Abstract
Functional changes in vocal organ morphology and motor control facilitate the evolution of acoustic signal diversity. Although many rodents produce vocalizations in a variety of social contexts, few studies have explored the underlying production mechanisms. Here, we describe mechanisms of audible and ultrasonic vocalizations (USVs) produced by grasshopper mice (genus Onychomys). Grasshopper mice are predatory rodents of the desert that produce both loud, long-distance advertisement calls and USVs in close-distance mating contexts. Using live-animal recording in normal air and heliox, laryngeal and vocal tract morphological investigations, and biomechanical modelling, we found that grasshopper mice employ two distinct vocal production mechanisms. In heliox, changes in higher-harmonic amplitudes of long-distance calls indicate an airflow-induced tissue vibration mechanism, whereas changes in fundamental frequency of USVs support a whistle mechanism. Vocal membranes and a thin lamina propria aid in the production of long-distance calls by increasing glottal efficiency and permitting high frequencies, respectively. In addition, tuning of fundamental frequency to the second resonance of a bell-shaped vocal tract increases call amplitude. Our findings indicate that grasshopper mice can dynamically adjust motor control to suit the social context and have novel morphological adaptations that facilitate long-distance communication.
Keywords: bioacoustics, source-filter theory, vocal production
1. Introduction
Acoustic signals are shaped by social selection [1,2] and constrained by the signalling environment [3–6] or ecological adaptation [7,8] in a broad range of taxa. Mechanistically, acoustic divergence is determined by several variables in senders: morphological adaptations of the sound source (larynx) and/or vocal tract filter (pharynx, nasal and oral cavities), changes in motor control of the source and filter, and selection on driving force (lung pressure). Identifying the relative contributions of each component to acoustic variation is critical to understanding the evolution of vocal communication systems.
Most terrestrial mammals produce sounds by airflow-induced vibrations of vocal folds [9]. By contrast, rodents produce ultrasonic vocalizations (USVs; greater than 20 kHz) in close-distance mating contexts via an aerodynamic whistle mechanism whereby tightly constricted larynges serve as an orifice to pass expired air [10–12]. However, our understanding of vocal functional morphology in rodents is limited to laboratory mice (Mus musculus) and rats (Rattus domesticus; [10–15]). The great diversity of rodent mating and social systems make the clade an excellent system to examine production mechanisms underlying signal diversification [16].
Cricetid rodents in the subfamily Neotominae [17] commonly produce audible (less than 20 kHz) vocalizations [18,19]. In particular, grasshopper mice (Onychomys) are predatory rodents of western North America known for their ‘wolf's howl in miniature’ [20]. Both males and females produce long-distance advertisement vocalizations used in mate attraction and territorial advertisement ([21,22]; figure 1). Animals often assume an upright posture and open their mouths widely to generate a remarkably loud call (electronic supplementary material, videos S1 and S2; [22]). Grasshopper mice also produce USVs in close-distance mating bouts ([18]; herein), allowing exploration into how sound production mechanisms vary in distinct social contexts.
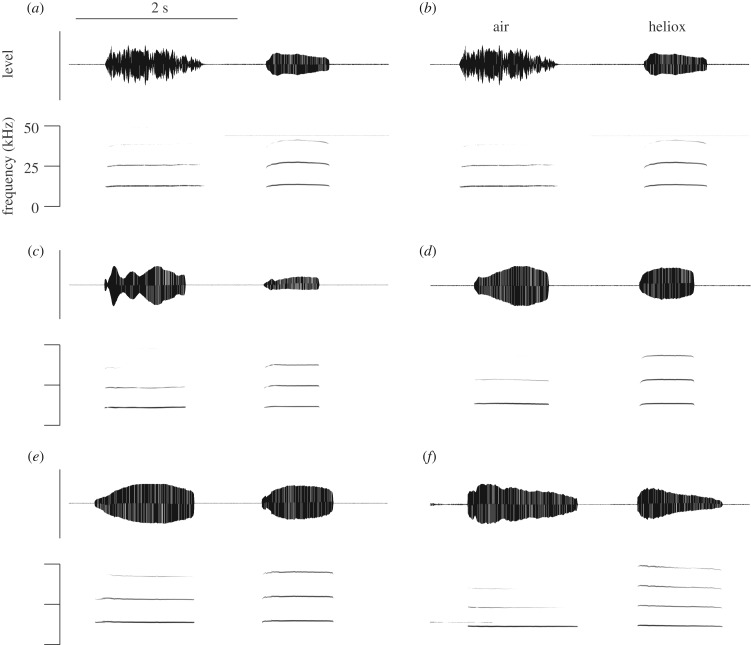
Figure 1.
Long-distance calls of Onychomys in normal air and heliox. Each panel (a–f) shows two calls from each of six mice in air (a,c,e) and heliox (b,d,f). Sound is depicted as a waveform (a–d) and spectrogram (e,f). The first call in (f) is preceded by a long-distance call of another animal in the same room.
In this study, we used heliox experiments, laryngeal and vocal tract morphological investigations, and biomechanical modelling to investigate how grasshopper mice produce spectacular long-distance calls. If vocalizations are produced via a whistle mechanism, fundamental frequency (F0) is predicted to increase under heliox atmosphere [23]. Conversely, if sound is produced via airflow-induced tissue vibration, then F0 is predicted to remain constant, but amplitudes of higher harmonics will change relative to F0 [24]. The morphological study identified adaptations that could facilitate long-distance call production. However, since vocal production is based on complex coupled mechanisms between airflow, vocal fold tissue properties and supraglottal structures, computational simulations are required [25]. Thus, our morphological findings were used to develop computational models of vocal fold movements to inform the physical mechanisms underlying call production.
2. Material and methods
(a). Animals
Grasshopper mice are predatory rodents that inhabit deserts, grasslands and prairies of the western United States and northern Mexico. We captured northern (Onychomys leucogaster), southern (Onychomys torridus) and Chihuahuan grasshopper mouse (Onychomys arenicola) in an area of sympatry near Tank Mountain (31°48'46.90″ N 108°48'49.90″ W) in the Animas Valley, New Mexico, USA [26,27]. Mice were transferred to animal facilities at Northern Arizona University, Flagstaff, AZ, USA, maintained on a 14 L : 10 D cycle (21 ± 2°C) and provided rodent chow and water ad libitum. Animals used in the experiments were F1 or F2 from same-species pairs or opposite-species pairs (hybrids). A subset of animals was transferred to Midwestern University, Glendale, AZ, USA, for heliox experiments and morphological investigations.
(b). Acoustic recording
We recorded long-distance calls and close-distance mating vocalizations in normal air and heliox atmosphere. Individual male mice (for spontaneous long-distance vocalizations) or a male–female pair (for close-distance USVs) were placed inside a custom-made cage consisting of 10 mm mesh inside a larger standard rat cage. The cage was equipped with bedding, a running wheel, food and water gel. Heliox gas (80% He, 20% O2) was injected into the rat cage at flow rates between 20 and 40 l min−1 through a 12 mm wide tube placed on the cage floor. Small holes along the tube facilitated uniform distribution of gas. In light gas, F0 of a whistle increases in proportion to the amount of gas present. Predicted effects of light gas concentrations were estimated with a small whistle placed at the floor of the cage and connected externally by a silastic tube. The whistle was blown and recorded at regular intervals in order to monitor the heliox concentration. The ratio of the frequency of the whistle in air and in heliox allowed an estimation of the expected effect for any given heliox concentration. We also recorded long-distance calls in air within individual male home cages to control for the introduction of animals into a novel environment.
Long-distance calls from seven males produced in normal air and in heliox atmosphere were analysed for total duration, F0 at mid-call and sound pressure level (decibel). Short-distance mating USVs from eight males were analysed for mean F0. All measurements were performed using PRAAT sound analysis software (v. 5.3.80 for Windows; www.praat.org). Reported means of maximum sound pressure level values are not relative to a common standard and were only compared within individuals between treatments.
(c). Histology
Four male mice (two O. torridus and two O arenicola) were euthanized with isoflurane and then transcardially perfused. Larynges were dissected and fixed in 10% buffered formalin phosphate (SF100-4; Fisher Scientific) for one week. Vocal folds were investigated for the presence of collagen, elastin and hyaluronan, all of which play an important role in vocal fold biomechanics [28–30]. Mid-membraneous coronal sections (5 mm thick) were stained with haematoxylin–eosin for a general overview, Masson's Trichrome for collagen fibre stain, Elastica–Van Gieson for elastic fibre stain and alcian blue (AB) stain (pH 2.5) for mucopolysaccharides and glycosaminoglycans. We also performed a digestion procedure with bovine testicular hyaluronidase (2 h at 37°C) in combination with a subsequent AB stain. Incubation with bovine testicular hyaluronidase increases specificity for various acid mucosubstances in the AB stain. If hyaluronan is a major component of the mucosubstances, AB stain fully degrades. Sections were scanned with an Aperio CS 2 slide scanner and processed with Imagescope software (v. 8.2.5.1263; Aperio Tech.).
(d). Computational simulation of the sound production mechanism
We expanded our previous biomechanical model on voice production [31] to investigate how morphological features affect the sound production mechanism and why long-distance calls reach high sound amplitude. The larynx model is based on a two-mass model of the vocal folds [32,33]. Acoustic characteristics of a flared vocal tract were estimated by using the transmission line model [34]. Details of the simulations are summarized in the electronic supplementary material.
3. Results
(a). Acoustic recordings
Long-distance calls were flat, stable vocalizations lasting between 0.8 and 1.4 s in air. Figure 1 shows examples of one call recorded in normal air and one in heliox for six animals. Long-distance calls recorded in heliox were slightly higher in F0 (515 ± 85 Hz; paired t-test, t6 = −6.03, p = 0.001) but much lower (−6.52 ± 0.6 kHz; paired t-test, t6 = 10.72, p < 0.0001) than predicted in the presence of He, indicating an airflow-induced tissue vibration mechanism (figure 2a). Interestingly, calls produced in heliox were slightly shorter in duration (−0.27 ± 0.03 s; paired t-test, t6 = 8.82, p < 0.0001; figure 2b), potentially indicating auditory-feedback-mediated vocal control [35].
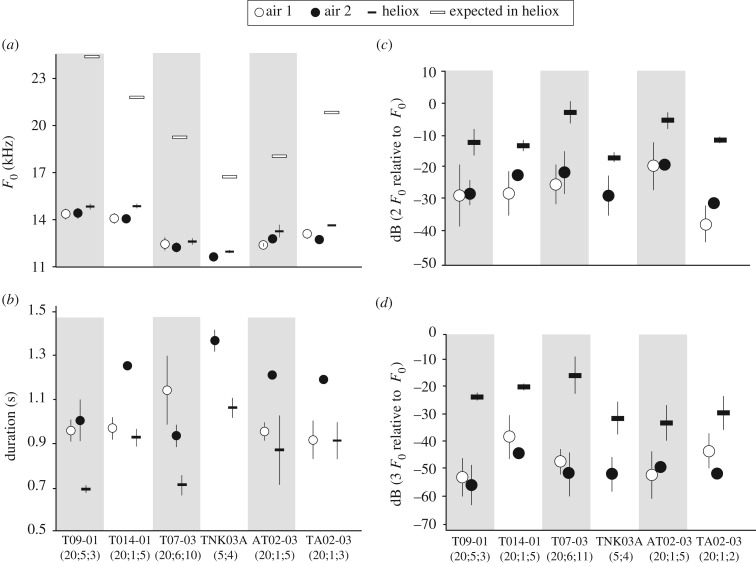
Figure 2.
Acoustic properties of Onychomys long-distance calls produced in normal air (‘air 1’, in home cage; ‘air 2’ in experimental cage) and heliox. (a) Centroid fundamental frequency (F0) of long-distance calls in air and heliox. Fundamental frequency was slightly higher in heliox but remained far below the predicted frequency estimated from the frequency response of a whistle (‘expected in heliox’; see Material and methods). (b) Duration of long-distance calls produced in air and heliox. Relative level (dB) of the second (c) and third (d) harmonics of long-distance calls in air and heliox. Error bars indicate mean ± s.d. for individual mice. Home cage recordings were not available for TNK03A.
Next, we tested how different filter properties of the oropharyngeal space affected sound characteristics in normal air and in heliox. Visual inspection of spectrograms suggested that upper harmonics in heliox were more prominent relative to F0 (figure 1). We measured the amplitude of 2F0 and 3F0 in normal air and in heliox relative to F0 at the center of the long-distance call. The data are summarized in table 1 and in figure 2c,d. In all animals, amplitude of 2F0 and 3F0 relative to F0 were significantly higher in heliox than in normal air (2F0: paired t-test, t6 = 8.9, p< 0.0001; 3F0: paired t-test, t6 = 9.9, p< 0.0001; figure 2c). The mean relative amplitude of 2F0 ranged from −18.7 to −30.7 dB in normal air compared with −2.8 to −16.7 dB in heliox (figure 2c; table 1). The mean relative amplitude of 3F0 ranged from −42.5 to −53.7 dB in normal air compared with −14.7 to −30 dB in heliox (figure 2d; table 1).
Table 1.
Fundamental frequency (F0) and dB levels of the second (2F0) and third (3F0) harmonic relative to F0 in normal air and in heliox.
| mouse ID | body mass (g) | F0 (kHz) of long-distance call in air | F0 (kHz) of long-distance call in heliox | 2F0 (dB) in air | 2F0 (dB) in heliox | 3F0 (dB) in air | 3F0 (dB) in heliox |
|---|---|---|---|---|---|---|---|
| T09-01 | 23.3 | 14.4 ± 0.3 | 14.8 ± 0.2 | −27.6 ± 3.8 | −11.9 ± 4.1 | −53.7 ± 7.3 | −22.2 ± 1.5 |
| T014-01 | 27.3 | 14.1 | 14.8 ± 0.1 | −21.8 | −13.0 ± 1.7 | −42.5 | −18.8 ± 1.3 |
| T07-03 | 36.1 | 12.2 ± 0.3 | 12.6 ± 0.2 | −21.4 ± 6.6 | −2.8 ± 3.5 | −50.0 ± 7.8 | −14.7 ± 6.6 |
| TANK03-A | 47.9 | 11.6 ± 0.1 | 11.9 ± 0.1 | −28.5 ± 6.2 | −16.7 ± 1.4 | −49.9 ± 6.3 | −30.0 ± 5.8 |
| AT02-03 | 28.9 | 12.8 | 13.3 ± 0.4 | −18.7 | −5.2 ± 2.6 | −47.6 | −31.5 ± 6.4 |
| TA02-03 | 29.8 | 12.7 | 13.6 ± 0.05 | −30.7 | −11.3 ± 1.0 | −49.9 | −27.9 ± 6.2 |
| L012-01 | 32.4 | 12.5 ± 0.2 | 12.8 ± 0.1 | −29.7 ± 7.1 | −7.8 ± 1.3 | −50.6 ± 4 | −24.1 ± 0.8 |
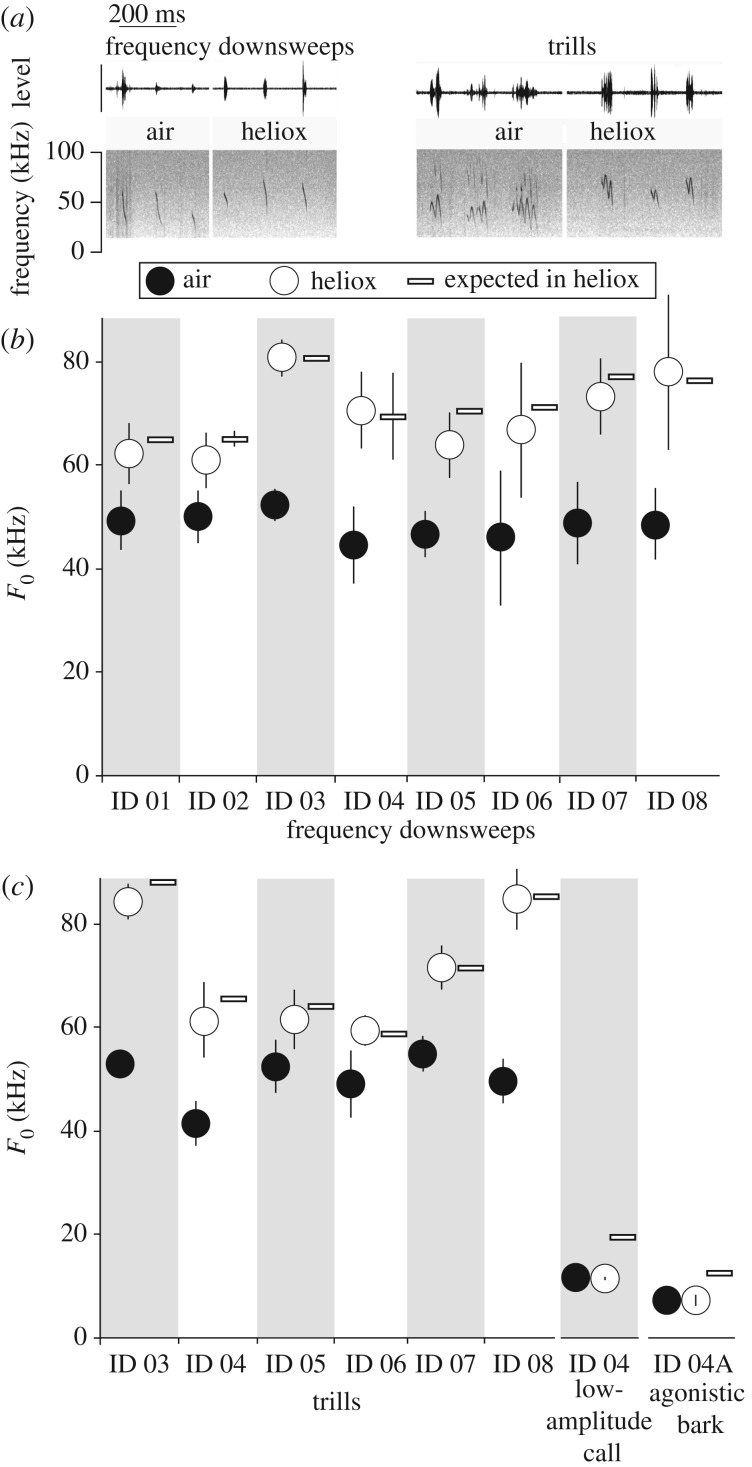
Mating vocalizations consisted of frequency downsweeps, trills, chevrons and a low-amplitude version of the long-distance call (figure 3). A non-receptive female also produced agonistic barks or ‘chits’ [21]. The F0 of downsweeps produced during the mating bouts increased to 69.2 ± 7.0 kHz in heliox from 48.3 ± 2.6 kHz in air (paired t-test, t7 = 8.9, p < 0.0001; figure 3). The F0 of trills and chevrons, which were produced less often, also increased in heliox compared with air (trills, air: 49.9 ± 4.6 kHz, heliox: 70.8 ± 11.7 kHz; paired t-test, t5 = 4.9, p = 0.004; chevron, air: 46.7 ± 4.5 kHz, heliox: 64.2 ± 7.0 kHz; paired t-test, t4 = 8.9, p > 0.001; figure 3). The results suggest that USVs are produced by an aerodynamic whistle mechanism, whereby airflow passes through the glottis, enters the supraglottal space, and impinges on a downstream structure [10,12,13]. By contrast, the low-amplitude version of the long-distance call and agonistic barks did not shift in frequency (figure 3), suggesting an airflow-induced tissue vibration mechanism [9]. In summary, animals appear capable of switching between two production mechanisms within the same social interaction.
Figure 3.
Ultrasonic vocalizations emitted during mating bouts are produced via an aerodynamic whistle mechanism. (a) Call types include frequency downsweeps and trills. Sound is depicted as a waveform (top panel; relative dB level) and a spectrogram (bottom panel). (b) Mean fundamental frequency (F0) of frequency downsweeps in air and heliox. (c) F0 of trills, low-amplitude calls, and agonistic barks (female) in air and heliox. Error bars indicate standard deviation. ‘Expected in heliox’ refers to the predicted frequency estimated from the frequency response of a whistle (see Material and methods).
(b). Laryngeal histology and vocal tract geometry
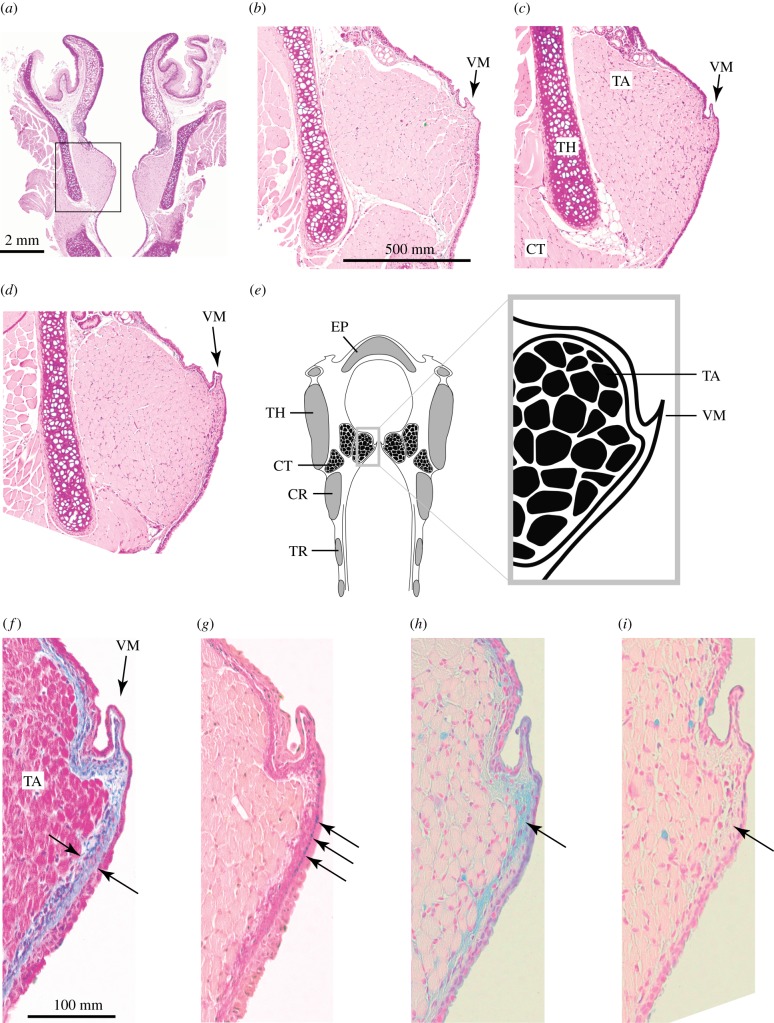
In order to better understand the vocal production mechanism in grasshopper mice, we investigated the anatomy of the laryngeal sound source and geometry of the vocal tract filter. Analysis of coronal sections from four grasshopper mice larynges revealed that vocal folds are composed of thyroarytenoid muscle, lamina propria and epithelium (figure 4). In grasshopper mice, the lamina propria is composed of a 19.7 µm (s.d. = 1.5 µm) thin layer of protein fibres, which are shaped into vocal membranes at the vibrating edge (figure 4a–e). Vocal membranes were symmetrical on the left and right vocal fold. Their length was measured by adding 5 µm coronal sections. The mean dorsoventral length of vocal membranes is 570 µm (s.d. = 90 µm) (O. arenicola: 660 and 610 µm; O. torridus: 480 and 510 µm). Histological preparations can cause some shrinkage of soft tissue (approx. 20% in laryngeal tissue according to Kimura et al. [36]), making a mean vocal membrane length of approximately 600 µm plausible.
Figure 4.
Grasshopper mouse larynx reveals vocal membranes. (a) Coronal cross section of a larynx. (b–d) Higher magnification images (from the square in (a) of three male mice vocal fold cross sections (H&E stain). On the medial edge of both vocal folds, all mice had vocal membranes consisting of a double layer of epithelium with a small amount of connective tissue between the epithelial layers. (e) Schematic of a grasshopper mouse larynx. (f,g) Lamina propria of vocal membranes contains collagen (blue stain in f), elastin (black stain in g) and hyaluronan (blue stain in h). (i) Removal of hyaluronan by hyaluronidase digestion with subsequent AB staining. CR, cricoid cartilage; CT, cricothyroid muscle; EP, epiglottis; TA, thyroarytenoid muscle; TH, thyroid cartilage; TR, tracheal rings; VM, vocal membrane. (Online version in colour.)
The lamina propria consists of collagen fibres (figure 4f), a thin layer of elastin fibres below the epithelium (figure 4g), and hyaluronan (figure 4h,i). The removal of hyaluronan by hyaluronidase digestion with subsequent AB staining indicated that much of the positive stain in the vocal membrane was attributed to hyaluronan (figure 4h,i).
We studied the geometry of oral and pharyngeal cavity in additional male specimens of O. leucogaster (n = 2) and O. arenicola (n = 1) by dissecting the thorax and injecting dental cast into the trachea and upper respiratory tract. Neck, head and mouth were positioned to resemble live animals with erect posture and wide mouth gape during long-distance vocalization (figure 5). Once the cast was solidified, specimens were deep-frozen and sectioned in the sagittal plane (figure 5). The total length of the oral and pharyngeal cavity measured between vocal folds and upper incisive was 20.3 ± 1.1 mm (mean ± s.d.; n = 3). The first segment of the vocal tract (hereafter ‘segment 1’) was narrow and tube-like (1 mm diameter) and measured 7.0 ± 1.0 mm (mean ± s.d.) in length. The second segment (segment 2) resembled a flared tube, with a diameter of 1 mm at the base and a 5 mm diameter at its opening, and a length of 13 mm.
Figure 5.
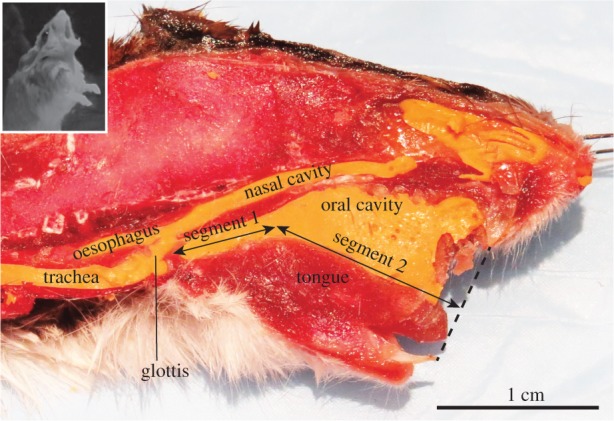
Adult males produce a long-distance call in an upright posture with wide-open mouths (inset). Midsagittal section of a grasshopper mouse specimen. The upper respiratory tract (trachea, part of oesophagus, nasal and oral cavity) are filled with yellow dental cast. Note that the vocal tract is narrow and uniform between the glottis and a point in the oral cavity about 7 mm upstream (segment 1). The remainder of the vocal tract (segment 2) widens like the bell of a horn from 1 mm to approximately 5 mm in diameter. (Online version in colour.)
(c). Computational simulation of the sound production mechanism
(i). The sound source
The two-mass model of vocal fold vibration (figure 6a) was simulated for grasshopper mice and house mice (Mus; see the electronic supplementary material for details) to determine the phonation threshold pressure (PTP) and F0 with varying Young's moduli. The lamina propria of a house mouse vocal fold is 80–100 µm thick mediolaterally and does not possess vocal membranes [37]. F0 at PTP is illustrated in a two-dimensional graph, wherein vocal production is realized only above the threshold line (figure 6b). In house mice, PTPs between 0.4 and 1.0 kPa are required to produce 1–5 kHz vocalizations. Interestingly, thinner lamina propria allows grasshopper mice to produce much higher frequency (10–17 kHz) calls with similar PTPs (0.5–1.2 kPa). The presence of vocal membranes increases glottal efficiency by reducing the PTP required to initiate phonation (figure 6b).
Figure 6.
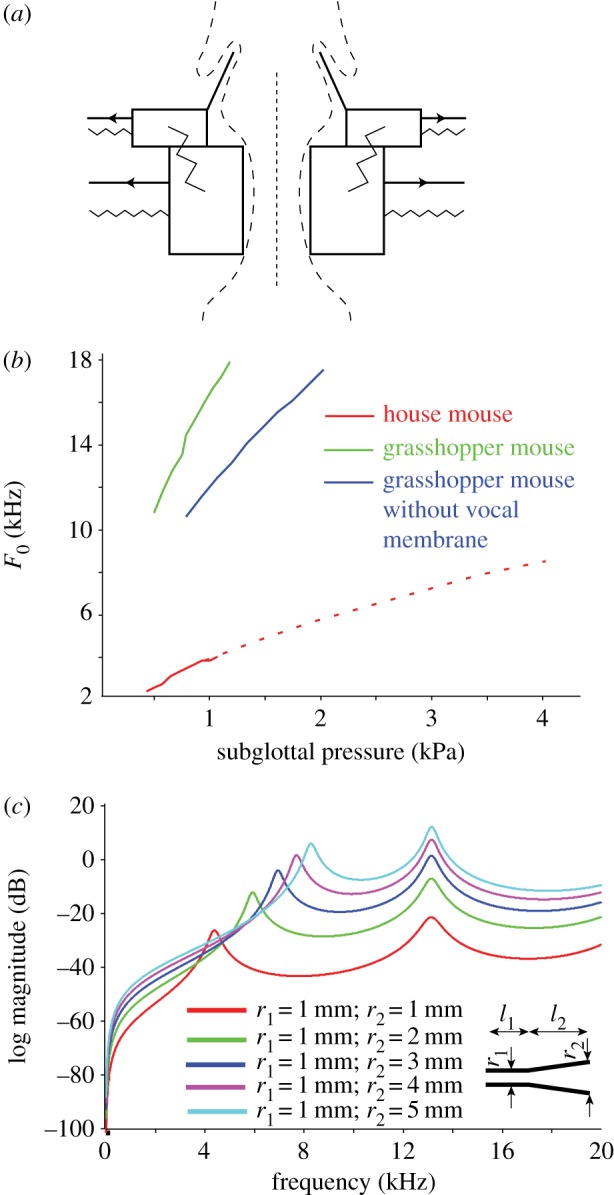
Sound production mechanism models in grasshopper mice (Onychomys). (a) Schematic of vocal membrane model. (b) Phonation threshold pressures (PTP) for house mouse and grasshopper mouse phonation. Note that the smaller lamina propria facilitates higher fundamental frequencies in grasshopper mice (blue line) than in house mice (red line). The dotted red line extrapolates the PTPs theoretically needed for house mice to produce high-frequency calls typical of Onychomys. The vocal membranes (green line) increase glottal efficiency by reducing the lung pressure required to initiate phonation. (c) Vocal tract models ranging between a uniform tube and a tube with bell-shaped mouth opening. The latter simulates a mouse producing a long-distance call with a wide-open mouth (figure 5). Relative radiated power level as a function of frequency, for a constant input volume flow at the larynx, with a vocal tract shape ranging from a uniform tube to vocal tract with flared bell-shaped opening. r1, radius of the vocal tract; l, length of segments 1 and 2 of the vocal tract; l1 = 7 mm and l2 = 13 mm; r1 = 1 mm for all experiments; r2 is flared and was altered from 1 to 5 mm. (Online version in colour.)
(ii). The vocal tract filter
A 20 mm long vocal tract (segment 1 + segment 2) shaped like a uniform tube with a 1 mm inner diameter generates resonances at 4.3 and approximately 13 kHz (red line in figure 6c). If segment 2 is modelled as a flared tube with a 1 mm diameter at the transition between segment 1 and 2, and an increasing diameter towards the open end, the first resonance increases to 8 kHz and the second remains close to 13 kHz (figure 6c). The simulation also suggests that the flared mouth opening radiates approximately 30 dB more sound power at the second formant than a uniform tube (figure 6c).
4. Discussion
Conspicuous acoustic displays are often matched by striking morphological innovations underlying vocal production [1]. As a consequence of their wide-ranging predatory lifestyle, grasshopper mice have evolved long-distance calls to advertise their presence over large distances. We found that long-distance call production is aided by laryngeal and supralaryngeal adaptations, including the presence of vocal membranes. In addition, we found that grasshopper mice alternate between an airflow-induced tissue vibration mechanism and a whistle mechanism to produce audible and USVs, respectively. We discuss our findings in relation to dynamic changes in motor control of vocal production necessary to produce distinct call types. We then consider functional adaptations of the vocal apparatus that promote energetic efficiency and social factors driving the evolution of long-distance signalling.
(a). Vocal motor control of distinct production mechanisms
Although production of audible vocalizations in rodents is well-documented in aversive contexts (Rattus; [38]; pine voles, Microtus pinetorum: [39]; Mongolian gerbil, Meriones unguiculatus: [40]; house mice: [41]) and more recently described in prosocial contexts ([18,19]; herein), the underlying production mechanisms are unknown. Our heliox experiments indicate that loud long-distance calls, softer pure tones and agonistic barks are all produced by an airflow-induced tissue vibration mechanism, whereas USVs are produced by a whistle mechanism. Alternation between two production mechanisms, in some cases within the same mating bout, probably necessitates recruitment of distinct motor programmes. For example, frequency patterns of rat USVs precisely match laryngeal muscle activation, requiring call-type specific motor programmes [14]. Furthermore, USVs are generated with lower subglottal pressures (2 kPa) than audible vocalizations produced by airflow-induced vibration of the vocal folds (5 kPa) in rats [11]. At the very least, grasshopper mice appear to dynamically adjust motor control of subglottal pressure to produce discrete call types. Further experimentation is needed to assess the boundaries and degrees to which laryngeal motor patterns are conserved or recombined to generate acoustic diversity [42,43].
(b). Adaptations for high vocal intensity facilitating long-distance communication
In general, lung pressure and morphological features within and above the larynx mediate the intensity of laryngeal vocal signals. Although we do not have empirical measures of lung pressure in grasshopper mice, we found two intralaryngeal adaptations that probably promote loud, high-frequency calls. First, grasshopper mouse vocal folds are composed of thin lamina propria (approx. 20 µm) compared with similarly sized (20–40 g) house mice (80–100 µm; [37]). Thin lamina propria enables faster tissue vibration, thus permitting higher F0. Indeed, the highest F0 for spontaneously produced audible calls in Mus (6 kHz; [44]) is much lower than grasshopper mice (11–14 kHz; table 1). Assuming identical vocal fold tissue density and viscoelasticity between Mus and Onychomys, lamina propria thickness probably accounts for the observed differences in F0. The degree to which lamina propria size contributes to species differences in call F0 within Onychomys awaits further investigation.
Second, grasshopper mice possess vocal membranes, or thin narrow upward extensions on the medial surface of the vocal fold. Vocal membranes have evolved independently in at least four mammalian orders (bats: [45]; non-human primates: [46]; cats: [47]; rodents: this study) and are associated with production of high F0 at high intensities. Similar to Mergell et al. [48], our model simulations confirm that vocal membranes increase glottal efficiency by lowering phonation threshold pressures, particularly at high frequencies [49,50]. Airborne acoustic signals are notorious for being energetically inefficient owing to heat loss and weak coupling between the sound source and the environment [51]. Thus, vocal membranes appear to be a common solution to producing high-frequency sounds with less effort, adding to other laryngeal innovations that facilitate specific acoustic objectives (e.g. fibrous mass in frogs: [52,53]; vocal fold composition in songbirds: [54]).
Finally, grasshopper mice possess supralaryngeal adaptations that facilitate loud calls. Grasshopper mice assume a conspicuous posture whereby the head is raised and the mouth is widely opened to produce a call that can purportedly travel in excess of 100 m [55]. Empirical and modelling results suggest that F0 of long-distance calls is tuned to the second resonance of the vocal tract (formant tuning), and that the bell-shaped mouth opening increases vocal intensity. The contribution of the mouth opening theoretically amplifies the signal by close to 30 dB, in accordance with empirical measures of upright calls (∼95 dB sound pressure level re: 20 µPa at 33 cm) and softer mating calls produced while prone with little mouth opening (∼52 dB; B. Pasch 2017, unpublished data). Such formant tuning is a strategy to adjust one of the vocal tract's lower resonances to match one of the lower harmonics. Songbirds, for example, tune the first vocal tract resonance to F0 during their songs by elaborate movements of the oropharyngeal–oesophageal cavity [8,14]. Similarly, human singers adjust the second or third resonance to the second or third harmonic while singing high notes [56,57]. Anatomical specializations of the head and neck associated with a predatory, carnivorous lifestyle may confer grasshopper mice with greater flexibility in mouth opening [58,59].
Simulation of the mouse's vocal tract transfer function also suggests increased radiation efficiency for frequencies between 8 and 15 kHz caused by a flare-shaped mouth. The amplifying effect of a bell-shaped mouth opening is based on improved coupling between the sound source and air, a phenomenon well-documented in horn acoustics, loudspeaker design [60], and mouth opening and lip configuration of singing humans [61]. The flared resonance chamber transfers higher frequencies better than lower frequencies [60]. A similar effect occurs in crickets [62], frogs [63] and bats [64] that exploit bell-shaped structures in the environment to improve sound propagation. Finer scale recordings coupled with detailed modelling efforts are needed to better quantify radiation efficiency.
In summary, our findings suggest that optimization of vocal fold morphology is associated with adjustments to vocal tract resonance to facilitate long-distance signalling. Comparative analyses across a wider range of vocal rodents of different body sizes and vocal tract lengths will clarify the relative importance of peripheral mechanisms in the evolution of acoustic displays. We predict that specializations for long-distance vocalizations are associated with morphological adaptations in the vocal apparatus to increase intensity, while USVs used in mating favour complexity arising from central control of breathing and laryngeal movements [65].
(c). Evolution of production mechanisms for long-distance communication in muroid rodents
Although USV production in close-distance social contexts is ubiquitous among muroid rodents [66–69], lower frequency audible vocalizations are common in at least one subfamily (Neotominae; [18,70]). What processes catalysed the evolution of such vocalizations? One hypothesis posits that USV production confers immunity from acoustically orienting predators [71,72], and evolutionary release from predators permitted production of lower frequencies. However, many mammalian predators are sensitive to frequencies of more than 50 kHz [73,74], and reptilian and avian predators can hear up to 5 and 10 kHz, respectively [75]. Thus, while ultrasonic signalling probably confers senders a relatively private channel owing to the high directionality, scattering and attenuation [13,76,77], factors other than predation probably contribute to the origin of long-distance audible vocalizations [78].
Advertisement vocalizations often function to mediate both intra- and intersexual interactions, implicating social structure and spatial organization as critical factors in the evolution of long-distance calls [1]. In non-human primates, the evolution of loud calls is associated with resource and/or mate defence and mate attraction [79]. Moreover, call F0 and the active space (carrying distance) of vocalizations are strongly associated with home range size [79,80]. Indeed, home ranges of grasshopper mice are five to nine times larger than predicted by body mass [81,82]. Wide spacing among individuals, either owing to resource availability and/or strong territoriality, necessitates a mechanism to facilitate detection of mates and/or competitors over large distances. Under this scenario, selection acted on small vocal organs to produce high intensity signals that propagate far distances. Indeed, biomechanical models suggest that sound pressure level increases with F0 at rates up to 10 dB per octave provided that lung pressure is raised proportionally [61,83]. Comparative analyses that explore the rich diversity of rodent social and spatial organization in relation to sound production mechanisms will help clarify the adaptations enabling this extraordinary mode of communication.
Supplementary Material
Acknowledgements
We thank Haley Szczublewski and Mandi Johnson for assistance with animal care, Aubrey Funke for initial exploratory histological preparations, and Polly Campbell and two anonymous reviewers for constructive feedback on the manuscript.
Ethics
Founder animals were captured with a permit from the New Mexico Department of Game and Fish (no. 3562) and all procedures were approved by the Institutional Animal Care and Use Committees at Northern Arizona University (no. 16-001) and Midwestern University, Glendale, AZ, USA (no. 2478).
Data accessibility
Data are available from Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.8m70g [84].
Authors' contributions
B.P., I.T.T. and T.R. conceived and designed the study. B.P. and T.R. acquired the acoustic and morphological data. I.T.T. developed the biomechanical model. B.P., I.T.T. and T.R. analysed and interpreted the data. B.P., I.T.T. and T.R. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by Northern Arizona University and Midwestern University, Glendale, AZ, USA.
References
- 1.Bradbury JW, Vehrencamp SL. 2011. Principles of animal communication, 2nd edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 2.Wilkins MR, Seddon N, Safran RJ. 2013. Evolutionary divergence in acoustic signals: causes and consequences. Trends. Ecol. Evol. 28, 156–166. ( 10.1016/j.tree.2012.10.002) [DOI] [PubMed] [Google Scholar]
- 3.Morton ES. 1975. Ecological sources of selection on avian sounds. Am. Nat. 109, 17–34. ( 10.1086/282971) [DOI] [Google Scholar]
- 4.Ryan MJ, Cocroft RB, Wilczynski W. 1990. The role of environmental selection in intraspecific divergence of mate recognition signals in the cricket frog, Acris crepitans. Evolution 44, 1869–1872. ( 10.1111/j.1558-5646.1990.tb05256.x) [DOI] [PubMed] [Google Scholar]
- 5.Brown CB, Gomez R, Waser PM. 1995. Old world monkey vocalizations: adaptation to the local habitat? Anim. Behav. 50, 945–961. ( 10.1016/0003-3472(95)80096-4) [DOI] [Google Scholar]
- 6.Seddon N. 2005. Ecological adaptation and species recognition drives vocal evolution in Neotropical suboscine birds. Evolution 59, 200–215. ( 10.1111/j.0014-3820.2005.tb00906.x) [DOI] [PubMed] [Google Scholar]
- 7.Podos J. 2001. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature 409, 185–188. ( 10.1038/35051570) [DOI] [PubMed] [Google Scholar]
- 8.Riede T, Suthers RA, Fletcher N, Blevins W. 2006. Songbirds tune their vocal tract to the fundamental frequency of their song. Proc. Natl Acad. Sci. USA 103, 5543–5548. ( 10.1073/pnas.0601262103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Titze IR. 2000. Principles of voice production. Iowa City, IA: National Center for Voice and Speech. [Google Scholar]
- 10.Roberts LH. 1975. The rodent ultrasound production mechanism. Ultrasonics 13, 83–88. ( 10.1016/0041-624X(75)90052-9) [DOI] [PubMed] [Google Scholar]
- 11.Riede T. 2011. Subglottal pressure, tracheal airflow and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J. Neurophysiol. 106, 2580–2592. ( 10.1152/jn.00478.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahrt E, Agarwal A, Perkel D, Portfors C, Elemans CP. 2016. Mice produce ultrasonic vocalizations by intra-laryngeal planar impinging jets. Curr. Biol. 26, R880–R881. ( 10.1016/j.cub.2016.08.032) [DOI] [PubMed] [Google Scholar]
- 13.Brudzynski SM, Fletcher NH. 2010. Rat ultrasonic vocalization: shortrange communication. In Handbook of mammalian vocalization. An integrative neuroscience approach (ed. Brudzynski SM.), pp. 69–76. Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 14.Riede T. 2013. Call type specific motor patterns in rat ultrasound vocalization. J. Exp. Zool. A 319, 213–224. ( 10.1002/jez.1785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sirotin YB, Costa ME, Laplagne DA. 2014. Rodent ultrasonic vocalizations are bound to active sniffing behavior. Front. Behav. Neurosci. 8, 1–12. ( 10.3389/fnbeh.2014.00399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff JO, Sherman PW. 2007. Rodent societies as model systems. In Rodent societies: an ecological and evolutionary approach (eds Wolff JO, Sherman PW), pp. 3–7. Chicago, IL: University of Chicago Press. [Google Scholar]
- 17.Merriam CH. 1894. A new subfamily of murine rodents—the Neotominae—with description of a new genus and species and a synopsis of the known forms. Proc. Phila. Acad. Sci., 46, 225–252. [Google Scholar]
- 18.Miller JR, Engstrom MD. 2012. Vocal stereotypy in the rodent genera Peromyscus and Onychomys (Neotominae): taxonomic signature and call design. Bioacous 21, 193–213. ( 10.1080/09524622.2012.675176) [DOI] [Google Scholar]
- 19.Pasch B, George AS, Campbell P, Phelps SM. 2011. Androgen-dependent male vocal performance influences female preference in neotropical singing mice. Anim. Behav. 82, 177–183. ( 10.1016/j.anbehav.2011.04.018) [DOI] [Google Scholar]
- 20.Bailey V, Sperry CC. 1929. Life history and habits of the grasshopper mice, genus Onychomys. U.S. Dep. Agric. Tech. Bull. 145, 1–19. [Google Scholar]
- 21.Hafner MS, Hafner DJ. 1979. Vocalizations of grasshopper mice (genus Onychomys). J. Mamm. 60, 85–94. ( 10.2307/1379761) [DOI] [Google Scholar]
- 22.Pasch B, Abbasi MZ, Wilson M, Zhao D, Searle JB, Webster MS, Rice AN. 2016. Cross-fostering alters advertisement vocalizations of grasshopper mice (Onychomys): evidence for the developmental stress hypothesis. Physiol. Behav. 157, 265–269. ( 10.1016/j.physbeh.2016.02.012) [DOI] [PubMed] [Google Scholar]
- 23.Hess DR, Fink JB, Venkataraman ST, Kim IK, Myers TR, Tano BD. 2006. The history and physics of heliox. Respir. Care 51, 608–612. [PubMed] [Google Scholar]
- 24.Spencer ML, Titze IR. 2001. An investigation of a modal-falsetto register transition hypothesis using helox gas. J. Voice 15, 15–24. ( 10.1016/S0892-1997(01)00003-0) [DOI] [PubMed] [Google Scholar]
- 25.Titze IR. 2006. The myoelastic-aerodynamic theory of phonation. Salt Lake City, UT: National Center for Voice and Speech. [Google Scholar]
- 26.McCarty R. 1975. Onychomys torridus. Mamm. Spp. 59, 1–5. ( 10.2307/3503863) [DOI] [Google Scholar]
- 27.Sullivan RM, Hafner DJ, Yates TL. 1986. Genetics of a contact zone between three chromosomal forms of the grasshopper mouse (genus Onychomys): a reassessment. J. Mamm. 67, 640–659. ( 10.2307/1381126) [DOI] [Google Scholar]
- 28.Gray SD, Titze IR, Alipour F, Hammond TH. 2000. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann. Otol. Rhinol. Laryngol. 109, 77–85. ( 10.1177/000348940010900115) [DOI] [PubMed] [Google Scholar]
- 29.Chan RW, Gray SD, Titze IR. 2001. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol. Head Neck Surg. 124, 607–614. ( 10.1067/mhn.2001.115906) [DOI] [PubMed] [Google Scholar]
- 30.Hahn MS, Kobler JB, Starcher BC, Zeitels SM, Langer RS. 2006. Quantitative and comparative studies of the vocal fold extracellular matrix. I: elastic fibers and hyaluronic acid. Ann. Otol. Rhinol. Laryngol. 115, 156–164. ( 10.1177/000348940611500213) [DOI] [PubMed] [Google Scholar]
- 31.Riede T, Li Z, Tokuda IT, Farmer C. 2015. Anatomy and mechanical properties of the Alligator mississipiensis larynx. J. Exp. Biol. 218, 991–998. ( 10.1242/jeb.117101) [DOI] [PubMed] [Google Scholar]
- 32.Ishizaka K, Flanagan JL. 1972. Synthesis of voiced sounds from a two-mass model of the vocal cords. Bell. Syst. Tech. J. 51, 1233–1268. ( 10.1002/j.1538-7305.1972.tb02651.x) [DOI] [Google Scholar]
- 33.Steinecke I, Herzel H. 1995. Bifurcations in an asymmetric vocal fold model. J. Acoust. Soc. Am. 97, 1874–1884. ( 10.1121/1.412061) [DOI] [PubMed] [Google Scholar]
- 34.Sohndi MM, Schroeter J. 1987. A hybrid time-frequency domain articulatory speech synthesizer. IEEE Trans. Acoust. Speech Signal Process. 35, 955–967. ( 10.1109/TASSP.1987.1165240) [DOI] [Google Scholar]
- 35.Smotherman M. 2007. Sensory feedback control of mammalian vocalizations. Behav. Brain Res. 182, 315–326. ( 10.1016/j.bbr.2007.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura M, Tayama N, Chan RW. 2003. Geometrical deformation of vocal fold tissue induced by formalin fixation. Laryngoscope 113, 607–613. ( 10.1097/00005537-200304000-00005) [DOI] [PubMed] [Google Scholar]
- 37.Yamashita M, Bless DM, Welham NV. 2010. Morphological and extracellular matrix changes following vocal fold injury in mice. Cells Tissues Organs 192, 262–271. ( 10.1159/000315476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson JW. 1954. The production of ultrasonic sounds by laboratory rats and other mammals. Science 119, 808–809. ( 10.1126/science.119.3101.808) [DOI] [PubMed] [Google Scholar]
- 39.Cherry JA, Lepri JJ. 1986. Sexual dimorphism and gonadal control of ultrasonic vocalizations in adult pine voles, Microtus pinetorum. Horm. Behav. 20, 34–48. ( 10.1016/0018-506X(86)90027-9) [DOI] [PubMed] [Google Scholar]
- 40.Kobayasi KI, Riqimaroux H. 2012. Classification of vocalizations in the Mongolian gerbil, Meriones unguiculatus. J. Acoust. Soc. Am. 131, 1622–1631. ( 10.1121/1.3672693) [DOI] [PubMed] [Google Scholar]
- 41.Grimsley JM, Sheth S, Vallabh N, Grimsley CA, Bhattal J, Latsko M, Jasnow A, Wenstrup J. 2016. Contextual modulation of vocal behavior in mouse: newly identified 12 kHz ‘mid-frequency’ vocalization emitted during restraint. Front. Behav. Neurosci. 10, 38 ( 10.3389/fnbeh.2016.00038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wetzel DM, Kelley DB, Campbell BA. 1980. Central control of ultrasonic vocalization in neonatal rats. I. Brain stem motor nuclei. J. Comp. Physiol. Psychol. 94, 596–605. ( 10.1037/h0077699) [DOI] [PubMed] [Google Scholar]
- 43.Jürgens U. 2009. The neural control of vocalization in mammals: a review. J. Voice 23, 1–10. ( 10.1016/j.jvoice.2007.07.005) [DOI] [PubMed] [Google Scholar]
- 44.Grimsley JM, Monaghan JJ, Wenstrup JJ. 2011. Development of social vocalizations in mice. PLoS ONE 6, e17460 ( 10.1371/journal.pone.0017460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suthers RA, Fattu JM. 1973. Mechanisms of sound production by echolocating bats. Am. Zool. 13, 1215–1226. ( 10.1093/icb/13.4.1215) [DOI] [Google Scholar]
- 46.Schön-Ybarra MA. 1995. A comparative approach to the nonhuman primate vocal tract: implications for sound production. In Current topics in primate vocal communication (eds Zimmermann E, Newman J, Jürgens U), pp. 185–198. New York, NY: Plenum. [Google Scholar]
- 47.Hast M. 1989. The larynx of roaring and non-roaring cats. J. Anat. 163, 117–121. [PMC free article] [PubMed] [Google Scholar]
- 48.Mergell P, Fitch WT, Herzel H. 1999. Modeling the role of non-human vocal membranes in phonation. J. Acoust. Soc. Am. 105, 2020–2028. ( 10.1121/1.426735) [DOI] [PubMed] [Google Scholar]
- 49.Titze IR. 1989. On the relation between subglottal pressure and fundamental frequency in phonation. J. Acoust. Soc. Am. 85, 901–906. ( 10.1121/1.397562) [DOI] [PubMed] [Google Scholar]
- 50.Titze IR. 1992. Phonation threshold pressure: a missing link in glottal aerodynamics. J. Acoust. Soc. Am. 91, 2926–2935. ( 10.1121/1.402928) [DOI] [PubMed] [Google Scholar]
- 51.Ryan MJ, Kime NM. 2003. Selection on long distance acoustic signals. In Springer handbook of auditory research; acoustic communication (eds Simmons A, Fay R, Popper A), pp. 225–274. Berlin, Germany: Springer Verlag. [Google Scholar]
- 52.Ryan MJ, Drewes RC. 1990. Vocal morphology of the Physalaemus pustulosus species group (Family Leptodactylidae): morphological response to sexual selection for complex calls. Biol. J. Linn. Soc. 40, 37–52. ( 10.1111/j.1095-8312.1990.tb00533.x) [DOI] [Google Scholar]
- 53.Griddi-Papp M, Rand AS, Ryan MJ. 2006. Complex call production in túngara frogs. Nature 441, 38 ( 10.1038/441038a) [DOI] [PubMed] [Google Scholar]
- 54.Riede T, Goller F. 2014. Morphological basis for the evolution of acoustic diversity in oscine songbirds. Proc. R. Soc. B 281, 20132306 ( 10.1098/rspb.2013.2306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langley WM. 2008. Grasshopper mouse: evolution of a carnivorous lifestyle. Raleigh, NC: Lulu Press. [Google Scholar]
- 56.Schutte HK, Miller DG, Duijnstee M. 2005. Resonance strategies revealed in recorded tenor high notes. Folia Phoniatr. Logop. 57, 292–307. ( 10.1159/000087082) [DOI] [PubMed] [Google Scholar]
- 57.Sundberg J, La FMB, Gill BP. 2013. Formant tuning strategies in professional male opera singers. J. Voice 27, 278–288. ( 10.1016/j.jvoice.2012.12.002) [DOI] [PubMed] [Google Scholar]
- 58.Satoh K, Iwaku F. 2006. Jaw muscle functional anatomy in northern grasshopper mouse, Onychomys leucogaster, a carnivorous murid. J. Morphol. 267, 987–99. ( 10.1002/jmor.10443) [DOI] [PubMed] [Google Scholar]
- 59.Williams SH, Pfeiffer E, Ford S. 2009. Gape and bite force in the rodents Onychomys leucogaster and Peromyscus maniculatus: does jaw-muscle anatomy predict performance? J. Morphol. 270, 1338–1347. ( 10.1002/jmor.10761) [DOI] [PubMed] [Google Scholar]
- 60.Kolbrek B.2008. Horn theory: an introduction part 1 & 2. See www.audioxpress.com .
- 61.Titze IR, Sundberg J. 1992. Vocal intensity in speakers and singers. J. Acoust. Soc. Am. 91, 2936–2946. ( 10.1121/1.402929) [DOI] [PubMed] [Google Scholar]
- 62.Bennet-Clark HC. 1987. The tuned singing burrow of mole crickets. J. Exp. Biol. 128, 383–409. [Google Scholar]
- 63.Lardner B, bin Lakim M. 2002. Animal communication: tree-hole frogs exploit resonance effects. Nature 420, 475 ( 10.1038/420475a) [DOI] [PubMed] [Google Scholar]
- 64.Chaverri G, Gillam EH. 2013. Sound amplification by means of a horn-like roosting structure in Spix's disc-winged bat. Proc. R. Soc. B 280, 20132362 ( 10.1098/rspb.2013.2362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riede T. 2014. Rat ultrasonic vocalization shows features of a modular behavior. J. Neurosci. 34, 6874–6878. ( 10.1523/JNEUROSCI.0262-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holy TE, Guo Z. 2005. Ultrasonic songs of male mice. PLOS Biol. 3, e386 ( 10.1371/journal.pbio.0030386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalcounis-Rüppell MC, Metheny JD, Vonhof MJ. 2006. Production of ultrasonic vocalizations by Peromyscus mice in the wild. Front. Zool. 3, 3 ( 10.1186/1742-9994-3-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musolf K, Hoffmann F, Penn DJ. 2010. Ultrasonic courtship vocalizations in wild house mice, Mus musculus. Anim. Behav. 79, 757–764. ( 10.1016/j.anbehav.2009.12.034) [DOI] [PubMed] [Google Scholar]
- 69.Sales GD. 2010. Ultrasonic calls of wild and wild-type rodents. In Handbook of mammalian vocalization: an integrative neuroscience approach (ed. Brudzynski SM.), pp. 77–88. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 70.Campbell P, Pasch B, Pino JL, Crino OL, Phillips M, Phelps SM. 2010. Geographic variation in the songs of neotropical singing mice: testing the relative importance of drift and local adaptation. Evolution 64, 1955–1972. ( 10.1111/j.1558-5646.2010.00962.x) [DOI] [PubMed] [Google Scholar]
- 71.Blanchard RJ, Blanchard DC. 1989. Antipredator defensive behaviors in a visible burrow system. J. Comp. Psychol. 103, 70–82. ( 10.1037/0735-7036.103.1.70) [DOI] [PubMed] [Google Scholar]
- 72.Brudzynski SM. 2014. Social origin of vocal communication in rodents. In Biocommunication of animals (ed. Witzany G.), pp. 63–79. New York, NY: Springer. [Google Scholar]
- 73.Pasch B, Pino JL. 2013. The cost of advertisement: long-tailed weasels as potential acoustically-orienting predators of Neotropical singing mice. Southwest. Nat. 58, 320–324. ( 10.1894/0038-4909-58.3.363) [DOI] [Google Scholar]
- 74.Heffner HE, Heffner RS. 2016. The evolution of mammalian sound localization. Acoust. Today 12, 20–27. [Google Scholar]
- 75.Dooling RJ, Popper AN. 2000. Hearing in birds and reptiles: an overview. In Comparative hearing: birds and reptiles (eds Dooling RJ, Fay RR, Popper AN), pp. 1–12. New York, NY: Springer. [Google Scholar]
- 76.Wiley RH, Richards DG. 1978. Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav. Ecol. Sociobiol. 3, 69–94. ( 10.1007/BF00300047) [DOI] [Google Scholar]
- 77.Musolf K, Penn DJ. 2012. Ultrasonic vocalizations in house mice: a cryptic mode of acoustic communication. In Evolution of the house mouse (eds Macholan M, Baird SJE, Munclinger P, Pialek J), pp. 253–77. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 78.Arch VS, Narins PM. 2008. ‘Silent’ signals: selective forces acting on ultrasonic communication systems in terrestrial vertebrates. Anim. Behav. 76, 1423–1428. ( 10.1016/j.anbehav.2008.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wich SA, Nunn CL. 2002. Do male ‘long-distance calls’ function in mate defense? A comparative study of long-distance calls in primates. Behav. Ecol. Sociobiol. 52, 474–484. ( 10.1007/s00265-002-0541-8) [DOI] [Google Scholar]
- 80.Mitani JC, Stuht J. 1998. The evolution of nonhuman primate loud calls: acoustic adaptation for long-distance transmission. Primates 39, 171–182. ( 10.1007/BF02557729) [DOI] [Google Scholar]
- 81.Frank DH, Heske EJ. 1992. Seasonal changes in space use patterns in the southern grasshopper mice, Onychomys torridus torridus. J. Mamm. 73, 292–298. ( 10.2307/1382059) [DOI] [Google Scholar]
- 82.Stapp P. 1999. Size and habitat characteristics of home ranges of northern grasshopper mice (Onychomys leucogaster). Southwest. Nat. 44, 101–105. [Google Scholar]
- 83.Titze IR, Riede T. 2010. A cervid vocal fold model suggests greater glottal efficiency in calling at high frequencies. PLoS Comp. Biol. 6, e1000897 ( 10.1371/journal.pcbi.1000897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasch B, Tokuda IT, Riede T. 2017. Data from: Grasshopper mice employ distinct vocal production mechanisms in different social contexts. Dryad Digital Repository. ( 10.5061/dryad.8m70g) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Pasch B, Tokuda IT, Riede T. 2017. Data from: Grasshopper mice employ distinct vocal production mechanisms in different social contexts. Dryad Digital Repository. ( 10.5061/dryad.8m70g) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.8m70g [84].