Abstract
Hippocampal inhibitory interneurons play important roles in controlling the excitability and synchronization of pyramidal cells, but whether they express long-term synaptic plasticity that contributes to hippocampal network function remains uncertain. We found that pairing postsynaptic depolarization with θ-burst stimulation induced long-term potentiation (LTP) of putative single-fiber excitatory postsynaptic currents in interneurons. Either postsynaptic depolarization or θ-burst stimulation alone failed to induce LTP. LTP was expressed as a decrease in failure rates and an increase in excitatory postsynaptic current amplitude, independent of N-methyl-d-aspartate receptors, and dependent on metabotropic glutamate receptors subtype 1a. LTP was induced specifically in interneurons in stratum oriens and not in interneurons of stratum radiatum/lacunosum-moleculare. Thus, excitatory synapses onto specific subtypes of inhibitory interneurons express a new form of hebbian LTP that will contribute to hippocampal network plasticity.
Long-term potentiation (LTP) is the enduring increase in strength of synaptic transmission observed after tetanization of afferents (1). This synaptic plasticity has been extensively studied at glutamate synapses onto CA1 pyramidal cells of the hippocampus, where multiple forms of LTP can be induced by activation of N-methyl-d-aspartate receptors (NMDARs) (1, 2), metabotropic glutamate receptors (mGluRs) (3), and voltage-dependent Ca2+ channels (4, 5).
The hippocampal neuronal network is also composed of inhibitory interneurons, which control the excitability and synchronization of projection cells (6–8). Modeling studies suggest that plasticity at interneuron synapses is important for learning and recall by hippocampal neuronal networks (9). However, experimental evidence of long-term synaptic plasticity at input and/or output synapses of hippocampal interneurons has been controversial (10–16). This uncertainty has been due, in part, to the heterogeneity of interneuron types present in the hippocampus and to the complexity of the network (6, 17, 18). An important confounding factor has been that LTP induced at pyramidal cell synapses by tetanic stimulation of Schaffer collaterals can passively propagate to interneurons through recurrent excitatory collaterals of pyramidal cells and can be confused with direct potentiation of excitatory inputs onto interneurons (19). In addition, the apparent absence in interneurons of Ca2+-calmodulin-dependent protein kinase II and calcineurin (20), two major components of the Ca2+-signaling cascades involved in the induction of NMDAR-dependent LTP and depression, has been argued to support the lack of synaptic plasticity in interneurons (19, 20). Recently, however, long-term depression has been observed at excitatory synapses onto interneurons after trains of high-frequency stimulation, suggesting that synaptic plasticity can indeed occur directly in interneurons (13, 15).
In the present study, the issue of long-term synaptic plasticity at synapses directly on interneurons was addressed by using experimental conditions to avoid passive propagation of LTP during whole-cell recordings from visually identified interneurons in rat hippocampal slices. The induction of LTP was investigated directly at excitatory synapses on interneurons by using a minimal stimulation paradigm to activate mainly single fiber responses (21, 22) in interneurons, effectively avoiding the polysynaptic contribution of passive propagation of LTP from pyramidal cells. In addition, a protocol pairing postsynaptic depolarization with presynaptic stimulation was used, in combination with biocytin cell labeling. In these conditions, we found that putative single-fiber excitatory postsynaptic currents (EPSCs) evoked by minimal stimulation showed LTP in CA1 interneurons of stratum oriens.
Methods
Hippocampal Slices.
Transverse hippocampal slices (300-μm thick) were obtained (12) from 18- to 21-day-old rats and transferred to oxygenated artificial cerebrospinal fluid (ACSF) containing 124 mM NaCl, 5 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 2 mM CaCl2, 26 mM NaHCO3, and 10 mM dextrose. After a 1-h recovery period, a slice was maintained submerged in a recording chamber mounted on an upright microscope equipped with a long-range water immersion objective (×40), Nomarski optics, and an IR video camera. CA1 and CA3 regions were surgically isolated and slices were perfused with oxygenated ACSF (2–4 ml/min) at room temperature (22–24°C). Pyramidal cells and interneurons located in different layers were easily distinguishable visually and were selected for whole-cell recordings.
Electrophysiological Recording and Pharmacology.
Tight-seals (>1 GΩ) were formed on somata of interneurons in stratum oriens or in stratum radiatum near lacunosum-moleculare (R/LM), as well as pyramidal cells. Whole-cell voltage-clamp recordings were obtained by using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA). Data were low-pass filtered at 1 KHz, displayed on an oscilloscope, and digitized at 22 KHz for storage on a videocassette recorder. Responses also were digitized at 20 KHz by using a microcomputer and a data acquisition board (TL-1–125, Axon Instruments), and analyzed by using PCLAMP6 (Axon Instruments). Recordings were accepted if holding current was stable. The ACSF also contained 10 μM bicuculline to block γ-aminobutyric acid type A receptors and elevated Ca2+ and Mg2+ (4 mM each). As noted, in some experiments normal Ca2+ and Mg2+ concentrations (2 mM each, see Fig. 4) were used. When indicated, 10 μM N-(4-hydroxyphenylpropanoyl)spermine (NHPP)-spermine, 100 μM dl-2-amino-5-phosphonopentanoic acid (AP5), 500 μM (RS)-α-ethyl-4-carboxyphenylglycine (E4CPG), 500 μM (RS)-α-methyl-4-carboxyphenylglycine (MCPG), or 100 μM (+)-2-methyl-4-carboxyphenylglycine (LY367385) was added. The patch solution contained 140 mM cesium-methane sulfonate, 5 mM NaCl, 1 mM MgCl2, 10 mM Hepes, 2 mM ATP-Tris, 0.4 mM GTP-Tris, and 0.1% biocytin, pH adjusted to 7.2–7.3 with CsOH. Cells were held at −60 mV, and series resistance was monitored every 2 min during recordings. For visualization of biocytin-filled cells, slices were processed histologically without resectioning, as described (8).
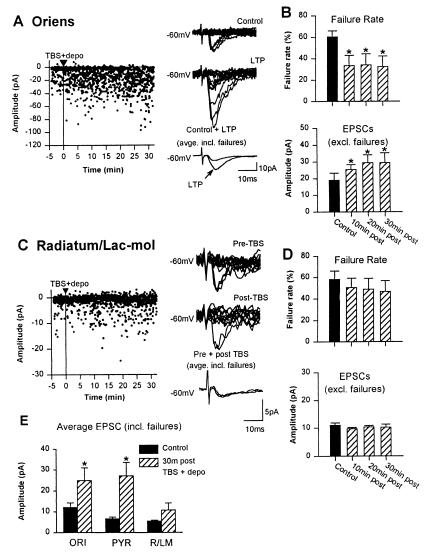
Figure 4.
LTP is cell-type specific. (A) Graph and traces from a representative oriens interneuron, showing LTP at 30 min after TBS + depo in ACSF with 2 mM Ca2+/Mg2+. (B) Histograms for all oriens cells in 2 mM Ca2+/Mg2+, showing the significant decrease in failure rate and significant increase in amplitude of EPSCs (failures excluded) after TBS + depo. (C) Representative example of the absence of LTP in a radiatum interneuron. Graph and traces show that EPSCs were unchanged after TBS + depo. (D) Histograms for all radiatum interneurons showing that the failure rate and amplitude of EPSCs (failures excluded) were unaffected by TBS + depo. (E) Summary histogram for all cells in 2 mM Ca2+/Mg2+, showing that the amplitude of average EPSCs (failures included) was significantly increased 30 min after TBS + depo in oriens interneurons (ORI) and pyramidal cells (PYR) but not in radiatum interneurons (R/LM).
Stimulation Procedures.
Synaptic responses were evoked with constant current pulses (50 μs) via an ACSF-filled bipolar θ glass electrode positioned ≈100 μm from the cell body in stratum oriens (for oriens interneurons) or in stratum radiatum (for R/LM interneurons and pyramidal cells). Criteria for single-fiber minimal stimulation were as follows (21, 22): EPSC failure rate >50% (responses and failures identified by visual inspection), invariant latency and shape of EPSC, average amplitude and failure rate independent of stimulus intensity over a range of approximately ± 5%, and lower stimulus intensities giving only failures. The success rate for obtaining such EPSCs within 10–15 min of gaining whole-cell access was ≈25%. When these criteria were met, we assumed that we were predominantly recording monosynaptic EPSCs evoked by activation of a single fiber. Minimally evoked EPSCs were recorded at 0.5 Hz. After a 4-min stable baseline period, LTP was induced by applying θ-burst stimulation [TBS; five bursts at 200-ms intervals (5 Hz), each burst consisting of four pulses at 100 Hz], paired with five 60-ms long depolarizing steps to −20 mV (Fig. 1A). Three episodes of TBS paired with depolarization (TBS + depo) were given at 30-s intervals. EPSCs were recorded for at least 35 min after the induction protocol. It is unclear whether minimal stimulation conditions were maintained during TBS; however, they were maintained before and after TBS (see Results and Fig. 2). LTP was examined in one cell per slice, and different experimental conditions were interleaved.
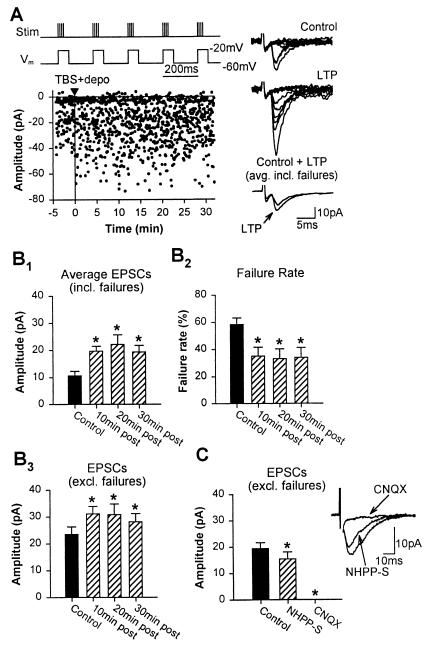
Figure 1.
LTP in oriens interneurons. (A) Graph of EPSCs amplitude from a representative cell showing LTP after TBS paired with depolarization (TBS + depo; protocol shown above and delivered at time indicated by black triangle and vertical line). Examples of control and potentiated EPSCs (10 consecutive responses) at 30 min after induction (Right). Superimposed average EPSCs (bottom traces; n = 64 responses each, failures included) from control and 30 min after induction also show LTP. (B) Histograms for all cells showing the significant increase in amplitude of average EPSCs (failures included) up to 30 min after induction (B1), significant decrease in failure rate (B2), and significant increase in EPSC amplitude (failures excluded) (B3). (C) Significant reduction in EPSC amplitude (excluding failures) by 10 μM NHPP-spermine and complete block by 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (n = 5 oriens cells). Representative example of EPSCs partially mediated by Ca2+-permeable AMPA receptors and entirely by AMPA/KA receptors. In this and the following figures, * indicate significantly different from control.
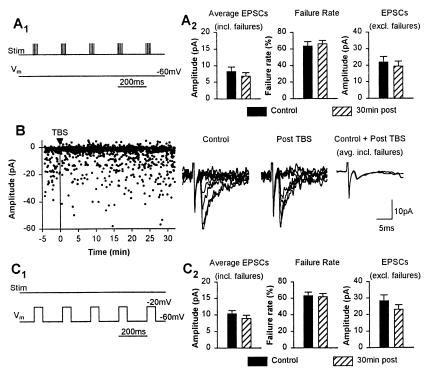
Figure 2.
No LTP with synaptic stimulation or postsynaptic depolarization alone. (A) TBS alone (A1) did not significantly change the amplitude of average EPSCs, or the failure rate and amplitude of EPSCs (A2). (B) Example from an oriens cell showing no effect of TBS on EPSC amplitude. Consecutive individual traces and superimposed average EPSCs (failures included) illustrate the absence of LTP 30 min after TBS alone. (C) Postsynaptic depolarization alone (C1) did not induce LTP in oriens interneurons. The amplitude of average EPSCs or the failure rate and amplitude of EPSCs were not significantly changed 30 min after depolarization (C2).
Measurements and Statistical Analyses.
The EPSC amplitude was determined from the peak amplitude minus the average of baseline points preceding the stimulation artifact. For failures, the amplitude measure was taken as the largest value of inward current within the same poststimulus time window minus baseline. Because amplitude measures for failures were affected by background noise, and occasionally by stimulation artifacts (which were not subtracted), they were not always normally distributed around zero. However, responses pre-TBS and post-TBS were always measured in identical fashion and consistently across cells.
Group comparisons for mean amplitude of average EPSCs (including failures), failure rates (number of failures as percentage of total number of events), and amplitude of EPSCs (excluding failures) were calculated from 4-min bins taken during the baseline period, at 10, 20, and 30 min after TBS. Repeated measures ANOVAs (rmANOVAs) were performed followed by posthoc Student-Newman–Keuls tests to compare the baseline control period to 10, 20, and 30 min after TBS. A paired t test was used to test the effect of NHPP-spermine. Significance levels were set at P < 0.05, and data are expressed as mean ± SEM.
Results
To examine plasticity directly at excitatory synapses on interneurons, putative single-fiber EPSCs evoked by minimal stimulation (21, 22) were recorded from CA1 interneurons in stratum oriens of hippocampal slices by using whole-cell voltage-clamp techniques in combination with biocytin cell labeling. This stimulation method allowed us to activate mainly single fiber responses (21, 22) in interneurons. In addition, a protocol pairing postsynaptic depolarization with presynaptic TBS was used (Fig. 1A). TBS was chosen based on its analogy to the naturally occurring hippocampal θ rhythm and its ability to potentiate polysynaptic inhibitory responses in pyramidal cells (10), as well as to minimize the long-term depression that is induced at interneuron excitatory synapses by 100-Hz tetanization (13, 15). Finally, to reduce the level of spontaneous activity and polysynaptic activation of oriens interneurons, a medium containing elevated (4 mM) Ca2+/Mg2+ was used. Under these conditions, TBS paired with postsynaptic depolarization resulted in LTP of average EPSCs (failures included) lasting at least 35 min (Fig. 1A; rmANOVA). This potentiation of average EPSC amplitude (failures included; 234.7 ± 53.2% of control) was associated with a significant decrease in failure rate (56.0 ± 8.5% of control) and a significant increase in EPSC amplitude (failures excluded; also called potency; 128.2 ± 8.4% of control) (Fig. 1 A and B; n = 8 cells, rmANOVA). These results indicate that pairing postsynaptic depolarization with presynaptic θ-burst activation produces LTP of putative single fiber EPSCs in oriens interneurons.
In hippocampal interneurons, Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) receptors lacking GluR2 subunits are found at specific synapses or are intermixed with Ca2+-impermeable AMPA receptors at other synapses (13, 23). To determine whether synapses showing LTP in oriens interneurons consisted of Ca2+-permeable AMPA receptors, 10 μM NHPP-spermine, a polyamine antagonist of GluR2-lacking AMPA receptors (23), was bath-applied at the end of LTP experiments. NHPP-spermine significantly reduced the amplitude of EPSCs (failures excluded) in interneurons (Fig. 1C). The NHPP-spermine-resistant components of EPSCs were abolished by the AMPA/kainate (AMPA/KA) receptor antagonist, 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (Fig. 1C). Therefore, LTP is induced at excitatory synapses of oriens interneurons that consist of AMPA/KA receptors and partly include Ca2+-permeable AMPA receptors.
To determine whether the induction of LTP in interneurons required joint presynaptic activity and postsynaptic depolarization, experiments were performed with either TBS (Fig. 2A1) or postsynaptic depolarization (Fig. 2C1) alone. Neither of these paradigms alone resulted in the induction of LTP. No significant changes were seen in average EPSCs (failures included), failure rates or amplitude of EPSCs (failures excluded) at 30 min post-TBS, or postdepolarization alone (Fig. 2; n = 8 cells each; P > 0.05, rmANOVA; data not shown for 10 and 20 min post-TBS). These results indicate that stimulation and recording conditions for EPSCs were stable throughout the experimental paradigm and that conjoint presynaptic activity and postsynaptic depolarization are necessary for the induction of LTP at excitatory synapses onto interneurons.
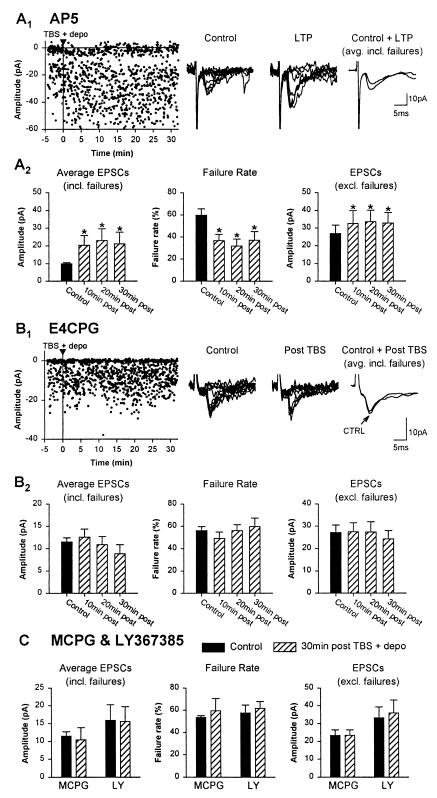
The types of glutamate receptors involved in the induction of LTP in oriens interneurons were investigated next. In CA1 pyramidal cells, a form of LTP requires postsynaptic depolarization and the activation of NMDAR (1, 2). Because we found that the induction of LTP in oriens interneurons also required postsynaptic depolarization, we examined whether NMDAR activation was necessary for LTP in interneurons. In the presence of the NMDAR antagonist AP5 (100 μM), which was effective in antagonizing the NMDAR component of EPSCs (data not shown), LTP of EPSCs was still induced in oriens interneurons and lasted at least 30 min (Fig. 3A; n = 8 cells). In AP5, LTP was again associated with a significant reduction in failure rate and a significant increase in EPSC amplitude. These results indicate that NMDARs are not necessary for LTP in oriens interneurons. Because group I/II mGluRs are coupled to specific intracellular Ca2+ mechanisms in oriens interneurons (24, 25), we investigated whether these receptors were involved in LTP induction. In the presence of the group I/II mGluR antagonist (RS)-α-ethyl-4-carboxyphenylglycine (26) (E4CPG; 500 μM), LTP was blocked in interneurons. The amplitude of average EPSCs, as well as the failure rate and amplitude of EPSCs were not significantly changed after TBS paired with depolarization in E4CPG (Fig. 3B; n = 8 cells). Similarly, LTP was prevented by the other group I/II mGluR antagonist MCPG (500 μM, n = 5 cells). Because oriens interneurons express high levels of the mGluR1a subtype of group I receptors (27, 28), we examined the effects of its selective antagonist (+)-2-methyl-4-carboxyphenylglycine (29) (LY367385; 100 μM) and found that it also prevented LTP induction in interneurons (n = 7 cells; Fig. 3C). These results indicate that excitatory synapses of oriens interneurons exhibit a unique NMDAR-independent form of synaptic plasticity, which requires activation of mGluR1a subtype of group I receptors.
Figure 3.
LTP is NMDAR-independent and mGluR1a-dependent. (A1) Graph from a representative cell showing LTP of EPSCs induced by TBS + depo in 100 μM AP5. Individual traces and average EPSCs (failures included) show the increase in EPSCs at 30 min after induction relative to control. (A2) Data for all cells in AP5, illustrating the significant change in amplitude of average EPSCs (failures included), failure rate, and amplitude of EPSCs (failures excluded) up to 30 min after TBS + depo. (B) Block of LTP by the group I/II mGluR antagonist E4CPG. Data from a representative cell (B1) and histograms for all oriens interneurons (B2) show that in 500 μM E4CPG the amplitude of average EPSCs, as well as the failure rate and amplitude of EPSCs, were unchanged after TBS + depo. (C) Block of LTP by the other group I/II antagonist MCPG and the mGluR1a antagonist LY367385. Histograms for all oriens cells showing that, in MCPG or LY367385, TBS + depo did not induce significant change in amplitude of average EPSCs (failures included), as well as failure rate or amplitude of EPSCs (failures excluded).
Hippocampal interneurons are a heterogeneous cell population in terms of local connectivity and cellular properties (6, 17). To examine whether LTP was a property of excitatory synapses specific to certain types of interneurons, the paradigm of TBS paired with depolarization was applied to interneurons in R/LM (12, 14) and to pyramidal cells. In addition, this series of experiments was conducted in ACSF containing normal (2 mM) Ca2+/Mg2+ levels to verify that LTP was not inducible solely in elevated extracellular Ca2+/Mg2+ levels. Under these conditions, the level of spontaneous synaptic activity in oriens interneurons was higher, often sufficiently to interfere with the analysis of EPSCs and require the exclusion of cells. However, in oriens interneurons with low levels of spontaneous activity (n = 7 cells), TBS paired with depolarization induced a similar LTP of EPSCs, which lasted at least 35 min (Fig. 4 A and B). In contrast, when the same stimulation paradigm was applied to interneurons in R/LM (n = 8 cells), there was no significant change in EPSCs (Fig. 4 C and D). As expected, when TBS was paired with depolarization in CA1 pyramidal cells, LTP also was induced (n = 8 cells; Fig. 4E). These results indicate that LTP can be induced in oriens interneurons by using a pairing protocol in ACSF containing normal Ca2+/Mg2+, and further, this LTP is specific to CA1 interneurons in stratum oriens as well as pyramidal cells and is not expressed in other interneurons in R/LM.
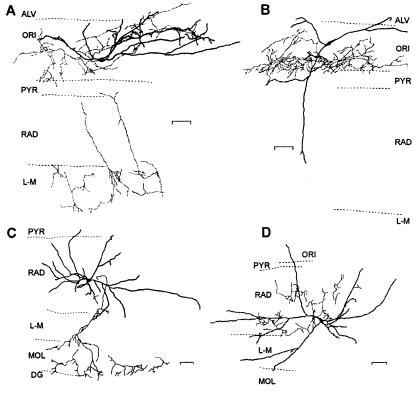
In all experiments, biocytin was included in the patch solution and the identity of interneurons was confirmed morphologically. For interneurons recorded in stratum oriens, both horizontal and vertical types of inhibitory interneurons previously identified in stratum oriens (24, 30) were found to show LTP (Fig. 5 A and B). Interneurons in R/LM that did not show LTP were similar to stellate cells (31) with dendritic and axonal arborizations in stratum radiatum and lacunosum-moleculare (Fig. 5 C and D). In one instance, a presumed interneuron in stratum radiatum was later morphologically identified as a giant cell (14). This particular cell showed LTP but was excluded from the R/LM interneuron group because these cells are nonpyramidal projection cells that show NMDAR-dependent LTP (14). These results indicate LTP is a property of excitatory synapses of specific subtypes of inhibitory interneurons, including horizontal and vertical cells of stratum oriens.
Figure 5.
Camera lucida reconstructions of biocytin-filled interneurons. (A and B) Representative examples of horizontal (A) and vertical (B) subtypes of oriens interneurons showing LTP. Data from the cell in A shown in Fig 1A. (C and D) Examples of stratum radiatum interneurons without LTP. Data from the cell in D shown in Fig 4C. ALV, alveus; ORI, oriens; PYR, pyramidale; RAD, radiatum; L-M, lacunosum-moleculare; MOL, dentate gyrus molecular layer, and DG, granule cell layer. (Scale bars = 50 μm.)
Discussion
The main finding of this study was that putative single fiber excitatory synapses on hippocampal oriens interneurons exhibit a hebbian form of LTP with induction mechanisms distinct from LTP at Schaffer collateral synapses in CA1 pyramidal cells. LTP was produced by concurrent TBS and postsynaptic depolarization, required the activation of mGluR1a, and was expressed as a decrease in failure rate and increase in amplitude (potency) of EPSCs. It was specifically induced in vertical and horizontal interneurons of stratum oriens but was absent in interneurons of R/LM.
These results provide clear evidence of LTP occurring directly at excitatory synapses on specific hippocampal interneurons, under conditions that avoid the passive propagation of LTP from pyramidal cells (19), and thus help resolve the controversy over the presence of LTP in hippocampal interneurons. First, a minimal stimulation paradigm was used in elevated extracellular Ca2+/Mg2+ levels to record putative single fiber EPSCs in interneurons (21, 22). Second, the induction of LTP was found to depend on the pairing of postsynaptic depolarization with synaptic stimulation. Because TBS of presynaptic fibers alone was not sufficient to induce LTP in oriens interneurons, our results exclude a possible contribution of passively propagated LTP from pyramidal cells to interneurons (19) to this form of LTP. The observations that LTP at interneuron synapses requires coincident presynaptic and postsynaptic activity indicates that its induction is hebbian in nature (32). These findings are consistent with the recent demonstration that dendrites of oriens interneurons actively generate action potentials (33). However, the precise cellular events that are triggered by postsynaptic depolarization and the potential role of active propagation of dendritic action potentials remain to be determined.
The induction of LTP at interneuron synapses was NMDAR-independent and mGluR1a-dependent. These results indicate that LTP at excitatory synapses on interneurons involves different induction mechanisms than the NMDAR-dependent LTP at Schaffer collateral synapses on CA1 pyramidal cells (1, 2). The requirement for activation of mGluR1a in LTP induction is consistent with the high level of expression of mGluR1a in oriens interneurons (27, 28). It is also compatible with the prominent intracellular Ca2+ rise that is elicited in oriens interneurons by activation of group I/II mGluRs and that involves a coupling of voltage-dependent Ca2+ channels and intracellular Ca2+ release from ryanodine-sensitive stores (25). Further, these group I/II mGluR-coupled mechanisms were specifically found in oriens but not in stratum radiatum and lacunosum-moleculare interneurons (25). Thus, the selective induction of LTP in certain interneurons (stratum oriens vs. radiatum/lacunosum-moleculare) may be related to the selective expression of these mGluR-linked mechanisms in specific interneuron subtypes (24, 25).
The finding of NMDAR-independent LTP in interneurons is consistent with the absence in these cells of calcineurin and Ca2+/calmodulin-dependent kinase II, key enzymes mediating NMDAR-dependent plasticity in pyramidal cells (20). Interestingly, NMDAR-independent forms of LTP also have been reported at Schaffer collateral synapses in CA1 pyramidal cells (4, 34), as well as at synapses composed of Ca2+-permeable AMPA receptors in amygdala interneurons (35). This NMDAR-independent LTP in pyramidal cells involves voltage-dependent Ca2+ channel and group II/III mGluR (4, 5) activity but requires intense tetanization for its induction (4, 34). The NMDAR-independent LTP found in interneurons in the present study appears dissimilar in some aspects. First, it is induced in interneurons by short episodes of TBS. Second, LTP in interneurons involves the mGluR1a subtype of receptors. Finally, our preliminary data† and recent work of others (36) suggest that group I mGluR activity is associated with Ca2+ responses and inward currents in oriens interneurons. Thus, mGluR1a, and not group II/III mGluRs, may be critical for NMDAR-independent LTP in interneurons.
Our results also imply that long-term plasticity at interneuron excitatory synapses composed of Ca2+-permeable AMPA receptors may be bi-directional because long-term depression is induced at such synapses by 100-Hz tetanization (13). Thus, the type of plasticity observed at these synapses may be stimulus-dependent: intense stimulation, like tetanization, may induce long-term depression, whereas less intense stimulation, like θ-burst activation, may produce LTP. It is noteworthy that the stimulus dependency of these bi-directional changes in synaptic strength is different for interneurons and pyramidal cells. In contrast, in hippocampal pyramidal cells, both TBS and tetanization induce LTP, whereas much lower frequency stimulation, typically 1 Hz, elicits long-term depression (37). These observations provide further evidence that different mechanisms govern long-term plasticity at interneuron and pyramidal cell synapses. The exact mechanisms responsible for LTP induction in interneurons during TBS stimulation, and whether single vs. few axons need to be activated, remain to be determined. Furthermore, given that the activity-dependent relief of the polyamine block of Ca2+-permeable AMPA receptors endows these channels with the capacity to undergo short-term potentiation during repetitive activation (38), it would appear that excitatory synapses containing Ca2+ permeable AMPA receptors in interneurons may be subject to considerable functional plasticity.
Finally, previous work indicates that interneurons in stratum oriens are largely involved in feedback inhibition (30, 39), whereas those in stratum radiatum/lacunosum-moleculare mainly participate in feed-forward inhibition (31). The presence of LTP specifically in oriens but not radiatum/lacunosum-moleculare interneurons indicates that synaptic plasticity may be a specific characteristic of excitatory inputs in feedback inhibitory interneuron networks and not a property of excitatory synapses on all types of interneurons. Interestingly, modeling studies have suggested that long-term synaptic changes at excitatory synapses in feedback inhibitory networks may play a critical role in long-term information processing in hippocampal networks (9). In this context, our results provide a hebbian mechanism for long-term plasticity directly at interneuron excitatory synapses that can contribute to such hippocampal information processing, in addition to the passive propagation of NMDAR-dependent LTP from pyramidal cells to interneurons (9). Our results are also compatible with experimental evidence that sustained activation of single pyramidal cells produces a γ-aminobutyric acid-mediated long-term suppression of excitatory synaptic transmission in pyramidal cells, via NMDAR and mGluR-dependent mechanisms (40). Therefore, these long-term increases in feedback inhibitory function may be partly due to long-term plastic changes directly at interneuron excitatory synapses that depend on mGluR and hebbian mechanisms as described here.
In conclusion, the plasticity observed directly at interneuron excitatory synapses indicates that synaptic efficacy in hippocampal inhibitory networks is modifiable according to hebbian rules and thus represents a mechanism by which long-term synaptic plasticity in inhibitory networks might contribute to complex information processing in the hippocampus (10, 11). The observation that TBS paired with depolarization is an effective stimulation paradigm for its induction further suggests that such synaptic plasticity may take place in a behaviorally relevant context, for instance, during hippocampal theta activity (41).
Acknowledgments
This work was supported by the Canadian Institutes for Health Research (J.-C.L.), the Fonds pour la Formation de Chercheurs et l'Aide à la Recherche (J.-C.L. and Y.P.), the Fonds de la Recherche en Santé du Québec (J.-C.L.), and the Savoy Foundation (Y.P. and F.M.).
Abbreviations
- LTP
long-term potentiation
- EPSC
excitatory postsynaptic current
- NMDAR
N-methyl-d-aspartate receptor
- ACSF
artificial cerebrospinal fluid
- AP5
dl-2-amino-5-phosphonopentanoic acid
- E4CPG
(RS)-α-ethyl-4-carboxyphenylglycine
- MCPG
(RS)-α-methyl-4-carboxyphenylglycine
- LY367385
(+)-2-methyl-4-carboxyphenylglycine
- TBS
θ-burst stimulation
- rmANOVA
repeated measures ANOVA
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate
- KA
kainate
- mGluR
metabotropic glutamate receptor
- R/LM
stratum radiatum near lacunosum-moleculare
- NHPP
N-(4-hydroxyphenylpropanoyl)spermine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Gee, C. & Lacaille, J.-C. (2000) Soc. Neurosci. Abstr. 26, 2342.
References
- 1.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Malenka R, Nicoll R. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 3.Bashir Z I, Bortolotto Z A, Davies C H, Berretta N, Irving A J, Seal A J, Henley J M, Jane D E, Watkins J C, Collingridge G L. Nature (London) 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- 4.Cavus I, Teyler T J. J Neurophysiol. 1996;76:3038–3047. doi: 10.1152/jn.1996.76.5.3038. [DOI] [PubMed] [Google Scholar]
- 5.Grover L M, Yan C. J Neurophysiol. 1999;82:2956–2969. doi: 10.1152/jn.1999.82.6.2956. [DOI] [PubMed] [Google Scholar]
- 6.Freund T F, Buzsaki G. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Buzsaki G, Chrobak J J. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 8.Chapman C A, Lacaille J-C. J Neurosci. 1999;19:8637–8645. doi: 10.1523/JNEUROSCI.19-19-08637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunze H C, Rainnie D G, Hasselmo M E, Barkai E, Hearn E F, McCarley R W, Greene R W. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez Y, Chapman C A, Woodhall G, Robitaille R, Lacaille J-C. Neuroscience. 1999;90:747–757. doi: 10.1016/s0306-4522(98)00531-4. [DOI] [PubMed] [Google Scholar]
- 11.Stelzer A, Slater N T, ten Bruccengate G. Nature (London) 1987;326:698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- 12.Ouardouz M, Lacaille J-C. J Neurophysiol. 1995;73:810–819. doi: 10.1152/jn.1995.73.2.810. [DOI] [PubMed] [Google Scholar]
- 13.Laezza F, Doherty J J, Dingledine R. Science. 1999;285:1411–1414. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- 14.Maccaferri G, McBain C J. J Neurosci. 1996;16:5334–5343. doi: 10.1523/JNEUROSCI.16-17-05334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon L L, Kauer J A. Neuron. 1997;18:295–305. doi: 10.1016/s0896-6273(00)80269-x. [DOI] [PubMed] [Google Scholar]
- 16.Cowan A I, Stricker C, Reece L J, Redman S J. J Neurophysiol. 1998;79:13–20. doi: 10.1152/jn.1998.79.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Lacaille J-C, Kunkel D D, Schwartzkroin P A. In: Neurology and Neurobiology, the Hippocampus: New Vistas. Kohler C, Chan-Palay V, editors. New York: Liss; 1989. pp. 285–303. [Google Scholar]
- 18.Parra P, Gulyas A I, Miles R. Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- 19.McBain C J, Freund T F, Mody I. Trends Neurosci. 1999;22:228–235. doi: 10.1016/s0166-2236(98)01347-2. [DOI] [PubMed] [Google Scholar]
- 20.Sik A, Hajos N, Gulacsi A, Mody I, Freund T F. Proc Natl Acad Sci USA. 1998;95:3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raastad M. Eur J Neurosci. 1995;7:1882–1888. doi: 10.1111/j.1460-9568.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 22.Stevens C F, Wang Y. Nature (London) 1994;371:704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- 23.Washburn M S, Numberger M, Zhang S, Dingledine R. J Neurosci. 1997;17:9393–9406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBain C J, Di Chiara T J, Kauer J A. J Neurosci. 1994;14:4433–4445. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodhall G, Gee C E, Robitaille R, Lacaille J-C. J Neurophysiol. 1999;81:371–382. doi: 10.1152/jn.1999.81.1.371. [DOI] [PubMed] [Google Scholar]
- 26.Bedingfield J S, Kemp M C, Jane D E, Tse H W, Roberts P J, Watkins J C. Br J Pharmacol. 1995;116:3323–3329. doi: 10.1111/j.1476-5381.1995.tb15142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Nature (London) 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- 28.Baude A, Nusser Z, Roberts J D, Mulvihill E, McIlhinney R A, Somogyi P. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- 29.Clark B P, Baker S R, Goldsworthy J, Harris J R, Kingston A E. Bioorg Med Chem Lett. 1997;7:2777–2780. [Google Scholar]
- 30.Lacaille J-C, Mueller A L, Kunkel D D, Schwartzkroin P A. J Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacaille J-C, Schwartzkroin P A. J Neurosci. 1988;8:1411–1424. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linden D J. Neuron. 1999;22:661–666. doi: 10.1016/s0896-6273(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 33.Martina M, Vida I, Jonas P. Science. 2000;287:295–300. doi: 10.1126/science.287.5451.295. [DOI] [PubMed] [Google Scholar]
- 34.Stricker C, Cowan A I, Field A C, Redman S J. J Physiol (London) 1999;520:513–525. doi: 10.1111/j.1469-7793.1999.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahanty N K, Sah P. Nature (London) 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- 36.van Hooft J A, Giuffrida R, Blatow M, Monyer H. J Neurosci. 2000;20:3544–3551. doi: 10.1523/JNEUROSCI.20-10-03544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bear M, Malenka R C. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 38.Rozov A, Zilberter Y, Wollmuth L P, Burnashev N. J Physiol (London) 1998;511:361–377. doi: 10.1111/j.1469-7793.1998.361bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blasco-Ibanez J M, Freund T F. Eur J Neurosci. 1995;7:2170–2180. doi: 10.1111/j.1460-9568.1995.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 40.Yanovsky Y, Haas H L. J Physiol (London) 1999;515:757–767. doi: 10.1111/j.1469-7793.1999.757ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larson J, Wong D, Lynch G. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]