Abstract
Human class I major histocompatibility antigens (HLA-A, -B and -C) are integral membrane glycoprotein heterodimers. A mutagenized B lymphoblastoid cell line has been previously shown to synthesize two forms of the HLA-A2 antigen; a minor form which remains cell-associated at all times, and an abundant form, which is secreted. The present study reports the isolation of cDNA clones for both the wild-type HLA-A2 molecule synthesized by the parent cell line and the secreted molecule synthesized by the mutant cell line. A comparison of their structures indicates that transcripts encoding the mutant HLA-A2 molecule lack the 117 nucleotides encoded by exon five of the HLA-A2 gene. This exon encodes the hydrophobic amino acids which are thought to anchor the polypeptide in the plasma membrane. This result supports an alternative splicing model to explain the phenotype of the mutant cell line. Further, it implies that information encoded in exon five is essential for anchoring class I antigens in the plasma membrane. The potential for a similar splicing mechanism to generate soluble forms of class I antigens in vivo is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnot D., Lillie J. W., Auffray C., Kappes D., Strominger J. L. Inter-locus and intra-allelic polymorphisms of HLA class I antigen gene mRNA. Immunogenetics. 1984;20(3):237–252. doi: 10.1007/BF00364206. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing R. J., Safani M., Peterson P. Soluble HLA antigens present in normal human serum. Tissue Antigens. 1977 Aug;10(2):75–82. doi: 10.1111/j.1399-0039.1977.tb01122.x. [DOI] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. Activation of a Qa/Tla class I major histocompatibility antigen gene is a general feature of oncogenesis in the mouse. Nature. 1983 Dec 22;306(5945):756–760. doi: 10.1038/306756a0. [DOI] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. Activation of a Qa/Tla class I major histocompatibility antigen gene is a general feature of oncogenesis in the mouse. Nature. 1983 Dec 22;306(5945):756–760. doi: 10.1038/306756a0. [DOI] [PubMed] [Google Scholar]
- Cosman D., Kress M., Khoury G., Jay G. Tissue-specific expression of an unusual H-2 (class I)-related gene. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4947–4951. doi: 10.1073/pnas.79.16.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. G., Cone R. E. I-Kk and H-2Kk antigens are shed as supramolecular particles in association with membrane lipids. J Immunol. 1981 Aug;127(2):482–486. [PubMed] [Google Scholar]
- Fukumaki Y., Collins F., Kole R., Stoeckert C. J., Jr, Jagadeeswaran P., Duncan C. H., Weissman S. M., Jagadeeswaran P., Pan J., Forget B. G. Sequences of human repetitive DNA, non-alpha-globin genes, and major histocompatibility locus genes. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1079–1086. [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982 Dec 16;300(5893):598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Goldman D. W., Pober J. S., White J., Bayley H. Selective labelling of the hydrophobic segments of intrinsic membrane proteins with a lipophilic photogenerated carbene. Nature. 1979 Aug 30;280(5725):841–843. doi: 10.1038/280841a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
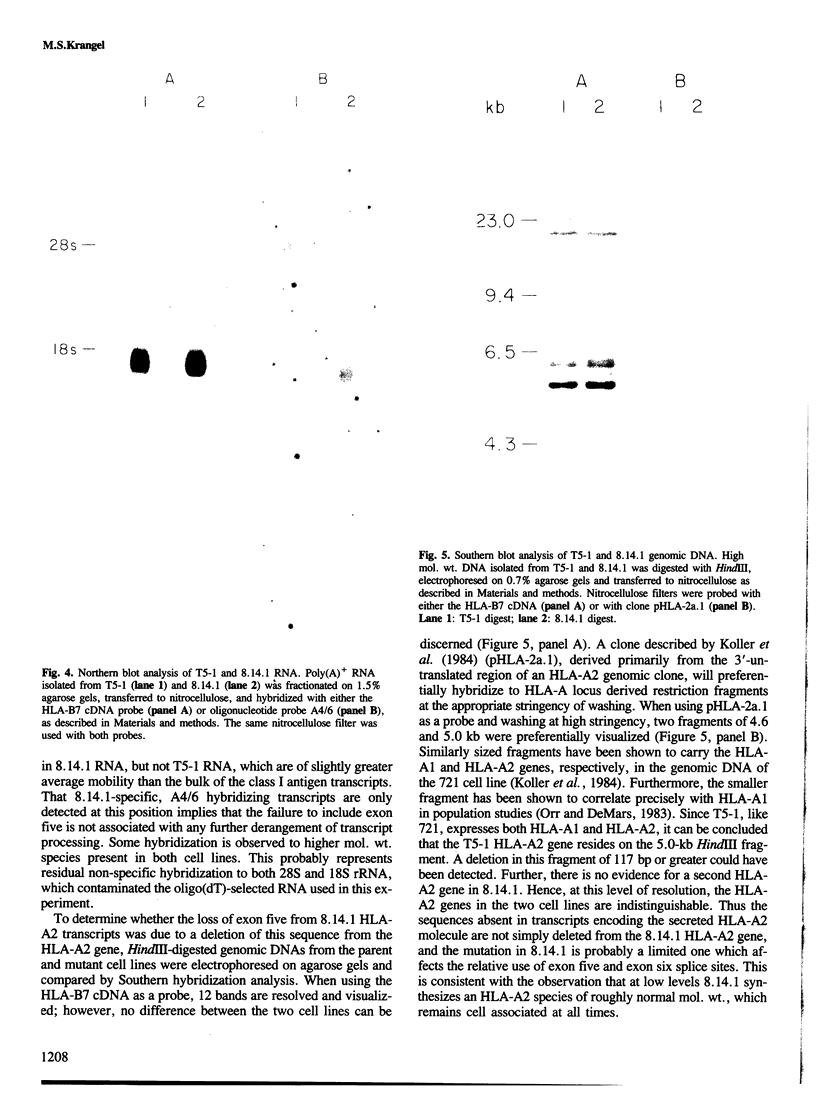
- Koller B. H., Sidwell B., DeMars R., Orr H. T. Isolation of HLA locus-specific DNA probes from the 3'-untranslated region. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5175–5178. doi: 10.1073/pnas.81.16.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krangel M. S., Pious D., Strominger J. L. Characterization of a B lymphoblastoid cell line mutant that secretes HLA-A2. J Immunol. 1984 Jun;132(6):2984–2991. [PubMed] [Google Scholar]
- Kress M., Cosman D., Khoury G., Jay G. Secretion of a transplantation-related antigen. Cell. 1983 Aug;34(1):189–196. doi: 10.1016/0092-8674(83)90149-6. [DOI] [PubMed] [Google Scholar]
- Kvist S., Peterson P. A. Isolation and partial characterization of a beta2-microglobulin-containing, H-2 antigen-like murine serum protein. Biochemistry. 1978 Oct 31;17(22):4794–4801. doi: 10.1021/bi00615a029. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Schneider W. J., Südhof T. C., Brown M. S., Goldstein J. L., Russell D. W. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science. 1985 Jan 11;227(4683):140–146. doi: 10.1126/science.3155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Malissen B., Jordan B. R. Exon/intron organization and complete nucleotide sequence of an HLA gene. Proc Natl Acad Sci U S A. 1982 Feb;79(3):893–897. doi: 10.1073/pnas.79.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy W. L., Coligan J. E., Barra Y., Jay G. Detection of a secreted form of the murine H-2 class I antigen with an antibody against its predicted carboxyl terminus. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1216–1220. doi: 10.1073/pnas.81.4.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes E. L., Waterfield M. D. Biosynthesis of the epidermal growth factor receptor in A431 cells. EMBO J. 1984 Mar;3(3):531–537. doi: 10.1002/j.1460-2075.1984.tb01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor A. L., Weiss E. H., Kress M., Jay G., Flavell R. A. A nonpolymorphic class I gene in the murine major histocompatibility complex. Cell. 1984 Jan;36(1):139–144. doi: 10.1016/0092-8674(84)90082-5. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., Tanigaki N., Kreiter V. P., Moore G. E., Pressman D. Characterization of soluble substances in plasma carrying HL-A alloantigenic activity and HL-A common antigenic activity. Transplantation. 1973 Mar;15(3):312–319. doi: 10.1097/00007890-197303000-00008. [DOI] [PubMed] [Google Scholar]
- Natori T., Tanigaki N., Pressman D. A mouse plasma substance carrying beta2-microglobulin activity and lacking in H-2 alloantigenic activity. J Immunogenet. 1976 Apr;3(2):123–134. doi: 10.1111/j.1744-313x.1976.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Orr H. T., DeMars R. Mapping of class I DNA sequences within the human major histocompatibility complex. Immunogenetics. 1983;18(5):489–502. doi: 10.1007/BF00364390. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Russo C., Allison J. P. HLA antigens in serum. Methods Enzymol. 1984;108:614–624. doi: 10.1016/s0076-6879(84)08122-2. [DOI] [PubMed] [Google Scholar]
- Pious D., Krangel M. S., Dixon L. L., Parham P., Strominger J. L. HLA antigen structural gene mutants selected with an allospecific monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7832–7836. doi: 10.1073/pnas.79.24.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Ramanathan L., Rogers M. J., Robinson E. A., Hearing V. J., Tanigaki N., Appella E. Biochemical analysis of a 40,000 mol. wt mouse serum protein which binds beta 2-microglobulin and has serological cross-reactivity with H-2 antigens. Mol Immunol. 1982 Sep;19(9):1075–1086. doi: 10.1016/0161-5890(82)90318-2. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Terhorst C., Strominger J. L. Sequence of the COOH-terminal hydrophilic region of histocompatibility antigens HLA-A2 and HLA-B7. J Biol Chem. 1978 Aug 10;253(15):5319–5324. [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Expression from cloned cDNA of cell-surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982 Oct;30(3):753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodoyer R., Damotte M., Delovitch T. L., Trucy J., Jordan B. R., Strachan T. Complete nucleotide sequence of a gene encoding a functional human class I histocompatibility antigen (HLA-CW3). EMBO J. 1984 Apr;3(4):879–885. doi: 10.1002/j.1460-2075.1984.tb01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Hood L. Genes of the major histocompatibility complex in mouse and man. Science. 1983 Nov 18;222(4625):727–733. doi: 10.1126/science.6356354. [DOI] [PubMed] [Google Scholar]
- Strachan T., Sodoyer R., Damotte M., Jordan B. R. Complete nucleotide sequence of a functional class I HLA gene, HLA-A3: implications for the evolution of HLA genes. EMBO J. 1984 Apr;3(4):887–894. doi: 10.1002/j.1460-2075.1984.tb01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transy C., Lalanne J. L., Kourilsky P. Alternative splicing in the 5' moiety of the H-2Kd gene transcript. EMBO J. 1984 Oct;3(10):2383–2386. doi: 10.1002/j.1460-2075.1984.tb02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Srinivas R. V., Hunter E. Mutations of the Rous sarcoma virus env gene that affect the transport and subcellular location of the glycoprotein products. J Cell Biol. 1984 Dec;99(6):2011–2023. doi: 10.1083/jcb.99.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]