Abstract
Although non-muscle invasive bladder cancer (NMIBC) is widely seen in men, most laboratory studies of new intravesical therapies to prevent NMIBC have been conducted on female animals. In addition, ozone (O3) has been shown to be a beneficial agent as an intravesical application in the treatment of various disorders. In the current study, we evaluated the immunohistopathological and oxidative-antioxidative effects of intravesical O3 treatment on n-methyl-n-nitrosourea (MNU)-induced NMIBC. Male Wistar-Albino rats (n=51) were divided into four groups: sham (n=6), O3 only (n=15), MNU only (n=15), and MNU+O3 (n=15). The MNU-only and MNU+O3 groups received MNU, and the O3-only group received saline every other week for 10 weeks. The MNU-only group received 1 ml saline in place of O3 treatment, whereas the O3-only and MNU+O3 groups were treated with 1 ml 25 µg/ml O3 between the 7th and 12th weeks. Rat bladders were collected in the 15th week for immunohistopathology and oxidant-antioxidant quantitation. Oxidant-antioxidant parameters were determined by ELISA. Although all surviving rats in the MNU-only group had preneoplastic (4/11, 36.4%) or neoplastic changes (7/11, 63.6%), a completely normal urothelium was observed in 2 rats (2/12, 16.7%) in the MNU+O3-group (P=0.478). More high-grade lesions were observed in the MNU-only group (4/11, 36.4%) than in the MNU+O3 group (1/12, 8.3%) (P=0.120). All oxidant-antioxidant parameters significantly increased (P<0.05) in the O3-only group compared with the sham group. However, only antioxidant superoxide dismutase was remarkably higher (178.9%, P=0.060) in the MNU+O3 group compared with the MNU-only group. This is the first methodologically and pathologically well-described male rat orthotopic bladder carcinogenesis model with intravesical MNU and administration of O3 in NMIBC.
Keywords: antioxidant response, bladder cancer model, intravesical ozone, male rat, MNU
Introduction
Tobacco smoking is the most important risk factor for bladder cancer (BC), accounting for approximately 50% of cases [4]. BC models chemically induced by N-butyl-N(4-hydroxybutyl)-nitrosamine (OH-BBN), N-[4-(5-nitro-2-furyl)-2-thiazolyl] formamide (FANFT), and n-methyl-n-nitrosourea (MNU) resemble human BC induced by tobacco smoking. Furthermore, animal models provide appropriate sources for the investigation of new promising intravesical or systemic agents for the treatment of BC. Although, women have a lower BC incidence rate than men [4], female rats are commonly used for new intravesical treatment modalities.
Antioxidant enzyme defects and elevated oxidative stress are associated with increased cancer incidence in animal cells. Studies showed that antioxidants such as vitamins A, C, and E play a role in preventing and delaying carcinogenesis [1]. The preventive characteristics of antioxidant agents in cancer treatment may be utilized intravesically on non-muscle invasive bladder cancer (NMIBC).
Ozone (O3) creates a dose-related antioxidative-oxidative response in tissue [3] and has been used as a therapeutic agent for the treatment of tissue necrosis, peritonitis, infected wounds, chronic skin ulcers, burns, and advanced ischemic diseases with local or systemic administration [29]. On the other hand, O3 has been shown to be beneficial as an intravesical agent in the treatment of bacterial [28], interstitial [16], and radiation cystitis [5] and has been shown to inhibit some cancer cells like breast and lung cancer cells in vitro [25].
The purposes of this research were to characterize MNU-induced NMIBC in male rats and to evaluate oxidative-antioxidative effects of intravesical O3 treatment in this model. In this study, the oxidants malondialdehyde (MDA), nitric oxide (NO), and myeloperoxidase (MPO) and antioxidants superoxide dismutase (SOD) and glutathione (GSH) were quantitated without and after intravesical O3 instillation therapy. To date, the oxidative-antioxidative effects of intravesical O3 instillation have not been evaluated in NMIBC.
Materials and Methods
Animals
Male Wistar-Albino rats (n=51) weighing 200–250 g were used in our experiment at 7–8 weeks of age. Animals were housed at a temperature of 21 ± 2°C with a 12-h dark-light cycle. They were fed a standard pellet diet and water ad libitum. The study was approved by the Animal Experiments Local Ethical Committee of Kocaeli University (25 March 2014, Number: 3/4–2014).
Experimental design and groups
Rats were randomized into four groups: sham, instillation with O3 only, MNU only, or MNU+O3. Anesthesia, cancer induction, and fluids used in the groups are shown in Table 1.
Table 1. Treatment schedules according to study groups.
| Group | Anesthesia | Carcinogen exposure | Used fluids |
|---|---|---|---|
| Sham (n=6) | Ketamine (90 mg/kg) Xylazine (10 mg/kg) |
None | None |
| O3 only (n=15) | Ketamine (90 mg/kg) Xylazine (10mg/kg) |
0.2 ml 0.9% NaCl at weeks 0, 2, 4, 6, 8, and 10¶ | 1 ml 25 µg/ml ozonated water twice a week at weeks 7, 8, 9, and 10 and three times a week at weeks 11 and 12 |
| MNU only (n=15) | Ketamine (90 mg/kg) Xylazine (10 mg/kg) |
2 mg MNU in 0.2 ml 0.9% NaCl at weeks 0, 2, 4, 6, 8, and 10 | 1 ml 0.9% NaCl twice a week at weeks 7, 8, 9, and 10 and three times a week at weeks 11 and 12§ |
| MNU+O3 (n=15) | Ketamine (90 mg/kg) Xylazine (10 mg/kg) |
2 mg MNU in 0.2 ml 0.9% NaCl at weeks 0, 2, 4, 6, 8, and 10 | 1 ml 25 µg/ml ozonated water twice a week at weeks 7, 8, 9, and 10 and three times a week at weeks 11 and 12 |
¶Vehicle control of MNU treatment. §Vehicle control of O3 treatment.
Cancer induction
MNU (Sigma-Aldrich, St. Louis, MO, USA) was used to chemically induce orthotopic bladder cancer. In the present study, 2 mg of MNU was dissolved in 0.2 ml 0.9% NaCl as proposed previously [10]. MNU instillation for cancer induction was performed every other week in weeks 0, 2, 4, 6, 8, and 10. Our dose and frequency were more than those in the protocols of Hicks and Wakefield [10] and Steinberg et al. [24]. Unlike females, bladder drainage in male rats can only be performed incompletely, which therefore results in a somewhat diluted drug concentration. In the present study, spontaneous micturition was generally observed around 20 min after instillation.
O3 generation and treatment schedule
O3 was generated by Ozonasan Photonik 1014® device (Dr. J. Hänsler GmbH, Iffezheim, Germany). Our calibrated ozonized water concentration was 25 µg/ml 0.9% NaCl as described previously [28]. After preparation, O3 solution was instilled immediately because of its short half-life and unstable structure [3]. Hyperplasia and dysplastic changes have been shown to start at week 6 [10], and urothelial carcinoma has been found at week 10 after MNU instillation [19]. For this reason, O3 instillation was started in the 7th week and maintained until the 12th week in this study. In weeks 7 to 10, O3 was instilled twice, and in the 11th and 12th weeks, it was instilled three times a week. MNU and O3 instillations were performed on separate days.
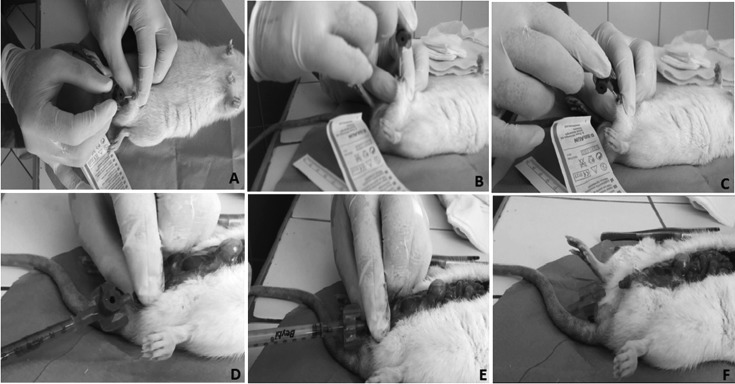
Anesthesia and male transurethral catheterization protocols (Fig. 1)
For anesthesia and erection prevention, 90 mg/kg ketamine (Ketalar®, Pfizer) and 10 mg/kg xylazine (Alfasan, Woerden, Netherlands) were injected intraperitoneally. All sedated rats were laid in a dorsal recumbency position, and the genital area was cleaned with 10% povidone iodine. The foreskin was retracted, penis was stretched and bent in the direction of the medulla spinalis to form a right angle, and the external urethral meatus was catheterized with a 22G 25 mm angio-catheter (Braun-Melsungen, Germany) (Figs. 1A and 1B). After catheter insertion, the penis was angled such that is was parallel to the medulla spinalis, and the catheter was advanced through the posterior urethra (Figs. 1C and 1D). The bladder was filled (Figs. 1E and 1F) with study fluids (with methylene blue in the figure). When optimal anesthesia is not achieved, a penile erection may prevent catheter insertion. In the case of a penile erection, an additional dose of 10 mg/kg ketamine should be administered. When the catheter was placed in the present study, the nondominant hand’s thumb and index fingers secured the catheter in position, and saline flow could be felt by the nondominant hand’s remaining fingers, which were placed on the suprapubic region.
Fig. 1.
Representation of instillation of methylene blue in alive male rat with its abdomen open (methylene-blue was used in this figure for demonstrative purposes). A, B) Angiocatheter insertion at a right angle. C, D) Angulation of the penis, maintenance of the angiocatheter parallel to the medulla spinalis, and advancement of the catheter through the posterior urethra. E) Methylene blue instillation shows the correct positioning of the angiocatheter. F) Methylene blue filled the bladder.
Sacrification and harvesting a cystectomy specimen
Sacrification of rats and cystectomy were performed in the 15th week. After removal, the bladder was randomly divided into two halves by longitudinal section from the dome to trigonum vesicae in the sagittal plane. One half of the specimen was placed in 10% formalin solution for immune-histopathological examination, and the other half was weighed and placed into liquid nitrogen to be stored at −80°C until biochemical evaluation.
Histopathological and immunodetection analyses
Tissue sections embedded in paraffin blocks were cut from the dome to trigone at 5 µm thickness. The sections were stained with hematoxylin-eosin (H-E). Lesions were described as normal, preneoplastic (hyperplasia, low-grade intraepithelial neoplasia [dysplasia]), and NMIBC (high-grade intraepithelial neoplasia [carcinoma in situ], pTa, pT1) [14].
For proliferative index analysis, the primary rabbit antibody Ki-67 (RB-9043-R7, Thermo Fisher Scientific, Waltham, MA, USA) was used. Ki-67 was diluted in 1% Bovine Serum Albumin and applied to sections overnight at 4°C. Ki-67 staining was done only as an adjunct to pathological diagnosis [18].
Biochemical (Enzyme-linked immunosorbent assay [ELISA]) analyses
MDA levels were measured in bladder tissue homogenates. A Sunred Biological Technology Rat MDA ELISA (SRB/Shanghai, China) kit was used for the ELISA assay. MDA results were expressed as nmol/mg protein in homogenate. NO concentrations were determined with a Northwest Life Science Specialties, LLC (Vancouver, WA, USA), ELISA kit, and tissue NO was expressed as nmol/mg protein. Tissue MPO activity was determined by ELISA assay with a Sunred Biological Technology (SRB/Shanghai, China) kit, and MPO activity was expressed as ng/mg protein in tissue. GSH levels were determined by the Ellman method [7]. A Sunred Biological Technology (SRB/Shanghai, China) GSH kit was used for the ELISA assay, and tissue GSH levels were expressed as µg/mg protein. SOD concentrations were determined by ELISA assay with a Cayman Chemical Co. (Ann Arbor, MI, USA) kit, and the amount of SOD in extract was reported as U/mg protein. The protein concentrations were determined with the Lowry method [13].
Statistical analyses
Results were expressed as median (interquartile range [IQR]) values. The Wilcoxon rank-sum (Mann-Whitney) test was used to compare two groups in continuous variables. All analyses were performed using Stata version 13.1 (StataCorp, College Station, TX, USA). A value of P<0.05 was accepted as statistically significant.
Results
Of the rats, 25.5% (n=13/51) were lost prematurely. All deaths were observed after anesthesia induction during the first six weeks of the study. At death, an autopsy was done for each rat. No pathologies were found that were considered the cause of death. So, the possible cause of death could have been anesthesia-related cardiopulmonary arrest. Because no deaths were observed after the start of the treatment weeks (7th week), we considered that intravesical O3 treatment had no additional toxic effects on rats. The premature death rates for the sham, O3-only, MNU-only, and MNU+O3 groups were 16.7% (n=1/6), 33.3% (n=5/15), 26.7% (n=4/15), and 20% (n=3/15), respectively.
Different stages of MNU-induced male rat bladder carcinogenesis were observed (Fig. 2). Histopathological results according to study groups are summarized in Table 2. At the end of the study, the urothelium was normal in all sham rats. Muscle invasive bladder cancer (MIBC) was not observed in any rat in the present study. In the O3-only group, hyperplasia was recorded in only one rat. This may have been because this rat had a stone at the trigonum vesicae. On the other hand, there was no normal urothelia (n=0/11) in the MNU-only group (all subjects in the MNU-only group had either preneoplastic or neoplastic lesions), but 16.7% (n=2/12) of the MNU+O3 group had a normal urothelium (P=0.478). In pairwise comparison between the MNU-only and MNU+O3 groups, the MNU-only group had three times more CIS than MNU+O3 (P=0.317). The MNU-only group had lower (36.4%) pTa or pT1 urothelial carcinoma rates compared with the MNU+O3 group (50%), but the difference was not statistically significant (P=0.680). When carcinoma grades were considered (Table 2), that rate of high-grade lesions was 36.4% (4/11; 1 pT1, 3 CIS) in the MNU-only group, but it was only 8.3% (1/12; 1 CIS) in the MNU+O3 group (P=0.120).
Fig. 2.
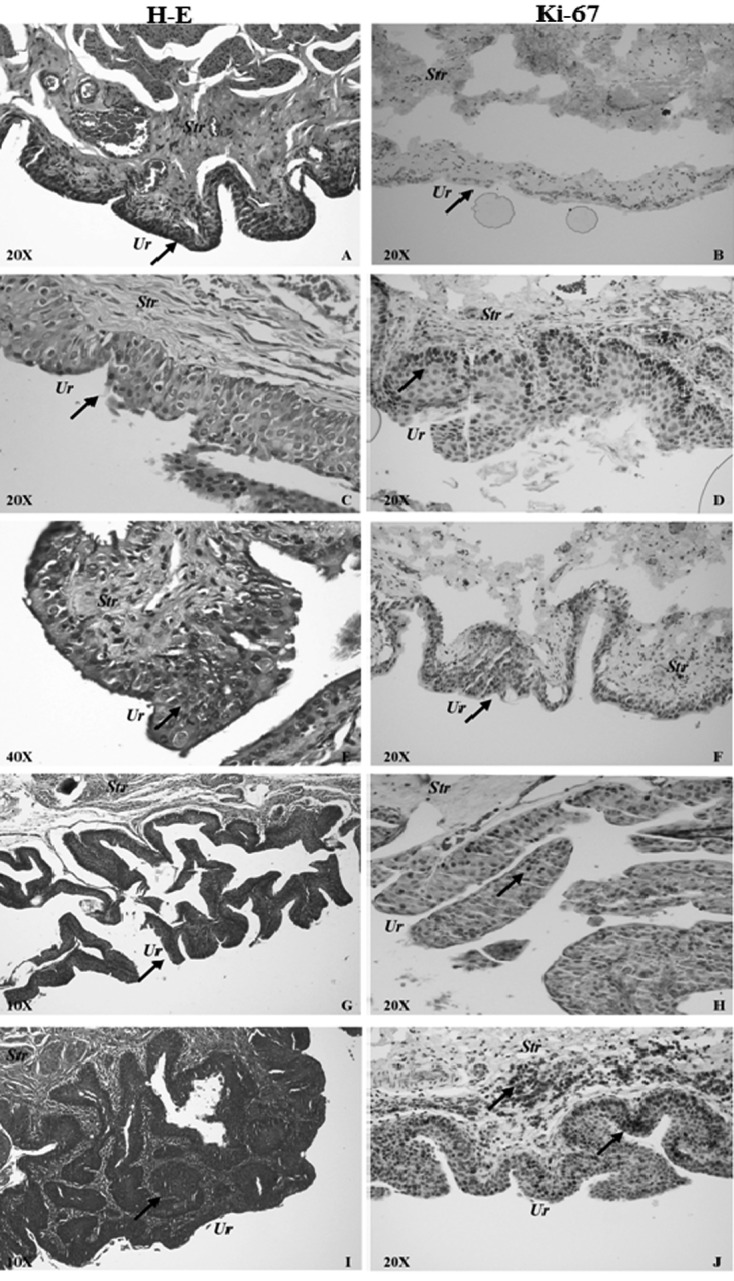
Normal urothelium and representative pictures of different stages of MNU-induced male rat bladder carcinogenesis in the MNU-only and MNU+O3 groups with H-E stain (left column) and Ki-67 immunolabeling (right column). A, B) Normal urothelium from the MNU+O3 group. Arrows show a normal urothelium. C, D) Dysplastic urothelium from the MNU+O3 group. Arrows show a dysplastic urothelium in cells stained with H-E (C) and Ki-67 (D). E, F) High-grade intraepithelial neoplasia (CIS) from the MNU-only group. Arrows show the CIS area (E) and CIS cells stained with Ki-67 (F). G, H) Low-grade pTa papillary urothelial carcinoma from the MNU+O3 group. Arrows show the pTa carcinoma area (G) and Ki-67 staining of it (H). I, J) High-grade pT1 papillary urothelial carcinoma from the MNU-only group. Arrows show the pT1 carcinoma area (I) and corresponding Ki-67 staining in the urothelium and submucosa (J). Str, Stroma; Ur, Urothelium.
Table 2. Histopathological results according to study groups (n=38) and distribution of high- and low-grade lesions in the MNU-only and MNU+O3 groups.
| MNU only (n=11) |
MNU+O3 (n=12) |
O3 only (n=10) |
Sham (n=5) | |
|---|---|---|---|---|
| Normal urothelium, n (%) | 0 (0) | 2 (16.7) | 9 (90) | 5 (100) |
| Preneoplastic lesions | ||||
| Hyperplasia, n (%) | 1 (9.1) | 1 (8.3) | 1 (10) | 0 (0) |
| Dysplasia / atypia (low-grade intraepithelial neoplasia), n (%) | 3 (27.3) | 2 (16.7) | 0 (0) | 0 (0) |
| NMIBC | ||||
| High-grade intraepithelial neoplasia (CIS), n (%) | 3 (27.3) | 1 (8.3) | 0 (0) | 0 (0) |
| Low-grade pTa papillary urothelial cancer, n (%) | 2 (18.2) | 3 (25.0) | 0 (0) | 0 (0) |
| Low-grade pT1 papillary urothelial cancer, n (%) | 2 (18.2) | 3 (25.0) | 0 (0) | 0 (0) |
| High-grade pT1 papillary urothelial cancer, n (%) | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) |
| All High-grade urothelial lesions (CIS, pTa, or pT1) | 4 (36.4) | 1 (8.3) | 0 (0) | 0 (0) |
The O3-only group showed 4- to 6-fold increases in oxidant parameters (P<0.05) and 2- to 3-fold increases in antioxidant parameters (P<0.05) compared with the sham group, implying a remarkable enhancing activity (more on oxidants) of O3 on all of these parameters. Although not significant, the median SOD value was remarkably (178.9%) higher in the MNU+O3 group than in the MNU-only group (P=0.060). Slight but not significant increases (all P>0.05) were observed in the MNU+O3 group compared with the MNU-only group in the rest of the oxidant (NO, MPO, MDA) and antioxidant (GSH) parameters. According to these results, it appears that bladder carcinogenesis may have suppressed enhancing effects of O3 on both oxidants and antioxidants; significant (all P<0.05) enhancing effects were only clearly obvious when the O3-only group was compared with the sham group (Table 3).
Table 3. Median values and interquartile ranges (IQRs) of tissue oxidants (NO, MPO, MDA) and antioxidants (GSH, SOD) according to study group and their percentage differences (%B-A and %D-C).
| A (MNU Only) | B (MNU+O3) | % B-A | P | C (Sham) | D (O3 Only) | % D-C | P | |
|---|---|---|---|---|---|---|---|---|
| NO (nmol/mg Prt) | 27.5 (14.05–81.22) | 35.0 (26.59–40.60) | 27.2 | 0.758 | 24.9 (22.11–33.43) | 114.5 (37.03–150.83) | 359.8 | 0.014 |
| MPO (ng/mg Prt) | 55.9 (34.82–165.81) | 57.0 (35.01–82.17) | 2.0 | 0.712 | 42.5 (25.49–58.44) | 151.3 (60.90–231.45) | 256.0 | 0.014 |
| MDA (nmol/mg Prt) | 34.9 (18.87–115.28) | 49.3 (35.74–66.98) | 41.3 | 0.758 | 36.4 (34.53–46.66) | 144.5 (95.35–248.91) | 297.0 | 0.003 |
| GSH (µg/mg Prt) | 1.0 (0.57–2.77) | 1.3 (0.81–2.02) | 30.0 | 0.878 | 1.0 (0.49–1.21) | 2.8 (1.64–6.40) | 180.0 | 0.014 |
| SOD (U/mg Prt) | 9.5 (6.26–26.67) | 26.5 (16.89–28.47) | 178.9 | 0.060 | 21.8 (11.28–24.68) | 61.9 (26.00–198.72) | 183.9 | 0.049 |
Discussion
Novel intravesical treatment agents have generally been investigated in female rats because of simple anatomical features and catheterization [17, 22]. However, estrogen is a natural antioxidant that prevents lipid peroxidation. For this reason, oxidative stress occurs less often in females than males. We preferred male rats to obviate the natural antioxidant effects of estrogens in female rats [27]. To our knowledge there is only one published study on a male rat bladder carcinogenesis model with intravesical MNU [30]. But the authors of that study acknowledged that their carcinogenetic success was not high. In the current study, we examined the effect of O3 treatment on a NMIBC model. We also examined oxidative-antioxidative properties.
Recent bladder carcinogenesis model (all with female rats) studies revealed that urethral stricture and urosepsis were significant mortal factors in the animals [23, 24]. However, in the current study, all deaths were observed within 1–2 h after anesthesia, and all of them were in the first six weeks. Therefore, we thought that animal deaths were caused by cardiopulmonary arrest or airway obstruction during anesthesia induction. On the other hand, antibiotics have previously been used to prevent commonly observed infections and squamous metaplasia in rats [20, 23, 24]. Since we thought that antibiotics might have some effect (positive or negative) on oxidant-antioxidant balance [6], we did not use any antibiotics. Despite this, our total loss rate was 25.5% (n=13/51), which was not different from similar studies [20, 24]. This implies that the use of antibiotics may not be necessary in intravesical studies with male rats.
Hormetic effects are the beneficial effects of low levels of agents that are harmful at high levels [9]. O3 has been described as one of the most oxidant agents at high levels (>80 µg/ml) [2], but at low levels (20–25 µg/ml), it is also known to have an antioxidant enhancing effect [28]. This is known as the paradoxal effect of O3 [29]. The biphasic hormetic dose response may be useful for dual pharmacological effects generated by O3 [3]. These redox-related effects of O3 may be used intravesically in NMIBC. There has been considerable interest in researching the role of free radicals in carcinogenesis and the role of antioxidants in the prevention and treatment of cancer and related side effects [1]. The current study and that of Tasdemir et al. [29] indicated that repeated low doses of O3 application results in increased antioxidant response. In the current study, O3 was used in a NMIBC treatment model for the first time. We found that two animals in the MNU+O3 group did not show any carcinogenesis (normal bladder), while all animals showed a form of preneoplastic or neoplastic proliferation in the MNU-only group (Table 2). Furthermore, more high-grade NMIBC lesions were found in the MNU-only group compared to MNU+O3 group (36.4% vs. 8.3%, Table 2). Although a statistical difference was not observed in our study, the above data could imply that O3 might statistically prove to be preventive of NMIBC and/or high-grade lesions in larger studies.
SOD is an enzymatic antioxidant and converts potent oxidative superoxide (O2−) to less oxidative hydrogen peroxide (H2O2) [3]. SOD’s antioxidant defense has been shown to be decreased in the blood of patients who have high-grade NMIBC and MIBC compared with patients who have low-grade NMIBC and healthy control patients [1]. Kaczmarek et al. investigated levels of SOD in peripheral blood platelets, before and after intravesical Bacillus Calmette-Guérin (BCG) treatment in 12 patients with NMIBC [12]. In all of these patients, SOD levels increased after BCG treatment [12]. On the other hand, tumor development is aggravated by oxidative stress after major surgery in a pulmonary metastasis model. In this model, authors implanted B16-BL6 malignant melanoma cells into the foot base [11]. When SOD derivatives were injected intravenously into these animals, remarkable prevention of pulmonary metastases was reported. However, in the present study, SOD levels were lower in the MNU-only group than in the sham group (9.5 vs. 21.8, P=0.390) and were remarkably higher in the MNU+O3 group than in the MNU-only group (26.5 vs. 9.5, P=0.060). Therefore, O3 seems to have restored the antioxidant (SOD) response in the MNU+O3 group to a level even higher than that of normal tissue in the sham group (26.5 vs. 21.8). On the other hand, despite development of preneoplastic and neoplastic lesions in all rats in the MNU-only group, a normal urothelium was observed in 16.7% (2/12) of the animals in the MNU+O3 group. Therefore, coupled with the increased antioxidant SOD levels in the MNU+O3 group, these findings imply that O3 may have a preventive effect on bladder carcinogenesis along with an antioxidant effect and that it can be considered a candidate supplementary agent in the treatment of NMIBC.
GSH is a nonenzymatic antioxidant and electron donor for glutathione peroxidase that catalyses the reaction from H2O2 to H2O [26]. NO is synthesized from arginine by nitric oxide synthase. Although low doses of NO induce proliferation of BC cells in vitro, high doses have a cytotoxic effect [15]. In addition, it has been shown that increased NO plays an important role in the cytotoxic effect of BCG on BC cells [15]. MPO is a lysosomal enzyme present both in neutrophils and monocytes. It also has a microbicidal effect and is a biochemical marker of inflammatory response [29]. MDA is a product of lipid peroxidation and exhibits cellular oxidative stress [28, 29]. In the present study, GSH, NO, MPO, and MDA levels were significantly increased in the O3-only group compared with the sham group. Their levels were also increased in the MNU+O3 group compared with the MNU-only group; however, this increase was not statistically significant (Table 3). Therefore, intravesical O3 therapy provided balanced increases in both oxidant and antioxidant systems and did not result in any abnormal histopathological change in normal (O3-only group) bladder tissue (Table 2).
Although both oxidants and antioxidants increased in the O3-only group compared with the sham group, the increase in oxidants was much higher than the increase in antioxidants (4- to 6-fold vs 2- to 3-fold). On the other hand, only the antioxidant SOD increased remarkably in the MNU+O3 group compared with the MNU-only group. The reason for this may be the resistance to the oxidative effects of O3 in MNU-induced proliferative lesions.
The limitations of this study are the limited number of experimental animals and a death rate close to the high end of the rates in the literature [8, 21, 30].
In conclusion, to our knowledge this is the first well-described (with respect to catheterization and detailed pathologic characterization of the lesions) male rat orthotopic bladder carcinogenesis model with intravesical MNU. Our results showed that oxidants are all increased potently in response to O3 and that this increase is counterbalanced by simultaneous increase of antioxidants in normal bladder tissue. O3 increased SOD remarkably in the MNU+O3 group compared with MNU-only group. It is noteworthy that no side effect was observed after O3 application. Although not statistically significant, the MNU+O3 group had 28.1% fewer high-grade lesions, and bladder carcinogenesis was completely prevented (bladders were completely normal) in 16.7% of the rats in this group when compared with the MNU-only group. These findings suggest that, when supported by larger studies, intravesical O3 may in the future prove to be a candidate supplementary agent for NMIBC.
Conflict of Interest
The authors declare that they have no conflicts of interest with respect to this research.
Acknowledgments
This work was supported by the Scientific and Investigational Projects Reserve of the University of Kocaeli (Project No: 2014/56). We would like thank to Mr. Kadir Akgül and Dr. Cüneyt Özer of the Animal Laboratory in the Kocaeli University Faculty of Medicine for their great assistance in handling and maintenance of the animals.
References
- 1.Badjatia N., Satyam A., Singh P., Seth A., Sharma A.2010. Altered antioxidant status and lipid peroxidation in Indian patients with urothelial bladder carcinoma. Urol. Oncol. 28: 360–367. doi: 10.1016/j.urolonc.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 2.Bocci V.2004. Ozone as Janus: this controversial gas can be either toxic or medically useful. Mediators Inflamm. 13: 3–11. doi: 10.1080/0962935062000197083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bocci V.A., Zanardi I., Travagli V.2011. Ozone acting on human blood yields a hormetic dose-response relationship. J. Transl. Med. 9: 66. doi: 10.1186/1479-5876-9-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger M., Catto J.W., Dalbagni G., Grossman H.B., Herr H., Karakiewicz P., Kassouf W., Kiemeney L.A., La Vecchia C., Shariat S., Lotan Y.2013. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 63: 234–241. doi: 10.1016/j.eururo.2012.07.033 [DOI] [PubMed] [Google Scholar]
- 5.Clavo B., Gutiérrez D., Martín D., Suárez G., Hernández M.A., Robaina F.2005. Intravesical ozone therapy for progressive radiation-induced hematuria. J. Altern. Complement. Med. 11: 539–541. doi: 10.1089/acm.2005.11.539 [DOI] [PubMed] [Google Scholar]
- 6.Dwyer D.J., Belenky P.A., Yang J.H., MacDonald I.C., Martell J.D., Takahashi N., Chan C.T., Lobritz M.A., Braff D., Schwarz E.G., Ye J.D., Pati M., Vercruysse M., Ralifo P.S., Allison K.R., Khalil A.S., Ting A.Y., Walker G.C., Collins J.J.2014. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 111: E2100–E2109. doi: 10.1073/pnas.1401876111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellman G.L.1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82: 70–77. doi: 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 8.Ferrari K.L., de Camargo J.A., Rocha G.Z., Carvalheira J.B., Saad M.J., Billis A., Reis L.O.2015. Intravesical bacillus Calmette-Guérin efficiently reduces p70S6K1 but not 4E-BP1 phosphorylation in nonmuscle invasive bladder cancer. J. Urol. 193: 682–689. doi: 10.1016/j.juro.2014.08.106 [DOI] [PubMed] [Google Scholar]
- 9.Goldman M.1996. Cancer risk of low-level exposure. Science 271: 1821–1822. doi: 10.1126/science.271.5257.1821 [DOI] [PubMed] [Google Scholar]
- 10.Hicks R.M., Wakefield J.S.1972. Rapid induction of bladder cancer in rats with N-methyl-N-nitrosourea. I. Histology. Chem. Biol. Interact. 5: 139–152. doi: 10.1016/0009-2797(72)90040-3 [DOI] [PubMed] [Google Scholar]
- 11.Hyoudou K., Nishikawa M., Kobayashi Y., Ikemura M., Yamashita F., Hashida M.2008. SOD derivatives prevent metastatic tumor growth aggravated by tumor removal. Clin. Exp. Metastasis 25: 531–536. doi: 10.1007/s10585-008-9165-3 [DOI] [PubMed] [Google Scholar]
- 12.Kaczmarek P., Buczynski A., Niemirowicz J., Gnitecki W., Kocur E., Karpinski J.2001. Lipids peroxidation in platelets in patients with bladder cancer treated with Mycobacterium suspension. Pol. Merkuriusz Lek. 11: 484–486. [PubMed] [Google Scholar]
- 13.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J.1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 14.Montironi R., Lopez-Beltran A.2005. The 2004 WHO classification of bladder tumors: a summary and commentary. Int. J. Surg. Pathol. 13: 143–153. doi: 10.1177/106689690501300203 [DOI] [PubMed] [Google Scholar]
- 15.Morcos E., Jansson O.T., Adolfsson J., Ehrén I., Wiklund N.P.2001. Bacillus Calmette-Guerin induces long-term local formation of nitric oxide in the bladder via the induction of nitric oxide synthase activity in urothelial cells. J. Urol. 165: 678–682. doi: 10.1097/00005392-200102000-00093 [DOI] [PubMed] [Google Scholar]
- 16.Neimark A.I., Nepomnyashchikh L.M., Lushnikova E.L., Bakarev M.A., Abdullaev N.A., Sizov K.A.2014. Microcirculation and structural reorganization of the bladder mucosa in chronic cystitis under conditions of ozone therapy. Bull. Exp. Biol. Med. 156: 399–405. doi: 10.1007/s10517-014-2358-7 [DOI] [PubMed] [Google Scholar]
- 17.Oliveira P.A., Nóbrega M.P.C., Arantes-Rodrigues R., Calado A.M., Carrola J., Ginja M., Colaço A.2009. Technical Report: Technique of Bladder Catheterization in Female Mice and Rats for Intravesical Instillation in Models of Bladder Cancer. Scand. J. Lab. Anim. Sci. 36: 5–9. [Google Scholar]
- 18.Palmeira C., Oliveira P.A., Lameiras C., Amaro T., Silva V.M., Lopes C., Santos L.2010. Biological similarities between murine chemical-induced and natural human bladder carcinogenesis. Oncol. Lett. 1: 373–377. doi: 10.3892/ol_00000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perabo F.G., Demant A.W., Wirger A., Schmidt D.H., Sitia M., Wardelmann E., Müller S.C., Kohn E.C.2005. Carboxyamido-triazole (CAI) reverses the balance between proliferation and apoptosis in a rat bladder cancer model. Anticancer Res. 25:(2A): 725–729. [PubMed] [Google Scholar]
- 20.Qian L., Ding H., Zhou J., Wang X., Shao P., Wu S., Yang J., Feugang J.M., Zou C.2006. Intravesical N-(4-hydroxyphenyl) retinamide and adriamycin induces apoptosis in bladder cancer. Front. Biosci. 11: 2045–2051. doi: 10.2741/1946 [DOI] [PubMed] [Google Scholar]
- 21.Reis L.O., Ferreira U., Billis A., Cagnon V.H., Fávaro W.J.2012. Anti-angiogenic effects of the superantigen staphylococcal enterotoxin B and bacillus Calmette-Guérin immunotherapy for nonmuscle invasive bladder cancer. J. Urol. 187: 438–445. doi: 10.1016/j.juro.2011.10.022 [DOI] [PubMed] [Google Scholar]
- 22.Reis L.O., Sopena J.M., Fávaro W.J., Martin M.C., Simão A.F., Reis R.B., Andrade M.F., Domenech J.D., Cardo C.C.2011. Anatomical features of the urethra and urinary bladder catheterization in female mice and rats. An essential translational tool. Acta Cir. Bras. 26:(Suppl 2): 106–110. doi: 10.1590/S0102-86502011000800019 [DOI] [PubMed] [Google Scholar]
- 23.Steinberg G.D., Brendler C.B., Ichikawa T., Squire R.A., Isaacs J.T.1990. Characterization of an N-methyl-N-nitrosourea-induced autochthonous rat bladder cancer model. Cancer Res. 50: 6668–6674. [PubMed] [Google Scholar]
- 24.Steinberg G.D., Brendler C.B., Squire R.A., Isaacs J.T.1991. Experimental intravesical therapy for superficial transitional cell carcinoma in a rat bladder tumor model. J. Urol. 145: 647–653. [DOI] [PubMed] [Google Scholar]
- 25.Sweet F., Kao M.S., Lee S.C., Hagar W.L., Sweet W.E.1980. Ozone selectively inhibits growth of human cancer cells. Science 209: 931–933. doi: 10.1126/science.7403859 [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M.2012. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J. Reprod. Dev. 58: 1–9. doi: 10.1262/jrd.11-138N [DOI] [PubMed] [Google Scholar]
- 27.Tang M., Abplanalp W., Ayres S., Subbiah M.T.1996. Superior and distinct antioxidant effects of selected estrogen metabolites on lipid peroxidation. Metabolism 45: 411–414. doi: 10.1016/S0026-0495(96)90212-7 [DOI] [PubMed] [Google Scholar]
- 28.Tasdemir C., Tasdemir S., Vardi N., Ates B., Onal Y., Erdogan S., Yucel A., Aglamis E., Yakupogullari Y., Altıntas R., Karaman A.2013. Evaluation of the effects of ozone therapy on Escherichia coli-induced cytitis in rat. Ir. J. Med. Sci. 182: 557–563. doi: 10.1007/s11845-013-0926-x [DOI] [PubMed] [Google Scholar]
- 29.Tasdemir S., Tasdemir C., Vardi N., Ates B., Taslidere E., Karaaslan M.G., Sapmaz H.I., Sagir M., Kurt A., Baser C.A.2013. Effects of ozone therapy on cyclophosphamide-induced urinary bladder toxicity in rats. Clin. Invest. Med. 36: E9–E17. doi: 10.25011/cim.v36i1.19400 [DOI] [PubMed] [Google Scholar]
- 30.Yaman O., Ozdiler E., Sözen S., Göğüş O.1999. Transmurally absorbed intravesical chemotherapy with dimethylsulfoxide in an animal model. Int. J. Urol. 6: 87–92. doi: 10.1046/j.1442-2042.1999.00623.x [DOI] [PubMed] [Google Scholar]