Abstract
Vocal learning from social partners is crucial for the successful development of communication in a wide range of species. Social interactions organize attention and enhance motivation to learn species-typical behaviour. However, the neurobiological mechanisms connecting social motivation and vocal learning are unknown. Using zebra finches (Taeniopygia guttata), a ubiquitous model for vocal learning, we show that manipulations of nonapeptide hormones in the vasopressin family (arginine vasotocin, AVT) early in development can promote or disrupt both song and social motivation. Young male zebra finches, like human infants, are socially gregarious and require interactive feedback from adult tutors to learn mature vocal forms. To investigate the role of social motivational mechanisms in song learning, in two studies, we injected hatchling males with AVT or Manning compound (MC, a nonapeptide receptor antagonist) on days 2–8 post-hatching and recorded song at maturity. In both studies, MC males produced a worse match to tutor song than controls. In study 2, which experimentally controlled for tutor and genetic factors, AVT males also learned song significantly better compared with controls. Furthermore, song similarity correlated with several measures of social motivation throughout development. These findings provide the first evidence that nonapeptides are critical to the development of vocal learning.
Keywords: arginine vasopressin (AVP), V1a receptor (V1aR), nonapeptides, vocal learning, social motivation, zebra finch
1. Introduction
From the earliest stages, language development in humans is guided by social interaction. For example, infants' prelinguistic vocalizations facilitate parental responses [1], and infants use those reactions to refine their vocal repertoires to match those of the ambient language [2,3]. Attention to social responses is thus an important component of vocal learning, and developmental disorders that affect social motivation, such as autism spectrum disorder (ASD), are associated with deficits in prelinguistic vocal development [4,5]. What mechanisms link social motivation and vocal learning? The neuroendocrine processes underlying affiliative behaviour may also mediate social influences on communicative development, but previous studies of vocal learning have not incorporated candidate neuroendocrine mechanisms. Social influences on vocal development are present in other vocal learners, such as songbirds [6–9], but specific pathways linking social interaction to developmental changes in song are not known.
Song learning in birds has become a ubiquitous model for understanding general principles underlying complex vocal learning across species, including language learning in humans [10,11]. Zebra finches (Taeniopygia guttata), like human infants, require interactive feedback from adult tutors to learn mature vocal forms [12,13]. Zebra finches are highly gregarious and experience a high degree of temporal overlap in the memorization and acquisition phases of song learning [14], allowing social processes to influence learning. Social interaction with a tutor is vital for normal song development [15–17], and young zebra finches cannot learn effectively from a passive tape-recorded song [12,18]. Zebra finches cross-fostered under Bengalese finches (Lonchura striata) will produce a good copy of their foster-parent's song, even if a zebra finch model is available in a neighbouring cage [19,20]. Non-singing female listeners are also known to affect song learning in the zebra finch [21]. Males raised with deaf adult females sing more frequently and develop more atypical songs than those raised with hearing females [17], and blindfolded males raised with a tutor develop more accurate song when also raised with a female sibling than without one [22]. While both vocal learning and neuroendocrine mechanisms of social behaviour have been investigated in the zebra finch, they have never been integrated.
It is well established that nonapeptide hormones in the vasopressin family—arginine vasopressin (AVP) and oxytocin (OT) in mammals; arginine vasotocin (AVT) and mesotocin (MT) in birds, reptiles and amphibians—are involved in social, motivational, sensory and motor processes, all of which may support vocal learning from social partners. These small peptide hormones, which derive from hypothalamic and smaller accessory cell groups, modulate social behaviours across taxa and have been identified as mediators of behavioural plasticity and diversity [23–26]. Changes to vocal behaviour are among the most common and pronounced effects of nonapeptides. AVT/AVP affects latency, duration and acoustic features of vocalizations in several vertebrate species, including fish [27,28], amphibians [29], rodents [30,31] and birds [32–36]. However, the effect of nonapeptides on vocal learning in social contexts is unknown.
In two separate experiments, we manipulated the nonapeptide system of zebra finch chicks on 2–8 days post-hatch (dph) via daily intracranial (IC) injections of AVT, Manning compound (MC)—a potent antagonist of the AVT/AVP 1a receptor (V1aR) and weak OT receptor antagonist—or a vehicle control, and assessed the effect on song learning, specifically the acoustic match to the social father. The first experiment was designed to focus on the effect of nonapeptide treatment on social development and pairing behaviour. Given the wide-ranging effect of treatment in the first study on a number of social behaviours, as well as adult song, we then designed the second experiment to more specifically focus on vocal learning in a naturalistic social environment. We predicted that AVT injected birds would show a better acoustic match to their social father's (tutor) song in adulthood than controls, whereas MC males would show a worse match. We further predicted that MC males would exhibit social behaviour deficits throughout development, which would predict corresponding vocal learning deficits.
2. Methods
(a). Study 1: organizational effects of nonapeptides on social development and song quality
Zebra finch offspring hatched in six large breeding aviaries within 40 days were used as experimental subjects. Starting on day 2 post-hatch through day 8, subjects received daily 2 µl IC injections of (i) AVT (10 ng), (ii) MC (50 ng) or (iii) 0.9% isotonic saline vehicle control [37–39]. This time point was chosen to target an important period of growth and maturation of the avian medial amygdala and, specifically, the predicted development of AVT neurons in both hypothalamic and medial amygdala structures [40–42]. Injections targeting more specific brain regions are not possible at this point in development because this is a major period of neurogenesis in the zebra finch brain. The forebrain, in particular, is increasing in volume by several orders of magnitude between days 2 and 8 post-hatch [40,43]. We followed the IC injection methodology detailed in [39,44]. Following injection, we verified that chicks exhibited normal begging behaviour (mouth gaping) in response to tactile stimulation before returning them to the nest. No behavioural data were collected from the chicks during the treatment period or prior to fledging. Both AVT and MC act at multiple receptor subtypes in the zebra finch brain, including the VT4 (V1aR), VT3 (OT-like) and V2 receptors [38,45].
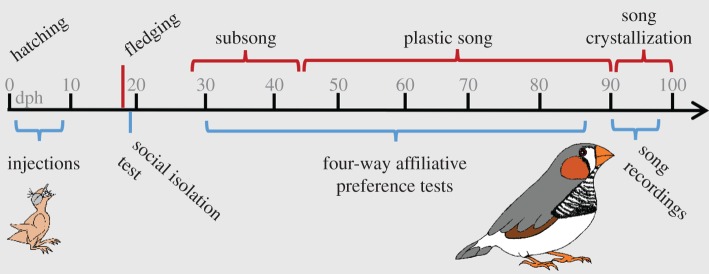
In order to study song development in a naturalistic social setting, subjects were cared for by the parents until approximately 40 days of age, when zebra finches normally become independent of parental feeding. After 39.8 ± 5.4 days, subjects were housed in same-sex aviaries in a separate room from the parents. To understand the effect of treatment on social motivation throughout development, we measured responsiveness to isolation from the family and subsequent reunion with the male parent the day after fledging. In addition, we assessed the changes in affiliation with the parents, unfamiliar males and unfamiliar females each week throughout juvenile development using a four-way affiliative preference test (see electronic supplementary material, Methods and materials) [39]. Results from this cohort showed widespread effects of nonapeptide treatment on social development in both male and female juveniles and on pairing behaviour and neural activity in adult males (and were previously published [39,46]). Here, we present new results, including song learning analyses and novel correlations, between measures of social motivation and song learning outcomes. As adults, males were assigned an unmanipulated, sexually naive and unpaired female pair partner. We performed all introductions between the subjects and their partner at 90 days post-hatch (dph) in a room with no other birds, after which they were moved into an aviary in a colony room and housed with the partner for 7 days. A large number of males, particularly in the two treatment groups, failed to sing during these introductions. Thus, additional recordings were obtained either by an observer in the colony room using a highly directional microphone or from recordings of reunion with the partner following a 1 h separation at 97 dph. We obtained quality song recordings for final analysis from a subset of all males (n = 4/11 AVT, n = 8/11 MC and n = 6/7 control). High-quality songs were recorded using a similar method from social fathers and all other adult males in the breeding aviaries for comparisons to juvenile songs. An experimental timeline for study 1 is shown in figure 1.
Figure 1.
Experimental timeline for study 1 with developmental events (hatching, fledging and song milestones) above the line and experimental events (injections, social isolation tests and four-way proximity tests, song recordings) below the line.
Zebra finch males sing a highly stereotyped song, each comprised several introductory notes followed by a single repeated motif with stable number of syllables [17]. To perform acoustic analysis, a single song motif excluding the introductory notes was randomly selected from subjects' recordings. We assessed song learning using Sound Analysis Pro 2.0 (SAP) to compare the acoustic features of juvenile and tutor song [47]. Our analysis focused primarily on the scores of song similarity, accuracy and sequential match percentage [48]. The motifs of each juvenile's song were compared with the respective tutor motif, using the same tutor motif for all of a juvenile's analyses. For all song analyses, we used linear mixed models to analyse the effect of treatment, with family included as a random effect. This allowed us to control for unobserved heterogeneity resulting from individual tutor song or family effects.
(b). Study 2: organizational effects of nonapeptides on vocal learning
In the second experiment, we used a within-family design to control for tutor and genetic factors which likely influence song learning. The genetic sex of the subjects was determined on the day of hatching and chicks were then cross-fostered at 2 dph to create families with three male subjects (one per treatment group, all unrelated to the social father) and one non-subject female sibling. We conducted the experiment with four family cohorts (n = 7 families), each with a total clutch size of four. One cohort (two families) was excluded due to high aggression by one adult male resulting in the death of two subjects and the male's female partner. An additional MC subject was not included due to incorrect genetic sexing, resulting in a total sample size of 14 subjects (n = 5 AVT, n = 4 MC and n = 5 control). Social rearing conditions and nonapeptide manipulations were identical to those described for study 1. Song was recorded every 3 days from 50 to 60 dph, every 10 days from 60 dph until 90 dph and on 120 dph for 1 h each day. Acoustic analyses followed the procedures of study 1, but additional song recording time at days 90 and 120 allowed us to obtain 10 motifs from each juvenile on each recording day. Collecting these additional motifs allowed us to perform more detailed song analyses, including analysis of Wiener entropy, pitch and harmonic structure, as well as syllable-level descriptions of song copying.
3. Results
(a). Song learning is sensitive to organizational effects of nonapeptides
(i). Study 1
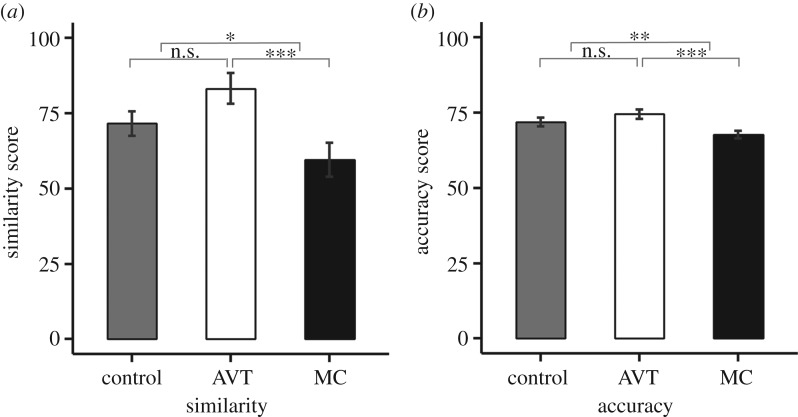
As predicted, nonapeptide treatment led to significant changes to males' crystallized song (figure 2; electronic supplementary material, video S1). Treatment affected the similarity score comparing subject and tutor song (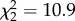 , p = 0.004; figure 3a). MC males had lower similarity than both control and AVT males, but the difference between AVT and control males did not reach significance. We found similar results for accuracy, a fine-grained measure of local similarity (
, p = 0.004; figure 3a). MC males had lower similarity than both control and AVT males, but the difference between AVT and control males did not reach significance. We found similar results for accuracy, a fine-grained measure of local similarity (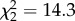 , p = 0.0008; figure 3b). There was no effect of treatment on the measure of sequential match (
, p = 0.0008; figure 3b). There was no effect of treatment on the measure of sequential match (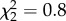 , p = 0.7).
, p = 0.7).
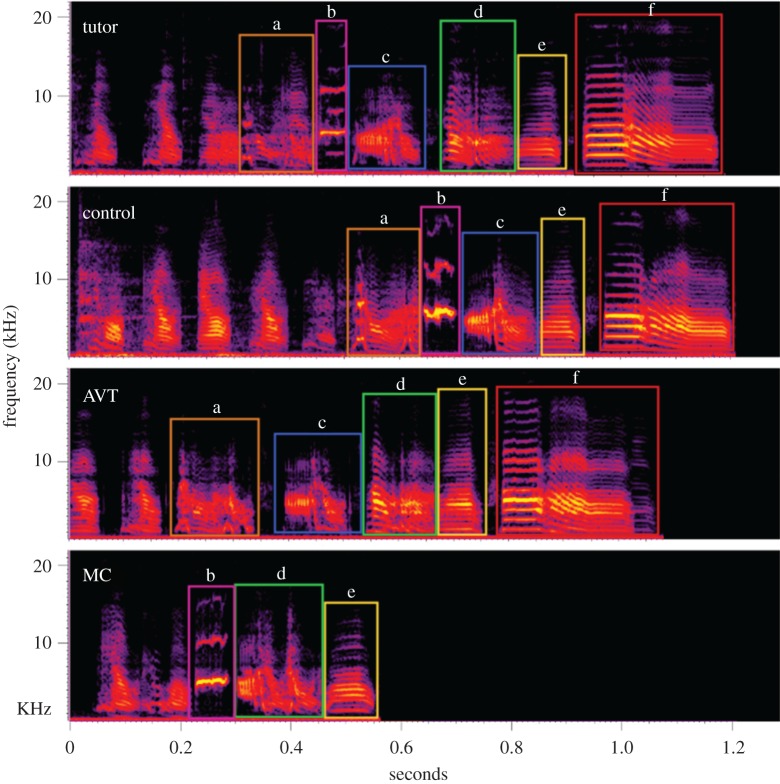
Figure 2.
Example spectrograms of the song of a tutor male and three subject males from each treatment group. Boxes outline individual song syllables. Letters label syllables that correspond between tutor and subject male song, as used for the syllable-level analyses in study 2.
Figure 3.
Study 1 similarity and accuracy scores at 90 dph. (a) Mean ± within-subject s.e. similarity score for study 1 males at day 90 when compared with social father's song as calculated using Sound Analysis Pro (SAP) (AVT–control: p = 0.07; MC–control: p = 0.03; AVT–MC: p = 0.0001). (b) Mean ± s.e. accuracy score for study 1 males at day 90 (AVT–control: p = 0.09; MC–control: p = 0.001; AVT–MC: p < 0.0001). *p < 0.05, **p < 0.01, ***p < 0.001.
(ii). Study 2
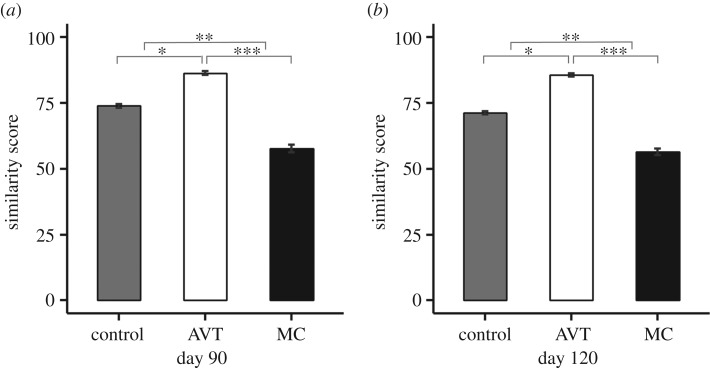
The effects of nonapeptide treatment on song similarity from study 1 were replicated and strengthened in the second study, which was designed to assess song development. Treatment predicted similarity both at 90 dph (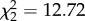 , p = 0.002; figure 4a) and at 120 dph, when zebra finch song is fully crystallized (
, p = 0.002; figure 4a) and at 120 dph, when zebra finch song is fully crystallized (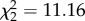 , p = 0.004; figure 4b). At both time points, all three treatment groups differed from each other, with AVT males having the highest similarity, MC males the lowest and control males intermediate. See electronic supplementary material, figure S1 for individual song similarity scores from both studies. Treatment did not impact either accuracy (90 dph:
, p = 0.004; figure 4b). At both time points, all three treatment groups differed from each other, with AVT males having the highest similarity, MC males the lowest and control males intermediate. See electronic supplementary material, figure S1 for individual song similarity scores from both studies. Treatment did not impact either accuracy (90 dph: 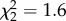 , p = 0.4; 120 dph:
, p = 0.4; 120 dph: 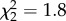 , p = 0.4) or sequential match (90 dph:
, p = 0.4) or sequential match (90 dph: 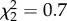 , p = 0.7); 120 dph:
, p = 0.7); 120 dph: 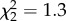 , p = 0.5).
, p = 0.5).
Figure 4.
Study 2 similarity scores at 90 and 120 dph. (a) Mean ± within-subject s.e. of the similarity score for study 2 males at day 90 (AVT–control: p = 0.02, MC–control: p = 0.006; AVT–MC: p < 0.0001). (b) Mean ± s.e. of the similarity score for study 2 males at day 120 (AVT–control: p = 0.02, MC–control: p = 0.03; AVT–MC: p < 0.0001). *p < 0.05, **p < 0.01, ***p < 0.001.
(b). Social motivation and attention to social cues influences song learning
(i). Study 1
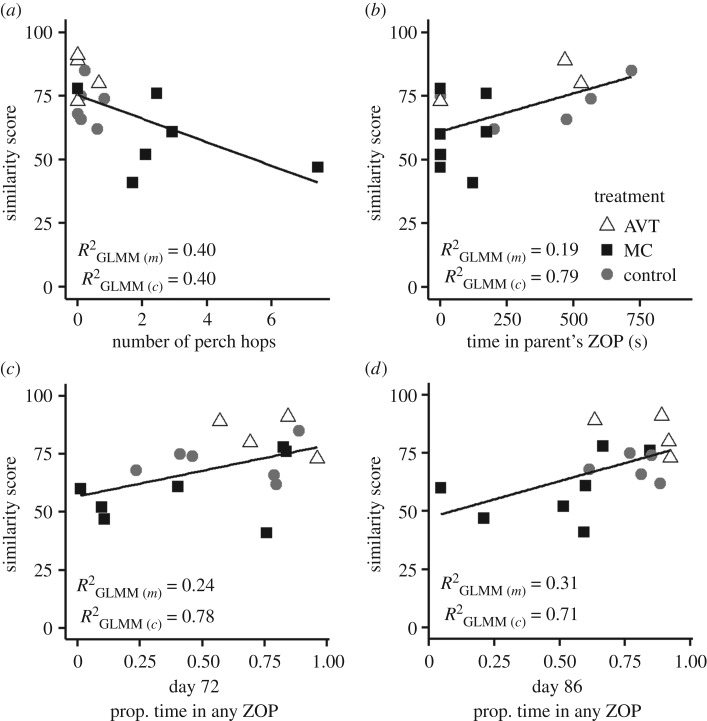
Song similarity was found to correlate with a number of measures of social motivation throughout development. There was a negative correlation between similarity and the number of perch hops (activity level) when newly fledged subjects were isolated from their parents and family (figure 5a; 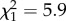 , p = 0.015). Increased activity during isolation is indicative of atypical social development; fledgling zebra finches typically remain silent and motionless when left alone during parental foraging bouts [49]. In addition, we found that the time spent in proximity to the parents in the four-way test of affiliative preferences at day 30 was positively correlated with similarity score (figure 5b;
, p = 0.015). Increased activity during isolation is indicative of atypical social development; fledgling zebra finches typically remain silent and motionless when left alone during parental foraging bouts [49]. In addition, we found that the time spent in proximity to the parents in the four-way test of affiliative preferences at day 30 was positively correlated with similarity score (figure 5b; 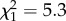 , p = 0.021). Additionally, increased time spent in proximity to any other birds (adult males, adult females or parents) at both days 72 and 86 post-hatch was associated with higher similarity scores (figure 5c,d; day 72,
, p = 0.021). Additionally, increased time spent in proximity to any other birds (adult males, adult females or parents) at both days 72 and 86 post-hatch was associated with higher similarity scores (figure 5c,d; day 72, 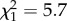 , p = 0.017; day 79,
, p = 0.017; day 79, 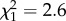 , p = 0.106; day 86,
, p = 0.106; day 86, 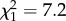 , p = 0.0074).
, p = 0.0074).
Figure 5.
Scatterplot of the similarity scores at 90 dph and (a) number of perch hops during isolation in the social isolation test, (b) time in seconds spent in the zone of proximity (ZOP) with the male and female parent during the four-way affiliative preference test on 30 dph, and (c,d) the proportion of total test time spent in any of the three ZOPs during the four-way affiliative preference tests on (c) 72 dph and (d) 86 dph. The lines depict significant general linear model fits.
(ii). Study 2
Several acoustic features of the songs differed between treatment groups, including amplitude, Weiner entropy, pitch and harmonic structure (electronic supplementary material, figure S2 and table S1). However, there was no evidence that our manipulation caused motor impairment, as acoustic measures of the songs produced by manipulated birds fell within normal ranges for zebra finches [50,51]. Additionally, we explored the possibility that nonapeptide treatment either accelerated or delayed the time for song to reach its stable mature form. Treatment groups did not differ in any measure of the amount of singing during the seven recording sessions between 50 and 80 dph (i.e. latency to sing, amount of singing, number of days in which singing occurred or earliest date of singing). Furthermore, there was no effect of treatment on individual variability across song bouts in any treatment group at day 120 (similarity, 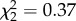 , p = 0.83; accuracy,
, p = 0.83; accuracy, 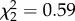 , p = 0.75), suggesting treatment did not impact the timing of song crystallization.
, p = 0.75), suggesting treatment did not impact the timing of song crystallization.
Thus, we sought to determine the factors that led to the differences in similarity across treatments by comparing numbers of tutor syllables copied in each group (figure 2). We found that MC males copied fewer syllables from their tutor than control and AVT males (55% versus 88% and 87%, respectively) (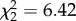 , p = 0.04; control–AVT: Z = 0.91, p = 0.76; control–MC: Z = 5.72, p = 0.03; and AVT–MC: Z = 7.17, p = 0.02). However, the treatment groups did not differ in either the similarity or accuracy of individual syllables (similarity,
, p = 0.04; control–AVT: Z = 0.91, p = 0.76; control–MC: Z = 5.72, p = 0.03; and AVT–MC: Z = 7.17, p = 0.02). However, the treatment groups did not differ in either the similarity or accuracy of individual syllables (similarity, 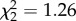 , p = 0.53; accuracy,
, p = 0.53; accuracy, 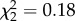 , p = 0.91). However, the lower accuracy of the whole song among MC males in study 1 suggests that the accuracy of individual syllables may have been affected in this study. Surprisingly, AVT males also did not differ from controls on any individual feature of acoustic similarity to the tutor's song. This indicates that AVT males' improved similarity score was a result of additive effects of multiple slight improvements in Wiener entropy, spectral continuity, pitch and frequency modulation, from which the similarity score is calculated [48].
, p = 0.91). However, the lower accuracy of the whole song among MC males in study 1 suggests that the accuracy of individual syllables may have been affected in this study. Surprisingly, AVT males also did not differ from controls on any individual feature of acoustic similarity to the tutor's song. This indicates that AVT males' improved similarity score was a result of additive effects of multiple slight improvements in Wiener entropy, spectral continuity, pitch and frequency modulation, from which the similarity score is calculated [48].
4. Discussion
To our knowledge, these are the first findings demonstrating the effects of early life manipulations of nonapeptides in a species that exhibits vocal learning. Our studies provide several converging lines of evidence suggesting that song learning outcomes were impacted by treatment-mediated changes to social motivation during development. First, we found several significant correlations between song learning and measures of social affiliation to both parents and conspecifics during development. Second, MC males only learned portions of their tutors' song, but did not differ in the acoustic match of individual syllables, suggesting that treatment effects were not driven by generalized effects on motor capacity. MC males copied fewer tutor syllables and several exhibited abnormal repeated notes characteristic of isolate-reared song at the beginning of their core motif [17]. Third, AVT males in study 2 exhibited improved skill in matching multiple features of their song to those of their tutors. In study 1, early life nonapeptide treatment was found to alter a whole suite of motivated social behaviours. MC males exhibited less—and AVT males more—affiliative interest in their parents throughout development [39]. Furthermore, although manipulated birds had longer latencies to sing to females (electronic supplementary material, figure S3), AVT males were several orders of magnitude more affiliative with their partner than both MC and control males [46].
Our neuroendocrine manipulation, conducted very early in development, demonstrated that a relatively non-localized IC administration of AVT or MC could create long-lasting effects. As the changes to social and vocal behaviour were observed over development, long after the injections were performed, our findings were not due to immediate activational effects on the AVT system during the first week of life. Instead, these results suggest that AVT plays an organizational role in the development of social and vocal circuits. Receptors for AVT, including V1aR and OTR, are widespread throughout the male zebra finch brain in adulthood. However, we know little about the development of the AVT system in songbirds, and there is limited evidence that the classic song learning anterior forebrain pathway (AFP) is directly modulated by AVT [45,52–54]. Thus, our findings suggest that other nonapeptide-sensitive sensorimotor and socio-motivational brain regions must be an important part of the vocal learning pathway [55,56]. Both AVT and V1aR appear to be involved in both sensory and motor components of vocal behaviour in adult songbirds. Several structures in the auditory forebrain, including the caudomedial mesopallium and the caudomedial nidopallium, highly express V1aR in zebra finches [53]. In addition to limited expression within RA, two nuclei involved in the motor pathway of song production contain high densities of AVT receptors: the intercollicular nucleus (ICo, a region implicated in vocal control) and nXIIts (the motor nucleus which innervates the syrinx) in several species [45,52,53,57,58].
In addition, our data suggest that changes to social motivation impact song learning outcomes, and that nonapeptides acting in the highly conserved mesolimbic reward and social behaviour networks provide a plausible neurobiological mechanism [23,24]. Numerous studies in other species provide evidence that nonapeptides may play an important role in experience-dependent development of social behaviour [59–71]. In zebra finches, AVT-immunoreactive fibres and V1aR are densely expressed in the ventral tegmental area (VTA), a region central in reward, motivation and reinforcement learning circuits [45,52,53,72]. AVT cell groups in the medial amygdala and medial bed nucleus of the stria terminalis (BSTm) send substantial projections to VTA. The subsequent connections between VTA and the nucleus accumbens (NAcc) form an important part of the mesolimbic reward pathway, which modulates the behavioural responses to rewarding or motivating stimuli. Our previous research showed that males treated with AVT have altered expression of V1aR and immediate early gene activity in the medial amygdala and BSTm, suggesting treatment changed the activity of this pathway [46].
The VTA also projects to the song learning system via dopaminergic input to the striatal Area X, innervating this nucleus most strongly during socially motivated singing [73]. Furthermore, the activity of dopaminergic cells in the VTA during song learning from a social partner is associated with better learning outcomes [9,74]. Thus, the pathway connecting the VTA to the AFP may allow for motivational modulation of song learning.
These studies provide a plausible neurobiological foundation for links between social motivation and song learning systems as an explanation for our findings. However, our data do not allow us to rule out the possibility that alterations to early vocal or social behaviour, rather than long-term organizational effects of nonapeptides on the brain, resulted in the observed changes to song development. We targeted our manipulation to alter the species-typical trajectory of the AVT system early in development, which probably resulted in widespread changes to multiple brain systems important in social function, including the hypothalamic–pituitary–adrenal (HPA) axis, sensorimotor systems and socio-motivational systems. For example, it is possible that treatment stimulated vocalization at the time of treatment, facilitating later vocal motor behaviour. However, zebra finches do not make vocalizations at all until at least 3 dph and then they may or may not vocalize during feeding from 4 to 12 dph [75]. It is also possible that, by altering interactions between parents and offspring at the time of treatment, these early life manipulations of the AVT system could have resulted in a self-reinforcing feedback loop in parent–offspring social interactions, leading to lifelong changes to social behaviour. For example, early life manipulations of the HPA axis via corticosterone administration in wild zebra finch chicks alter begging vocalizations, which in turn alters parental feeding behaviour [75]. Thus, AVT manipulations may alter early vocalizations or interactions between parents and offspring in the nest.
5. Conclusion
A major innovation necessary for the evolution of vocal learning is thought to be the linkage between the neural representation of social partners, motivational circuitry and communicative systems [56]. Consistent with this idea, we propose that nonapeptide treatment has altered males' motivation to attend to socially relevant cues, or has changed the salience of those cues, during vocal learning. The reduced similarity in MC males' song may result from reduced attention or sensitivity to behavioural feedback from tutors, whereas AVT males were more motivated to affiliate with and attend to social partners.
Our findings indicate that the developing brains of songbirds are modulated by nonapeptides in ways that are crucial for communicative development. Nonapeptides are known to play a role in diverse physiological functions including the stress response via regulation of the HPA axis, sensorimotor processes and social behaviours—all of which may impact the process of vocal learning. Despite well-known associations between social deficits and language impairments, this study is among the first to assess the effect of developmental exposure to AVT/AVP on a vocal learner of any species. Given the strong parallels between songbird and human vocal learning at multiple levels of organization [76], nonapeptides probably play a similarly important role in the communicative development of humans. Further investigation is urgently needed, as exogenous administration of nonapeptides is currently being tested in clinical trials in children diagnosed with ASD, a developmental disorder associated with deficits in social motivation [4,5]. Our findings open the door for further work on the neural and neuroendocrine mechanisms underlying social and communicative development.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful for the hands-on assistance of undergraduate students: N. C. Sklar, J. Mendez and J. Ridley. In addition, technical and logistical assistance was provided by J. K. Morrisey, DVM, T. J. DeVoogd, T. L. Van Deusen, S. M. Bogdanowicz and K. O. Smiley.
Ethics
All procedures were developed with veterinary supervision and approved by Cornell University's Institutional Animal Care and Use Committee (Protocol # 2011-0130).
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
N.M.B., S.C.P., T.H.K., M.H.G. and E.A.-R. designed the experiments. N.M.B. (study 1), T.H.K. (study 2) and S.C.P. (both studies) performed the experiments. S.C.P. performed all acoustic analyses. N.M.B. performed all statistical analyses. N.M.B, S.C.P., M.H.G. and E.A.-R. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by an NSF Doctoral Dissertation Improvement Grant (NSF, IOS—1310908; N.M.B.); NIH Training Grant 5T32HD055177-05 (N.M.B.); NSF Graduate Research Fellowship DGE-1650441 (S.C.P.), Einhorn Discovery Grant (T.H.K.); and NSF, IOS—1146891 (E.A.-R).
References
- 1.Goldstein MH, West MJ. 1999. Consistent responses of human mothers to prelinguistic infants: the effect of prelinguistic repertoire size. J. Comp. Psychol. 113, 52–58. ( 10.1037/0735-7036.113.1.52) [DOI] [PubMed] [Google Scholar]
- 2.Goldstein MH, King AP, West MJ. 2003. Social interaction shapes babbling: testing parallels between birdsong and speech. Proc. Natl Acad. Sci. USA 100, 8030–8035. ( 10.1073/pnas.1332441100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein MH, Schwade JA. 2008. Social feedback to infants' babbling facilitates rapid phonological learning. Psychol. Sci. 19, 515–523. ( 10.1111/j.1467-9280.2008.02117.x) [DOI] [PubMed] [Google Scholar]
- 4.Warlaumont AS, Richards JA, Gilkerson J, Oller DK. 2014. A social feedback loop for speech development and its reduction in autism. Psychol. Sci. 25, 1314–1324. ( 10.1177/0956797614531023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. 2012. The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239. ( 10.1016/j.tics.2012.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West MJ, King AP. 1988. Female visual displays affect the development of male song in the cowbird. Nature 334, 244–246. ( 10.1038/334244a0) [DOI] [PubMed] [Google Scholar]
- 7.Kojima S, Doupe AJ, Knudsen EI. 2011. Social performance reveals unexpected vocal competency in young songbirds. Proc. Natl Acad. Sci. USA 108, 1687–1692. ( 10.1073/pnas.1010502108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ljubičić I, Hyland Bruno J, Tchernichovski O. 2016. Social influences on song learning. Curr. Opin. Behav. Sci. 7, 101–107. ( 10.1016/j.cobeha.2015.12.006) [DOI] [Google Scholar]
- 9.Chen Y, Matheson LE, Sakata JT. 2016. Mechanisms underlying the social enhancement of vocal learning in songbirds. Proc. Natl Acad. Sci. USA 113, 6641–6646. ( 10.1073/pnas.1522306113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein MH, Schwade JA. 2009. From birds to words. In Oxford handbook of developmental behavioral neuroscience (eds Blumberg MS, Freeman JH, Robinson SR), pp. 708–729. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Lipkind D, et al. 2013. Stepwise acquisition of vocal combinatorial capacity in songbirds and human infants. Nature 498, 104–108. ( 10.1038/nature12173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derégnaucourt S, Poirier C, Kant AV der, Linden AV der, Gahr M. 2013. Comparisons of different methods to train a young zebra finch (Taeniopygia guttata) to learn a song. J. Physiol. Paris 107, 210–218. ( 10.1016/j.jphysparis.2012.08.003) [DOI] [PubMed] [Google Scholar]
- 13.Slater PJB, Eales LA, Clayton NS. 1988. Song learning in zebra finches (Taeniopygia guttata): progress and prospects. Adv. Study Behav. 18, 1–34. ( 10.1016/S0065-3454(08)60308-3) [DOI] [Google Scholar]
- 14.Roper A, Zann R. 2006. The onset of song learning and song tutor selection in fledgling zebra finches. Ethology 112, 458–470. ( 10.1111/j.1439-0310.2005.01169.x) [DOI] [Google Scholar]
- 15.Price PH. 1979. Developmental determinants of structure in zebra finch song. J. Comp. Physiol. Psychol. 93, 260–277. ( 10.1037/h0077553) [DOI] [Google Scholar]
- 16.Williams H, Kilander K, Sotanski ML. 1993. Untutored song, reproductive success and song learning. Anim. Behav. 45, 695–705. ( 10.1006/anbe.1993.1084) [DOI] [Google Scholar]
- 17.Williams H. 2004. Birdsong and singing behavior. Ann. N. Y. Acad. Sci. 1016, 1–30. ( 10.1196/annals.1298.029) [DOI] [PubMed] [Google Scholar]
- 18.Eales LA. 1989. The influences of visual and vocal interaction on song learning in zebra finches. Anim. Behav. 37, 507–508. ( 10.1016/0003-3472(89)90097-3) [DOI] [Google Scholar]
- 19.Immelmann K. 1969. Song development in the zebra finch and other estrildid finches. In Bird vocalizations (ed. Hinde RA.), pp. 61–74. London, UK: Cambridge University Press. [Google Scholar]
- 20.Böhner J. 1983. Song learning in the zebra finch (Taeniopygia guttata): selectivity in the choice of a tutor and accuracy of song copies. Anim. Behav. 31, 231–237. ( 10.1016/S0003-3472(83)80193-6) [DOI] [Google Scholar]
- 21.Jones AE, Slater PJB. 1993. Do young male zebra finches prefer to learn songs that are familiar to females with which they are housed? Anim. Behav. 46, 616–617. ( 10.1006/anbe.1993.1233) [DOI] [Google Scholar]
- 22.Adret P. 2003. Vocal imitation in blindfolded zebra finches (Taeniopygia guttata) is facilitated in the presence of a non-singing conspecific female. J. Ethol. 22, 29–35. ( 10.1007/s10164-003-0094-y) [DOI] [Google Scholar]
- 23.Goodson JL. 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11–22. ( 10.1016/j.yhbeh.2005.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connell LA, Hofmann HA. 2011. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599–3639. ( 10.1002/cne.22735) [DOI] [PubMed] [Google Scholar]
- 25.Insel TR. 2010. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65, 768–779. ( 10.1016/j.neuron.2010.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Insel TR, Young LJ. 2001. The neurobiology of attachment. Nat. Rev. Neurosci. 2, 129–136. ( 10.1038/35053579) [DOI] [PubMed] [Google Scholar]
- 27.Goodson JL, Bass AH. 2000. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J. Comp. Neurol. 422, 363–379. ( 10.1002/1096-9861(20000703)422:3%3C363::AID-CNE4%3E3.0.CO;2-8) [DOI] [PubMed] [Google Scholar]
- 28.Goodson JL, Bass AH. 2000. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature 403, 769–772. ( 10.1038/35001581) [DOI] [PubMed] [Google Scholar]
- 29.Boyd SK. 2013. Vasotocin modulation of social behaviors in amphibians. In Oxytocin, vasopressin and related peptides in the regulation of behavior (eds Choleris E, Pfaff DW, Kavaliers M), pp. 97–109. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 30.Lukas M, Wöhr M. 2015. Endogenous vasopressin, innate anxiety, and the emission of pro-social 50-kHz ultrasonic vocalizations during social play behavior in juvenile rats. Psychoneuroendocrinology 56, 35–44. ( 10.1016/j.psyneuen.2015.03.005) [DOI] [PubMed] [Google Scholar]
- 31.Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, Crawley JN. 2008. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav. Brain Res. 187, 371–378. ( 10.1016/j.bbr.2007.09.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voorhuis TAM, De Kloet ER, De Wied D. 1991. Effect of a vasotocin analog on singing behavior in the canary. Horm. Behav. 25, 549–559. ( 10.1016/0018-506X(91)90020-I) [DOI] [PubMed] [Google Scholar]
- 33.Harding CF, Rowe SA. 2003. Vasotocin treatment inhibits courtship in male zebra finches; concomitant androgen treatment inhibits this effect. Horm. Behav. 44, 413–418. ( 10.1016/j.yhbeh.2003.06.007) [DOI] [PubMed] [Google Scholar]
- 34.Maney DL, Goode CT, Wingfield JC. 1997. Intraventricular infusion of arginine vasotocin induces singing in a female songbird. J. Neuroendocrinol. 9, 487–491. ( 10.1046/j.1365-2826.1997.00635.x) [DOI] [PubMed] [Google Scholar]
- 35.Goodson JL. 1998. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla). Horm. Behav. 34, 67–77. ( 10.1006/hbeh.1998.1467) [DOI] [PubMed] [Google Scholar]
- 36.Goodson JL, Rinaldi J, Kelly AM. 2009. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm. Behav. 55, 197–202. ( 10.1016/j.yhbeh.2008.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodson JL, Lindberg L, Johnson P. 2004. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm. Behav. 45, 136–143. ( 10.1016/j.yhbeh.2003.08.006) [DOI] [PubMed] [Google Scholar]
- 38.Manning M, et al. 2012. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol. 24, 609–628. ( 10.1111/j.1365-2826.2012.02303.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baran NM, Sklar NC, Adkins-Regan E. 2016. Developmental effects of vasotocin and nonapeptide receptors on early social attachment and affiliative behavior in the zebra finch. Horm. Behav. 78, 20–31. ( 10.1016/j.yhbeh.2015.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikebuchi M, Nanbu S, Okanoya K, Suzuki R, Bischof H-J. 2013. Very early development of nucleus taeniae of the amygdala. Brain. Behav. Evol. 81, 12–26. ( 10.1159/000342785) [DOI] [PubMed] [Google Scholar]
- 41.Buijs RM, Velis DN, Swaab DF. 1980. Ontogeny of vasopressin and oxytocin in the fetal rat: early vasopressinergic innervation of the fetal brain. Peptides 1, 315–324. ( 10.1016/0196-9781(80)90009-1) [DOI] [PubMed] [Google Scholar]
- 42.Szot P, Dorsa DM. 1993. Differential timing and sexual dimorphism in the expression of the vasopressin gene in the developing rat brain. Dev. Brain Res. 73, 177–183. ( 10.1016/0165-3806(93)90136-X) [DOI] [PubMed] [Google Scholar]
- 43.Charvet CJ, Striedter GF. 2009. Developmental origins of mosaic brain evolution: morphometric analysis of the developing zebra finch brain. J. Comp. Neurol. 514, 203–213. ( 10.1002/cne.22005) [DOI] [PubMed] [Google Scholar]
- 44.Bender AT, Veney SL. 2008. Treatment with the specific estrogen receptor antagonist ICI 182,780 demasculinizes neuron soma size in the developing zebra finch brain. Brain Res. 1246, 47–53. ( 10.1016/j.brainres.2008.09.089) [DOI] [PubMed] [Google Scholar]
- 45.Leung CH, Goode CT, Young LJ, Maney DL. 2009. Neural distribution of nonapeptide binding sites in two species of songbird. J. Comp. Neurol. 513, 197–208. ( 10.1002/cne.21947) [DOI] [PubMed] [Google Scholar]
- 46.Baran NM, Tomaszycki ML, Adkins-Regan E. 2016. Early life manipulations of the nonapeptide system alter pair maintenance behaviors and neural activity in adult male zebra finches. Front. Behav. Neurosci. 10, 58 ( 10.3389/fnbeh.2016.00058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tchernichovski O, Mitra P. 2002. Towards quantification of vocal imitation in the zebra finch. J. Comp. Physiol. A 188, 867–878. ( 10.1007/s00359-002-0352-4) [DOI] [PubMed] [Google Scholar]
- 48.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. 2000. A procedure for an automated measurement of song similarity. Anim. Behav. 59, 1167–1176. ( 10.1006/anbe.1999.1416) [DOI] [PubMed] [Google Scholar]
- 49.Zann RA. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press. [Google Scholar]
- 50.Wood WE, Ii PJO, Roseberry TK, Perkel DJ. 2013. A daily oscillation in the fundamental frequency and amplitude of harmonic syllables of zebra finch song. PLoS ONE 8, e82327 ( 10.1371/journal.pone.0082327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riede T, Schilling N, Goller F. 2012. The acoustic effect of vocal tract adjustments in zebra finches. J. Comp. Physiol. A 199, 57–69. ( 10.1007/s00359-012-0768-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voorhuis TAM, De Kloet ER. 1992. Immunoreactive vasotocin in the zebra finch brain (Taeniopygia guttata). Dev. Brain Res. 69, 1–10. ( 10.1016/0165-3806(92)90116-E) [DOI] [PubMed] [Google Scholar]
- 53.Leung CH, Abebe DF, Earp SE, Goode CT, Grozhik AV, Mididoddi P, Maney DL. 2011. Neural distribution of vasotocin receptor mRNA in two species of songbird. Endocrinology 152, 4865–4881. ( 10.1210/en.2011-1394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura T, Okanoya K, Wada M. 1999. Effect of testosterone on the distribution of vasotocin immunoreactivity in the brain of the zebra finch, Taeniopygia guttata castanotis. Life Sci. 65, 1663–1670. ( 10.1016/S0024-3205(99)00415-4) [DOI] [PubMed] [Google Scholar]
- 55.Rose JD, Moore FL. 2002. Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front. Neuroendocrinol. 23, 317–341. ( 10.1016/S0091-3022(02)00004-3) [DOI] [PubMed] [Google Scholar]
- 56.Syal S, Finlay BL. 2011. Thinking outside the cortex: social motivation in the evolution and development of language. Dev. Sci. 14, 417–430. ( 10.1111/j.1467-7687.2010.00997.x) [DOI] [PubMed] [Google Scholar]
- 57.Kiss JZ, Voorhuis TA, Van Eekelen JAM, De Kloet ER, De Wied D. 1987. Organization of vasotocin-immunoreactive cells and fibers in the canary brain. J. Comp. Neurol. 263, 347–364. ( 10.1002/cne.902630304) [DOI] [PubMed] [Google Scholar]
- 58.Panzica GC, Plumari L, García-Ojeda E, Deviche P. 1999. Central vasotocin-immunoreactive system in a male passerine bird (Junco hyemalis). J. Comp. Neurol. 409, 105–117. ( 10.1002/(SICI)1096-9861(19990621)409:1%3C105::AID-CNE8%3E3.0.CO;2-8) [DOI] [PubMed] [Google Scholar]
- 59.Hammock EAD, Law CS, Levitt P. 2013. Vasopressin eliminates the expression of familiar odor bias in neonatal female mice through V1aR. Horm. Behav. 63, 352–360. ( 10.1016/j.yhbeh.2012.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boer GJ, Quak J, de Vries MC, Heinsbroek RPW. 1994. Mild sustained effects of neonatal vasopressin and oxytocin treatment on brain growth and behavior of the rat. Peptides 15, 229–236. ( 10.1016/0196-9781(94)90007-8) [DOI] [PubMed] [Google Scholar]
- 61.Winslow JT, Insel TR. 1993. Effects of central vasopressin administration to infant rats. Eur. J. Pharmacol. 233, 101–107. ( 10.1016/0014-2999(93)90354-K) [DOI] [PubMed] [Google Scholar]
- 62.Veenema AH, Bredewold R, De Vries GJ. 2012. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm. Behav. 61, 50–56. ( 10.1016/j.yhbeh.2011.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veenema AH, Bredewold R, De Vries GJ. 2013. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology 38, 2554–2561. ( 10.1016/j.psyneuen.2013.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bredewold R, Smith CJW, Dumais KM, Veenema AH. 2014. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front. Behav. Neurosci. 8, 216 ( 10.3389/fnbeh.2014.00216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bales KL, Carter CS. 2003. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster). Behav. Neurosci. 117, 854–859. ( 10.1037/0735-7044.117.4.854) [DOI] [PubMed] [Google Scholar]
- 66.Bales KL, Carter CS. 2003. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster). Horm. Behav. 44, 178–184. ( 10.1016/S0018-506X(03)00154-5) [DOI] [PubMed] [Google Scholar]
- 67.Bales KL, Abdelnabi M, Cushing BS, Ottinger MA, Carter CS. 2004. Effects of neonatal oxytocin manipulations on male reproductive potential in prairie voles. Physiol. Behav. 81, 519–526. ( 10.1016/j.physbeh.2004.02.016) [DOI] [PubMed] [Google Scholar]
- 68.Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. 2007. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience 144, 38–45. ( 10.1016/j.neuroscience.2006.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto Y, Cushing B, Kramer K, Epperson P, Hoffman G, Carter C. 2004. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 125, 947–955. ( 10.1016/j.neuroscience.2004.02.028) [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto Y, Carter CS, Cushing BS. 2006. Neonatal manipulation of oxytocin affects expression of estrogen receptor alpha. Neuroscience 137, 157–164. ( 10.1016/j.neuroscience.2005.08.065) [DOI] [PubMed] [Google Scholar]
- 71.Mogi K, Ooyama R, Nagasawa M, Kikusui T. 2014. Effects of neonatal oxytocin manipulation on development of social behaviors in mice. Physiol. Behav. 133, 68–75. ( 10.1016/j.physbeh.2014.05.010) [DOI] [PubMed] [Google Scholar]
- 72.Gale SD, Perkel DJ. 2010. A basal ganglia pathway drives selective auditory responses in songbird dopaminergic neurons via disinhibition. J. Neurosci. 30, 1027–1037. ( 10.1523/JNEUROSCI.3585-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kubikova Ľ, Košťál Ľ. 2010. Dopaminergic system in birdsong learning and maintenance. J. Chem. Neuroanat. 39, 112–123. ( 10.1016/j.jchemneu.2009.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gadagkar V, Puzerey PA, Chen R, Baird-Daniel E, Farhang AR, Goldberg JH. 2016. Dopamine neurons encode performance error in singing birds. Science 354, 1278–1282. ( 10.1126/science.aah6837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez EC, Mariette MM, Cochard P, Soulage CO, Griffith SC, Vignal C. 2016. Corticosterone triggers high-pitched nestlings' begging calls and affects parental behavior in the wild zebra finch. Behav. Ecol. 27, 1665–1675. ( 10.1093/beheco/arw069) [DOI] [Google Scholar]
- 76.Doupe AJ, Kuhl PK. 1999. Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci. 22, 567–631. ( 10.1146/annurev.neuro.22.1.567) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.