10.2307/2410731) [DOI] [PubMed] [Google Scholar]
- 6.Ewald P. 1993. The evolution of virulence. Sci. Am. 268, 86–93. ( 10.1038/scientificamerican0493-86) [DOI] [PubMed] [Google Scholar]
- 7.Myers JH, Kuken B. 1995. Changes in the fecundity of tent caterpillars: a correlated character of disease resistance or sublethal effect of disease? Oecologia 103, 475–480. ( 10.1007/BF00328686) [DOI] [PubMed] [Google Scholar]
- 8.Duron O, Bouchon D, Boutin SSS, Bellamy L, Zhou L, Engelstädter J, Hurst GD, Engelstädter J, Hurst GD. 2008. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 6, 27 ( 10.1186/1741-7007-6-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchon D, Cordaux R, Grève P. 2008. Feminizing Wolbachia and the evolution of sex determination in isopods. In Insect symbiosis, vol. 3 (eds Bourtzìs K, Miller TA), pp. 273–296. Boca Raton, FL: CRC Press. [Google Scholar]
- 10.Cordaux R, Bouchon D, Grève P. 2011. The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends Genet. 27, 332–341. ( 10.1016/j.tig.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 11.Hurst GDD, Frost CL. 2015. Reproductive parasitism: maternally inherited symbionts in a biparental world. Cold Spring Harb. Perspect. Biol. 7, 1–21. ( 10.1101/cshperspect.a017699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann AA, Turelli M. 1988. Unidirectional incompatibility in Drosophila simulans: inheritance, geographic variation and fitness effects. Genetics 119, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snook RR, Cleland SY, Wolfner MF, Karr TL. 2000. Offsetting effects of Wolbachia infection and heat shock on sperm production in Drosophila simulans: analyses of fecundity, fertility and accessory gland proteins. Genetics 155, 167–178. ( 10.1111/j.1558-5646.2010.01138.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braquart-Varnier C, Lachat M, Herbinière J, Johnson M, Caubet Y, Bouchon D, Sicard M. 2008. Wolbachia mediate variation of host immunocompetence. PLoS ONE 3, e3286 ( 10.1371/journal.pone.0003286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sicard M, Chevalier F, De Vlechouver M, Bouchon D, Grève P, Braquart-Varnier C. 2010. Variations of immune parameters in terrestrial isopods: a matter of gender, aging and Wolbachia. Naturwissenschaften 97, 819–826. ( 10.1007/s00114-010-0699-2) [DOI] [PubMed] [Google Scholar]
- 16.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science (80-.) 322, 702 ( 10.1126/science.1162418) [DOI] [PubMed] [Google Scholar]
- 17.Teixeira L, Ferreira Á, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6, 2753–2763. ( 10.1371/journal.pbio.1000002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braquart-Varnier C, Altinli M, Pigeault R, Chevalier FD, Grève P, Bouchon D, Sicard M. 2015. The mutualistic side of Wolbachia-isopod interactions: Wolbachia mediated protection against pathogenic intracellular bacteria. Front. Microbiol. 6, 1388 ( 10.3389/fmicb.2015.01388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sicard M, Dittmer J, Grève P, Bouchon D, Braquart-Varnier C.. 2014. A host as an ecosystem: Wolbachia coping with environmental constraints. Environ. Microbiol. 16, 3583–3607. ( 10.1111/1462-2920.12573) [DOI] [PubMed] [Google Scholar]
- 20.Saridaki A, Bourtzis K. 2010. Wolbachia: more than just a bug in insects genitals. Curr. Opin. Microbiol. 13, 67–72. ( 10.1016/j.mib.2009.11.005) [DOI] [PubMed] [Google Scholar]
- 21.Jiggins FM, Hurst GDD. 2011. Rapid insect evolution by symbiont transfer. Science 332, 185–186. ( 10.1126/science.1205386) [DOI] [PubMed] [Google Scholar]
- 22.Zug R, Hammerstein P.. 2014. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 49, 89–111. ( 10.1111/brv.12098) [DOI] [PubMed] [Google Scholar]
- 23.Levin BR, Bull JJ. 1994. Short-sighted evolution and the virulence of pathogenic microorganisms. Trends Microbiol. 2, 76–81. ( 10.1016/0966-842X(94)90538-X) [DOI] [PubMed] [Google Scholar]
- 24.Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. 2015. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B 282, 20150249 ( 10.1098/rspb.2015.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werren JH, Windsor D, Guo LR. 1995. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. Lond. B 262, 197–204. ( 10.1098/rspb.1995.0196) [DOI] [Google Scholar]
- 26.Vavre F, Fleury F, Lepetit D, Fouillet P, Boulétreau M. 1999. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol. Biol. Evol. 16, 1711–1723. ( 10.1093/oxfordjournals.molbev.a026084) [DOI] [PubMed] [Google Scholar]
- 27.Huigens ME, de Almeida RP, Boons PAH, Luck RF, Stouthamer R. 2004. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc. R. Soc. B 271, 509–515. ( 10.1098/rspb.2003.2640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes GL, Rasgon JL.. 2013. Transinfection: a method to investigate Wolbachia–host interactions and control arthropod-borne disease. Insect. Mol. Biol. 23, 141–151. ( 10.1111/imb.12066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S-J, Ahmed MZ, Lv N, Shi P-Q, Wang X-M, Huang J-L, Qiu B-L. 2016. Plant-mediated horizontal transmission of Wolbachia between whiteflies. ISME J. 11, 1019–1028. ( 10.1038/ismej.2016.164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasgon JL, Gamston CE, Ren X. 2006. Survival of Wolbachia pipientis in cell-free medium. Appl. Environ. Microbiol. 72, 6934–6937. ( 10.1128/AEM.01673-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson MF, Weinert LA, Welch JJ, Linheiro RS, Magwire MM, Jiggins FM, Bergman CM. 2012. Population genomics of the Wolbachia endosymbiont in Drosophila melanogaster. PLoS Genet. 8, e1003129 ( 10.1371/journal.pgen.1003129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atyame CM, Delsuc F, Pasteur N, Weill M, Duron O. 2011. Diversification of Wolbachia endosymbiont in the culex pipiens mosquito. Mol. Biol. Evol. 28, 2761–2772. ( 10.1093/molbev/msr083) [DOI] [PubMed] [Google Scholar]
- 33.Chrostek E, Marialva MSP, Yamada R, O'Neill SL, Teixeira L. 2014. High anti-viral protection without immune upregulation after interspecies Wolbachia transfer. PLoS ONE 9, e99025 ( 10.1371/journal.pone.0099025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chrostek E, Teixeira L.. 2015. Mutualism breakdown by amplification of Wolbachia genes. PLoS Biol. 13, e1002065 ( 10.1371/journal.pbio.1002065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. 2007. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 5, 0997–1005. ( 10.1371/journal.pbio.0050114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klasson L, et al. 2008. Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol. Biol. Evol. 25, 1877–1887. ( 10.1093/molbev/msn133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider DI, Riegler M, Arthofer W, Merçot H, Stauffer C, Miller WJ.. 2013. Uncovering Wolbachia diversity upon artificial host transfer. PLoS ONE 8, e82402 ( 10.1371/journal.pone.0082402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rigaud T, Pennings PS, Juchault P. 2001. Wolbachia bacteria effects after experimental interspecific transfers in terrestrial isopods. J. Invertebr. Pathol. 77, 251–257. (doi:06/jipa.2001.5026) [DOI] [PubMed] [Google Scholar]
- 39.Le Clec'h W, Raimond M, Guillot S, Bouchon D, Sicard M. 2013. Horizontal transfers of feminizing versus non-feminizing Wolbachia strains: from harmless passengers to pathogens. Environ. Microbiol. 15, 2922–2936. ( 10.1111/1462-2920.12172) [DOI] [PubMed] [Google Scholar]
- 40.Dittmer J, Beltran-Bech S, Lesobre J, Raimond M, Johnson M, Bouchon D. 2014. Host tissues as microhabitats for Wolbachia and quantitative insights into the bacterial community in terrestrial isopods. Mol. Ecol. 23, 2619–2635. ( 10.1111/mec.12760) [DOI] [PubMed] [Google Scholar]
- 41.Chevalier F, Herbinière-Gaboreau J, Bertaux J, Raimond M, Morel F, Bouchon D, Grève P, Braquart-Varnier C. 2011. The immune cellular effectors of terrestrial isopod Armadillidium vulgare: meeting with their invaders, Wolbachia. PLoS ONE 6, e18531 ( 10.1371/journal.pone.0018531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Clec'h W, Raimond M, Bouchon D, Sicard M. 2014. Strength of the pathogenicity caused by feminizing Wolbachia after transfer in a new host: strain or dose effect? J. Invertebr. Pathol. 116, 18–26. ( 10.1016/j.jip.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 43.Braquart-Varnier C, Raimond M, Mappa G, Chevalier FD, Le Clec'h W, Sicard M. 2015. The hematopoietic organ: a cornerstone for Wolbachia propagation between and within hosts. Front. Microbiol. 6, 1424 ( 10.3389/fmicb.2015.01424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dittmer J, Lesobre J, Moumen B, Bouchon D.. 2016. Host origin and tissue microhabitat shaping the microbiota of the terrestrial isopod Armadillidium vulgare. FEMS Microbiol. Ecol. 92, fiw063 ( 10.1093/femsec/fiw063) [DOI] [PubMed] [Google Scholar]
- 45.Rigaud T, Juchault P. 1995. Success and failure of horizontal transfers of feminizing Wolbachia endosymbionts in woodlice. J. Evol. Biol. 8, 249–255. ( 10.1046/j.1420-9101.1995.8020249.x) [DOI] [Google Scholar]
- 46.Genty LM, Bouchon D, Raimond M, Bertaux J.. 2014. Wolbachia infect ovaries in the course of their maturation: last minute passengers and priority travellers? PLoS ONE 9, e94577 ( 10.1371/journal.pone.0094577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Clec'h W, Braquart-Varnier C, Raimond M, Ferdy JB, Bouchon D, Sicard M.. 2012. High virulence of Wolbachia after host switching: when autophagy hurts. PLoS Pathog. 8, e1002844 ( 10.1371/journal.ppat.1002844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kocher TD, Thomas WK, Meyer A, Edwards SV, Paabo S, Villablanca FX, Wilson AC. 1989. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc. Natl Acad. Sci. USA 86, 6196–6200. ( 10.1073/pnas.86.16.6196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Clec'h W, Chevalier FD, Genty L, Bertaux J, Bouchon D, Sicard M.. 2013. Cannibalism and predation as paths for horizontal passage of Wolbachia between terrestrial isopods. PLoS ONE 8, e60232 ( 10.1371/journal.pone.0060232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rasband W. 1997. ImageJ. Bethesda, MD: National Institutes of Health. [Google Scholar]
- 51.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703. ( 10.1128/jb.173.2.697-703.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muyzer G, De Waal EC, Uitterlinden AG. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59, 695–700. (doi:0099-2240/93/030695-06$02.00/0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dittmer J, Lesobre J, Raimond R, Zimmer M, Bouchon D. 2012. Influence of changing plant food sources on the gut microbiota of saltmarsh detritivores. Microb. Ecol. 64, 814–825. ( 10.1007/s00248-012-0056-4) [DOI] [PubMed] [Google Scholar]
- 54.Dortmans JCFM, Rottier PJM, Koch G, Peeters BPH. 2011. Passaging of a newcastle disease virus pigeon variant in chickens results in selection of viruses with mutations in the polymerase complex enhancing virus replication and virulence. J. Gen. Virol. 92, 336–345. ( 10.1099/vir.0.026344-0) [DOI] [PubMed] [Google Scholar]
- 55.Hu G, Chen SH, Qiu J, Bennett JE, Myers TG, Williamson PR. 2014. Microevolution during serial mouse passage demonstrates FRE3 as a virulence adaptation gene in Cryptococcus neoformans. MBio 5, e00941–e009414.. ( 10.1128/mBio.00941-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsugawa T, Tatsumi M, Tsutsumi H. 2014. Virulence-associated genome mutations of murine rotavirus identified by alternating serial passages in mice and cell cultures. J. Virol. 88, 5543–5558. ( 10.1128/JVI.00041-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolodny-Hirsch DM, Van Beek NAM. 1997. Selection of a morphological variant of Autographa californica nuclear polyhedrosis virus with increased virulence following serial passage in Plutella xylostella. J. Invertebr. Pathol. 69, 205–211. ( 10.1006/jipa.1997.4659) [DOI] [Google Scholar]
- 58.Rafaluk C, Gildenhard M, Mitschke A, Telschow A, Schulenburg H, Joop G.. 2015. Rapid evolution of virulence leading to host extinction under host–parasite coevolution. BMC Evol. Biol. 15, 112 ( 10.1186/s12862-015-0407-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouchon D, Zimmer M, Dittmer J.. 2016. The terrestrial isopod microbiome: an all-in-one toolbox for animal-microbe interactions of ecological relevance. Front. Microbiol. 7, 1472 ( 10.3389/FMICB.2016.01472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Clec'h W, Dittmer J, Raimond M, Bouchon D, Sicard M. 2017. Data from: Phenotypic shift in Wolbachia virulence towards its native host across serial horizontal passages. Dryad Digital Repository. ( 10.5061/dryad.f6rc2) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Le Clec'h W, Dittmer J, Raimond M, Bouchon D, Sicard M. 2017. Data from: Phenotypic shift in Wolbachia virulence towards its native host across serial horizontal passages. Dryad Digital Repository. ( 10.5061/dryad.f6rc2) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting the analyses are deposited online on Dryad (http://dx.doi.org/10.5061/dryad.f6rc2) [60].